Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Somatostatin Receptor Scintigraphy with Somatostatin Analogues
| Somatostatine Analogues | Abbreviation | Sequence | Radiolabeled Compounds | Indication | SST Affinity | Refs. |
|---|---|---|---|---|---|---|
| EDDA-HYNIC-octreotide | HYNIC-TOC | HYNIC-DPhe-Cys-Tyr-DTrp-Lys-Thr-Cys-Thr-ol | 99mTc | PRS | 2, 3, 5 | [43] |
| DTPA-octreotide | DTPA-OC | DTPA-DPhe-Cys-Phe-DTrp-Lys-Thr-Cys-Thr-ol | 111In | PRRT | 2, 3, 5 | [6,44,45,46] |
| DOTA-octreotide | DOTA-OC | DOTA-DPhe-Cys-Phe-DTrp-Lys-Thr-Cys-Thr-ol | 111In, 90Y | PRRT | 3 | [42,47,48] |
| DOTA-Tyr3-octreotide | DOTA-TOC | DOTA-DPhe-Cys-Tyr-DTrp-Lys-Thr-Cys-Thr-ol | 123I, 64Cu, 68Ga, 90Y, 177Lu, 225Ac, 213Bi | PRRT | 2, 5 | [49,50,51,52] |
| DOTA-Tyr3-octreotate | DOTA-TATE | DOTA-DPhe-Cys-Tyr-DTrp-Lys-Thr-Cys-Thr-COOH | 131I, 64Cu, 68Ga, 177Lu, 90Y, 225Ac, 213Bi | PRRT, PRS | 2 | [52,53,54,55] |
| DOTAGA-Tyr3-octreotate | DOTAGA-TATE | DOTAGA-DPhe-Cys-Tyr-DTrp-Lys-Thr-Cys-Thr-COOH | 68Ga | PRS | NA | [56] |
| DOTAGA-octreotide | DOTAGA-TOC | DOTAGA-DPhe-Cys-Tyr-DTrp-Lys-Thr-Cys-Thr-ol | 68Ga | PRS | NA | [56] |
| DOTA-Nal3-octreotide | DOTA-NOC | DOTA-DPhe-Cys-Nal-DTrp-Lys-Thr-Cys-Thr-ol | 111In, 90Y, 68Ga | PRRT, PRS | 2, 3, 5 | [57,58] |
3. [111In]In-1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetraacetic Acid (DOTA)-Octreotide Derivatives
4. [99mTc]Tc-HYNIC-Octreotide Derivatives
5. PET in Diagnosis of NET
5.1. [68Ga]Ga-DOTA-Octreotide Derivatives
5.2. [64Cu]Cu-DOTA-Octreotide Derivatives
6. Antagonists versus Agonists
7. Conclusions and Future Research Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Kawasaki, K.; Fujii, M.; Sato, T. Gastroenteropancreatic neuroendocrine neoplasms: Genes, therapies and models. Dis. Models Mech. 2018, 11, dmm029595. [Google Scholar] [CrossRef] [Green Version]
- Klöppel, G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef]
- Miller, N.R.; Walsh, F.B.; Hoyt, W.F. Walsh and Hoyt’s Clinical Neuro-Ophthalmology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 2. [Google Scholar]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1441–1448. [Google Scholar] [CrossRef]
- Reubi, J.; Waser, B.; Schaer, J.-C.; Laissue, J.A. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef]
- Buchmann, I.; Henze, M.; Engelbrecht, S.; Eisenhut, M.; Runz, A.; Schäfer, M.; Schilling, T.; Haufe, S.; Herrmann, T.; Haberkorn, U. Comparison of 68Ga-DOTATOC PET and 111 In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1617–1626. [Google Scholar] [CrossRef]
- Ueberberg, B.; Tourne, H.; Redman, A.; Walz, M.; Schmid, K.; Mann, K.; Petersenn, S. Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm. Metab. Res. 2005, 37, 722–728. [Google Scholar] [CrossRef]
- Oda, Y.; Tanaka, Y.; Naruse, T.; Sasanabe, R.; Tsubamoto, M.; Funahashi, H. Expression of somatostatin receptor and effects of somatostatin analog on pancreatic endocrine tumors. Surg. Today 2002, 32, 690–694. [Google Scholar] [CrossRef]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allia, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.; Bussolati, G. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Cescato, R.; Gloor, B.; Stettler, C.; Christ, E. Internalized somatostatin receptor subtype 2 in neuroendocrine tumors of octreotide-treated patients. J. Clin. Endocrinol. Metab. 2010, 95, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Deng, X.; Duan, C.; Chen, C.; Xiang, J.; Lu, Y.; Ma, O. Somatostatin receptors SSTR2 and SSTR5 are expressed in the human thoracic duct. Lymphology 2011, 44, 21–28. [Google Scholar]
- Ito, T.; Jensen, R.T. Molecular imaging in neuroendocrine tumors: Recent advances, controversies, unresolved issues, and roles in management. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 15. [Google Scholar] [CrossRef] [Green Version]
- Öberg, K.; Sundin, A. Imaging of neuroendocrine tumors. Imaging Endocr. Disord. 2016, 45, 142–151. [Google Scholar]
- Toumpanakis, C.; Kim, M.K.; Rinke, A.; Bergestuen, D.S.; Thirlwell, C.; Khan, M.S.; Salazar, R.; Oberg, K. Combination of cross-sectional and molecular imaging studies in the localization of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 2014, 99, 63–74. [Google Scholar] [CrossRef]
- Baumann, T.; Rottenburger, C.; Nicolas, G.; Wild, D. Gastroenteropancreatic neuroendocrine tumours (GEP-NET)–imaging and staging. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 45–57. [Google Scholar] [CrossRef]
- Kjaer, A.; Knigge, U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand. J. Gastroenterol. 2015, 50, 740–747. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Xu, X.; Zhao, M.; Zhang, B.; Deng, S.; Wu, Y. 64Cu-based radiopharmaceuticals in molecular imaging. Technol. Cancer Res. Treat. 2019, 18, 1533033819830758. [Google Scholar] [CrossRef] [Green Version]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Loft, M.; Johnbeck, C.B.; Carlsen, E.A.; Johannesen, H.H.; Oturai, P.; Langer, S.W.; Knigge, U.; Kjaer, A. Initial experience with 64Cu-DOTATATE digital PET of patients with neuroendocrine neoplasms: Comparison with analog PET. Diagnostics 2021, 11, 350. [Google Scholar] [CrossRef]
- Mirzaei, S.; Revheim, M.-E.; Raynor, W.; Zehetner, W.; Knoll, P.; Zandieh, S.; Alavi, A. 64Cu-DOTATOC PET-CT in patients with neuroendocrine tumors. Oncol. Ther. 2020, 8, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Malmberg, C.; Ripa, R.S.; Johnbeck, C.B.; Knigge, U.; Langer, S.W.; Mortensen, J.; Oturai, P.; Loft, A.; Hag, A.M.; Kjaer, A. 64Cu-DOTATATE for noninvasive assessment of atherosclerosis in large arteries and its correlation with risk factors: Head-to-head comparison with 68Ga-DOTATOC in 60 patients. J. Nucl. Med. 2015, 56, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.A.; Johnbeck, C.B.; Binderup, T.; Loft, M.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W. 64Cu-DOTATATE PET/CT and prediction of overall and progression-free survival in patients with neuroendocrine neoplasms. J. Nucl. Med. 2020, 61, 1491–1497. [Google Scholar] [CrossRef]
- Vahidfar, N.; Aghanejad, A.; Ahmadzadehfar, H.; Farzanehfar, S.; Eppard, E. Theranostic advances in breast cancer in nuclear medicine. Int. J. Mol. Sci. 2021, 22, 4597. [Google Scholar] [CrossRef]
- Vahidfar, N.; Eppard, E.; Farzanehfar, S.; Yordanova, A.; Fallahpoor, M.; Ahmadzadehfar, H. An impressive approach in nuclear medicine: Theranostics. PET Clin. 2021, 16, 327–340. [Google Scholar] [CrossRef]
- Vahidfar, N.; Fallahpoor, M.; Farzanehfar, S.; Divband, G.; Ahmadzadehfar, H. Historical review of pharmacological development and dosimetry of PSMA-based theranostics for prostate cancer. J. Radioanal. Nucl. Chem. 2019, 322, 237–248. [Google Scholar] [CrossRef]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef]
- Dalm, S.U.; Nonnekens, J.; Doeswijk, G.N.; de Blois, E.; van Gent, D.C.; Konijnenberg, M.W.; de Jong, M. Comparison of the therapeutic response to treatment with a 177Lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J. Nucl. Med. 2016, 57, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Wild, D.; Fani, M.; Behe, M.; Brink, I.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J. Nucl. Med. 2011, 52, 1412–1417. [Google Scholar] [CrossRef] [Green Version]
- Gurusamy, K.S.; Ramamoorthy, R.; Sharma, D.; Davidson, B.R. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst. Rev. 2009, 2, CD007060. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Kam, B.L.; Van Essen, M.; Teunissen, J.J.; van Eijck, C.H.; Valkema, R.; De Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2010, 17, R53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenning, E.P.; Breeman, W.A.; Kooij, P.P.; Lameris, J.; Bakker, W.H.; Koper, J.; Ausema, L.; Reubi, J.C.; Lamberts, S.W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 333, 242–244. [Google Scholar] [CrossRef]
- Krenning, E.; Kwekkeboom, D.J.; Bakker, W.e.a.; Breeman, W.; Kooij, P.; Oei, H.; van Hagen, M.; Postema, P.; de Jong, M.; Reubi, J.-C. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe 1]-and [123I-Tyr 3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 1993, 20, 716–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, W.H.; Krenning, E.P.; Breeman, W.A.; Kooij, P.; Reubi, J.-C.; Koper, J.W.; de Jong, M.; Laméris, J.S.; Visser, T.J.; Lamberts, S.W. In vivo use of a radioiodinated somatostatin analogue: Dynamics, metabolism, and binding to somatostatin receptor-positive tumors in man. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1991, 32, 1184–1189. [Google Scholar]
- Hofland, L.J.; Lamberts, S.W. Somatostatin receptors and disease: Role of receptor subtypes. Bailliere’s Clin. Endocrinol. Metab. 1996, 10, 163–176. [Google Scholar] [CrossRef] [Green Version]
- O’Carroll, A.-M.; Raynor, K.; Lolait, S.J.; Reisine, T. Characterization of cloned human somatostatin receptor SSTR5. Mol. Pharmacol. 1994, 46, 291–298. [Google Scholar]
- Kumar, U.; Grant, M. Somatostatin and somatostatin receptors. Cell. Pept. Horm. Synth. Secret. Pathw. 2009, 50, 97–120. [Google Scholar]
- Petersenn, S.; Rasch, A.; Bohnke, C.; Schulte, H. Identification of an upstream pituitary-active promoter of human somatostatin receptor subtype 5. Endocrinology 2002, 143, 2626–2634. [Google Scholar] [CrossRef]
- Panetta, R.; Greenwood, M.T.; Warszynska, A.; Demchyshyn, L.L.; Day, R.; Niznik, H.B.; Srikant, C.B.; Patel, Y.C. Molecular cloning, functional characterization, and chromosomal localization of a human somatostatin receptor (somatostatin receptor type 5) with preferential affinity for somatostatin-28. Mol. Pharmacol. 1994, 45, 417–427. [Google Scholar]
- Ye, Y.; Xiao, C.; Li, Y.; Shan, Y.; Li, J.; Jiang, D.; Li, J.; Han, C.; Li, W. The Correlations between the Expression of SSTR2, SSTR5 Proteins and Clinicopathological Parameters as well as Prognosis of Gastric Neuroendocrine Neoplasms. 2020. Available online: https://www.researchsquare.com/article/rs-66797/v1 (accessed on 3 February 2021).
- Laznicek, M.; Laznickova, A.; Maecke, H.R. Receptor affinity and preclinical biodistribution of radiolabeled somatostatin analogs. Anticancer Res. 2012, 32, 761–766. [Google Scholar]
- Garai, I.; Barna, S.; Nagy, G.; Forgacs, A. Limitations and pitfalls of 99mTc-EDDA/HYNIC-TOC (Tektrotyd) scintigraphy. Nucl. Med. Rev. 2016, 19, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenning, E.; De Jong, M.; Kooij, P.; Breeman, W.; Bakker, W.; De Herder, W.; Van Eijck, C.; Kwekkeboom, D.; Jamar, F.; Pauwels, S. Radiolabelled somatostatin analogue (s) for peptide receptor scintigraphy and radionuclide therapy. Ann. Oncol. 1999, 10, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Serafini, A.N.; Telischi, F.F.; Civantos, F.J.; Falcone, S. 111In octreotide scintigraphy in the evaluation of head and neck lesions. Am. J. Neuroradiol. 1997, 18, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontico, M.; Frantellizzi, V.; Cosma, L.; De Vincentis, G. 111In-Octreoscan SPECT/CT hybrid imaging and 68Ga-DOTANOC PET/CT in neuroendocrine adenoma of the middle ear (NAME). Indian J. Radiol. Imaging 2020, 30, 400. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Schmitt, J.S.; Chen, J.; Waser, B.; Reubi, J.-C.; de Jong, M.; Schulz, S.; Maecke, H.R. Design, synthesis, and biological evaluation of somatostatin-based radiopeptides. Chem. Biol. 2006, 13, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Forrer, F.; Riedweg, I.; Maecke, H.R.; Mueller-Brand, J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 334. [Google Scholar]
- Kratochwil, C.; Giesel, F.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213 Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Ur, E.; Bomanji, J.; Mather, S.; Britton, K.; Wass, J.; Grossman, A.; Besser, G. Localization of neuroendocrine tumours and insulinomas using radiolabeled somatostatin analogues, 123I-Try3-octreotide and 111in-pentatreitide. Clin. Endocrinol. 1993, 38, 501–506. [Google Scholar] [CrossRef]
- Jamous, M.; Haberkorn, U.; Mier, W. Synthesis of peptide radiopharmaceuticals for the therapy and diagnosis of tumor diseases. Molecules 2013, 18, 3379–3409. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Breeman, W.A.; Klette, I.; Gottschaldt, M.; Odparlik, A.; Baehre, M.; Tworowska, I.; Schultz, M.K. Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat. Protoc. 2016, 11, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, E.; Caldeira Filho, J.; Nagamati, L.; Muramoto, E.; Colturato, M.; Couto, R.; Pujatti, P.; Mengatti, J.; Silva, C. A comparative study of 131I and 177Lu labeled somatostatin analogues for therapy of neuroendocrine tumours. Appl. Radiat. Isot. 2009, 67, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, H.; Yu, J.; Han, X.; Xie, Q.; Liu, T.; Xia, C.; Li, N.; Yang, Z. 68Ga/177Lu-labeled DOTA-TATE shows similar imaging and biodistribution in neuroendocrine tumor model. Tumor Biol. 2017, 39, 1010428317705519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satpati, D.; Shinto, A.; Kamaleshwaran, K.; Sarma, H.D.; Dash, A. Preliminary PET/CT imaging with somatostatin analogs [68Ga] DOTAGA-TATE and [68Ga] DOTAGA-TOC. Mol. Imaging Biol. 2017, 19, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Schmitt, J.S.; Ginj, M.; Mäcke, H.R.; Bernard, B.F.; Krenning, E.; De Jong, M.; Wenger, S.; Reubi, J.-C. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Baum, R. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: Characterization of uptake in normal organs and tumor lesions. QJ Nucl. Med. Mol. Imaging 2010, 54, 61–67. [Google Scholar]
- Virgolini, I.; Patri, P.; Novotny, C.; Traub, T.; Leimer, M.; Füger, B.; Li, S.; Angelberger, P.; Raderer, M.; Wogritsch, S. Comparative somatostatin receptor scintigraphy using in-111-DOTA-lanreotide and in-111-DOTA-Tyr3-octreotide versus F-18-FDG-PET for evaluation of somatostatin receptor-mediated radionuclide therapy. Ann. Oncol. 2001, 12, S41–S45. [Google Scholar] [CrossRef]
- Bakker, W.; Krenning, E.; Reubi, J.-C.; Breeman, W.; Setyono-Han, B.; De Jong, M.; Kooij, P.; Bruns, C.; Van Hagen, P.; Marbach, P. In vivo application of [111In-DTPA-D-Phe1]-octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci. 1991, 49, 1593–1601. [Google Scholar] [CrossRef]
- Krenning, E.; Bakker, W.; Kooij, P.; Breeman, W.; Oei, H.; De Jong, M.; Reubi, J.; Visser, T.; Bruns, C.; Kwekkeboom, D. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1992, 33, 652–658. [Google Scholar]
- Mikołajczak, R.; Maecke, H.R. Radiopharmaceuticals for somatostatin receptor imaging. Nucl. Med. Rev. 2016, 19, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: A systematic review and meta-analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decristoforo, C.; Maina, T.; Nock, B.; Gabriel, M.; Cordopatis, P.; Moncayo, R. 99mTc-Demotate 1: First data in tumour patients—Results of a pilot/phase I study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A.; Cordopatis, P.; Bernard, B.F.; Breeman, W.A.; van Gameren, A.; van den Berg, R.; Reubi, J.-C.; Krenning, E.P.; de Jong, M. [99mTc] Demotate 2 in the detection of sst2-positive tumours: A preclinical comparison with [111In] DOTA-tate. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Radioisotopes, I.; No, R.S. 1: Technetium 99m Radiopharmaceuticals: Status and Trends. 2009. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1405_web.pdf (accessed on 1 April 2022).
- Decristoforo, C.; Melendez-Alafort, L.; Sosabowski, J.K.; Mather, S.J. 99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: Preclinical evaluation and comparison with 111In-octreotide. J. Nucl. Med. 2000, 41, 1114–1119. [Google Scholar]
- Rufini, V.; Calcagni, M.L.; Baum, R.P. Imaging of neuroendocrine tumors. Semin. Nucl. Med. 2006, 36, 228–247. [Google Scholar] [CrossRef]
- Boutsikou, E.; Porpodis, K.; Chatzipavlidou, V.; Hardavella, G.; Gerasimou, G.; Domvri, K.; Papadopoulos, N.; Avramidou, V.; Spyratos, D.; Kontakiotis, T. Predictive value of 99MTC-hynic-toc scintigraphy in lung neuroendocrine tumor diagnosis. Technol. Cancer Res. Treat. 2019, 18, 1533033819842586. [Google Scholar] [CrossRef] [Green Version]
- Saponjski, J.; Macut, D.; Petrovic, N.; Ognjanovic, S.; Popovic, B.; Bukumiric, Z.; Saranović, D.S. Diagnostic and prognostic value of 99mTc-Tektrotyd scintigraphy and 18F-FDG PET/CT in a single-center cohort of neuroendocrine tumors. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Gabriel, M.; Muehllechner, P.; Decristoforo, C.; Guggenberg, E.V. 99-mTc-EDDA/HYNIC-Tyr (3)-octreotide for staging and follow-up of patients with neuroendocrine gastro-entero-pancreatic tumors. Q. J. Nucl. Med. Mol. Imaging 2005, 49, 237–244. [Google Scholar]
- Treglia, G.; Sadeghi, R.; Giovinazzo, F.; Galiandro, F.; Annunziata, S.; Muoio, B.; Kroiss, A.S. PET with different radiopharmaceuticals in neuroendocrine neoplasms: An umbrella review of published meta-analyses. Cancers 2021, 13, 5172. [Google Scholar] [CrossRef]
- Alevroudis, E.; Spei, M.-E.; Chatziioannou, S.N.; Tsoli, M.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Clinical utility of 18F-FDG PET in neuroendocrine tumors prior to peptide receptor radionuclide therapy: A systematic review and meta-analysis. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef]
- Barrio, M.; Czernin, J.; Fanti, S. The impact of SSTR-directed PET/CT on the management of patients with neuroendocrine tumor: A systematic review and meta-analysis. J. Nucl. Med. 2017, 5, 756–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Dosso, S.; Treglia, G.; Pascale, M.; Tamburello, A.; Santhanam, P.; Kroiss, A.S.; Pereira Mestre, R.; Saletti, P.; Giovanella, L. Detection rate of unknown primary tumour by using somatostatin receptor PET/CT in patients with metastatic neuroendocrine tumours: A meta-analysis. Endocrine 2019, 64, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, Y.-I. Prognostic value of maximum standardized uptake value in 68Ga-somatostatin receptor positron emission tomography for neuroendocrine tumors: A systematic review and meta-analysis. Clin. Nucl. Med. 2019, 44, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kan, Y.; Yang, J.-g. Clinical value of 68Ga-DOTA-SSTR PET/CT in the diagnosis and detection of neuroendocrine tumors of unknown primary origin: A systematic review and meta-analysis. Acta Radiol. 2021, 62, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kan, Y.; Ge, B.H.; Yuan, L.; Li, C.; Zhao, W. Diagnostic role of gallium-68 DOTATOC and gallium-68 DOTATATE PET in patients with neuroendocrine tumors: A meta-analysis. Acta Radiol. 2014, 55, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Fiz, F.; Bottoni, G.; Ugolini, M.; Noordzij, W.; Trimboli, P. Head-to-head comparison between 18F-DOPA PET/CT and 68Ga-DOTA peptides PET/CT in detecting intestinal neuroendocrine tumours: A systematic review and meta-analysis. Clin. Endocrinol. 2021, 95, 595–605. [Google Scholar] [CrossRef]
- De Camargo Etchebehere, E.C.S.; de Oliveira Santos, A.; Gumz, B.; Vicente, A.; Hoff, P.G.; Corradi, G.; Ichiki, W.A.; de Almeida Filho, J.G.; Cantoni, S.; Camargo, E.E. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: A prospective trial. J. Nucl. Med. 2014, 55, 1598–1604. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Graham, M.M.; Gu, X.; Ginader, T.; Breheny, P.; Sunderland, J.J. 68Ga-DOTATOC imaging of neuroendocrine tumors: A systematic review and metaanalysis. J. Nucl. Med. 2017, 58, 1452–1458. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-H.; Chang, Y.-C.; Hwang, T.-L.; Chen, J.-S.; Chou, W.-C.; Hsieh, C.-H.; Yeh, T.-S.; Hsu, J.-T.; Yeh, C.-N.; Tseng, J.-H. 68Ga-DOTATOC and 18F-FDG PET/CT for identifying the primary lesions of suspected and metastatic neuroendocrine tumors: A prospective study in Taiwan. J. Formos. Med. Assoc. 2018, 117, 480–487. [Google Scholar] [CrossRef]
- Hofmann, M.; Maecke, H.; Börner, A.; Weckesser, E.; Schöffski, P.; Oei, M.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, G. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Lee, B.N.; Hassan, S.Z.A. Diagnostic value of 68Ga-DOTATATE PET/CT in liver metastases of neuroendocrine tumours of unknown origin. Nucl. Med. Mol. Imaging 2014, 48, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Feghali, K.A.; Yeboa, D.N.; Chasen, B.; Gule, M.K.; Johnson, J.M.; Chung, C. The use of 68Ga-DOTATATE PET/CT in the non-invasive diagnosis of optic nerve sheath meningioma: A case report. Front. Oncol. 2018, 8, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. Differential uptake of 68Ga-DOTATOC and 68Ga-DOTATATE in PET/CT of gastroenteropancreatic neuroendocrine tumors. In Theranostics, Gallium-68, and Other Radionuclides; Springer: Berlin/Heidelberg, Germany, 2013; pp. 353–371. [Google Scholar]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: First-in-humans study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Jødal, L.; Le Loirec, C.; Champion, C. Positron range in PET imaging: Non-conventional isotopes. Phys. Med. Biol. 2014, 59, 7419. [Google Scholar] [CrossRef]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar]
- Delpassand, E.S.; Ranganathan, D.; Wagh, N.; Shafie, A.; Gaber, A.; Abbasi, A.; Kjaer, A.; Tworowska, I.; Núñez, R. 64Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor–positive neuroendocrine tumors: Results of the first US prospective, reader-masked clinical trial. J. Nucl. Med. 2020, 61, 890–896. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Kjær, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol. 2014, 10, 2259–2277. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.; Lipp, R.W. Peptide and pseudo-peptide (PSMA) Cu-64 radiopharmaceuticals. Q. J. Nucl. Med. Mol. Imaging 2020, 4, 364–370. [Google Scholar]
- Loft, M.; Carlsen, E.A.; Johnbeck, C.B.; Johannesen, H.H.; Binderup, T.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K. 64Cu-DOTATATE PET in patients with neuroendocrine neoplasms: Prospective, head-to-head comparison of imaging at 1 hour and 3 hours after injection. J. Nucl. Med. 2021, 62, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Overview of receptor interactions of agonists and antagonists. Curr. Protoc. Pharmacol. 2008, 42, 4.1.1–4.1.24. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of somatostatin receptor–positive tumors using 64Cu-and 68Ga-somatostatin antagonists: The chelate makes the difference. J. Nucl. Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cescato, R.; Schulz, S.; Waser, B.; Eltschinger, V.; Rivier, J.E.; Wester, H.-J.; Culler, M.; Ginj, M.; Liu, Q.; Schonbrunn, A. Internalization of sst2, sst3, and sst5 receptors: Effects of somatostatin agonists and antagonists. J. Nucl. Med. 2006, 47, 502–511. [Google Scholar] [PubMed]
- Fani, M.; Peitl, P.K.; Velikyan, I. Current status of radiopharmaceuticals for the theranostics of neuroendocrine neoplasms. Pharmaceuticals 2017, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B.; Mäcke, H.; Rivier, J. Highly increased 125I-JR11 antagonist binding in vitro reveals novel indications for sst2 targeting in human cancers. J. Nucl. Med. 2017, 58, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, S.; Pandit-Taskar, N.; Reidy, D.; Beattie, B.J.; Lyashchenko, S.K.; Lewis, J.S.; Bodei, L.; Weber, W.A.; O’Donoghue, J.A. Biodistribution and radiation dose estimates for 68Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 677–685. [Google Scholar] [CrossRef]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O’Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gönen, M.; Lohrmann, C. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist 177Lu-satoreotide tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef] [Green Version]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [Green Version]
- Fani, M.; Nicolas, G.P.; Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med. 2017, 58, 61S–66S. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
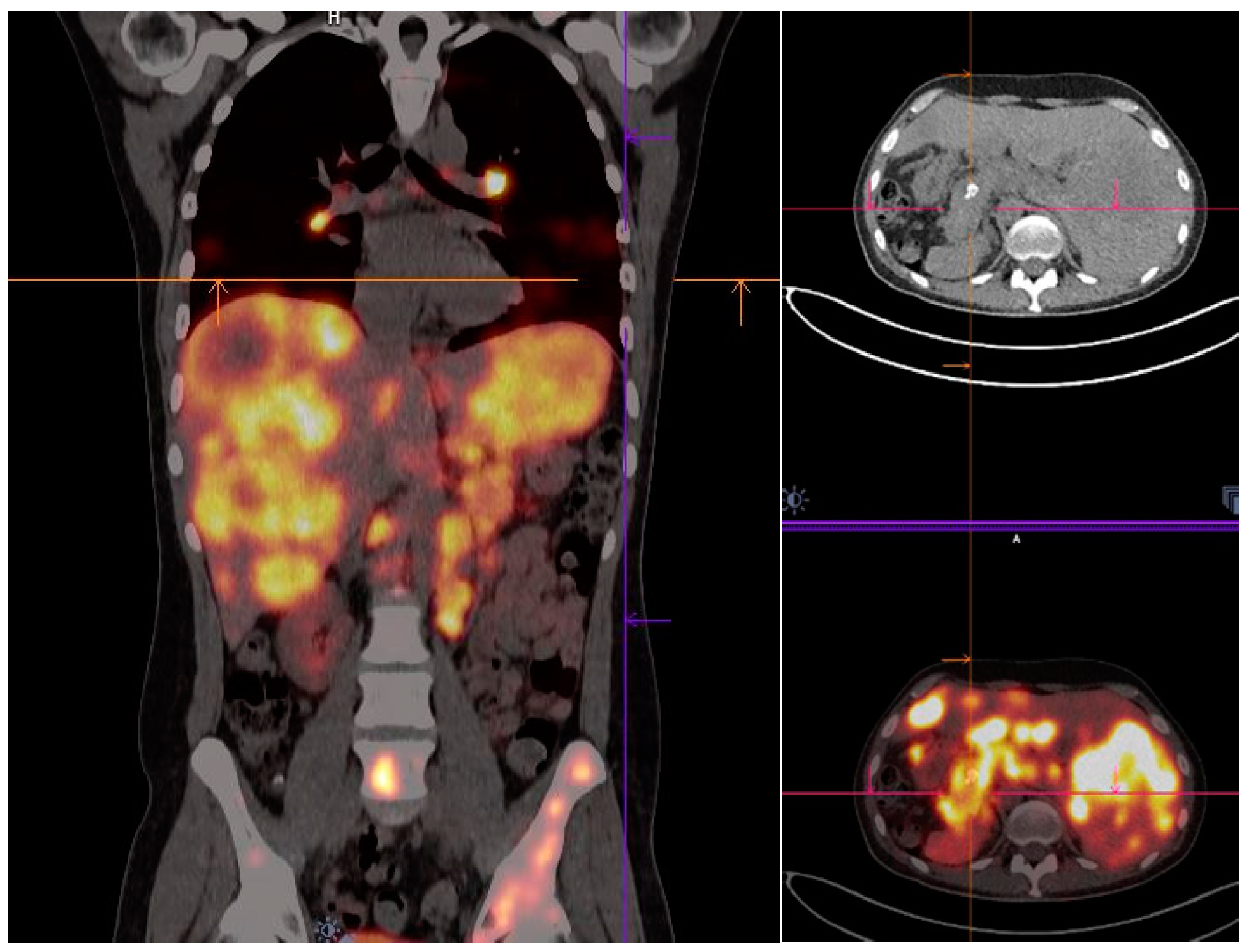

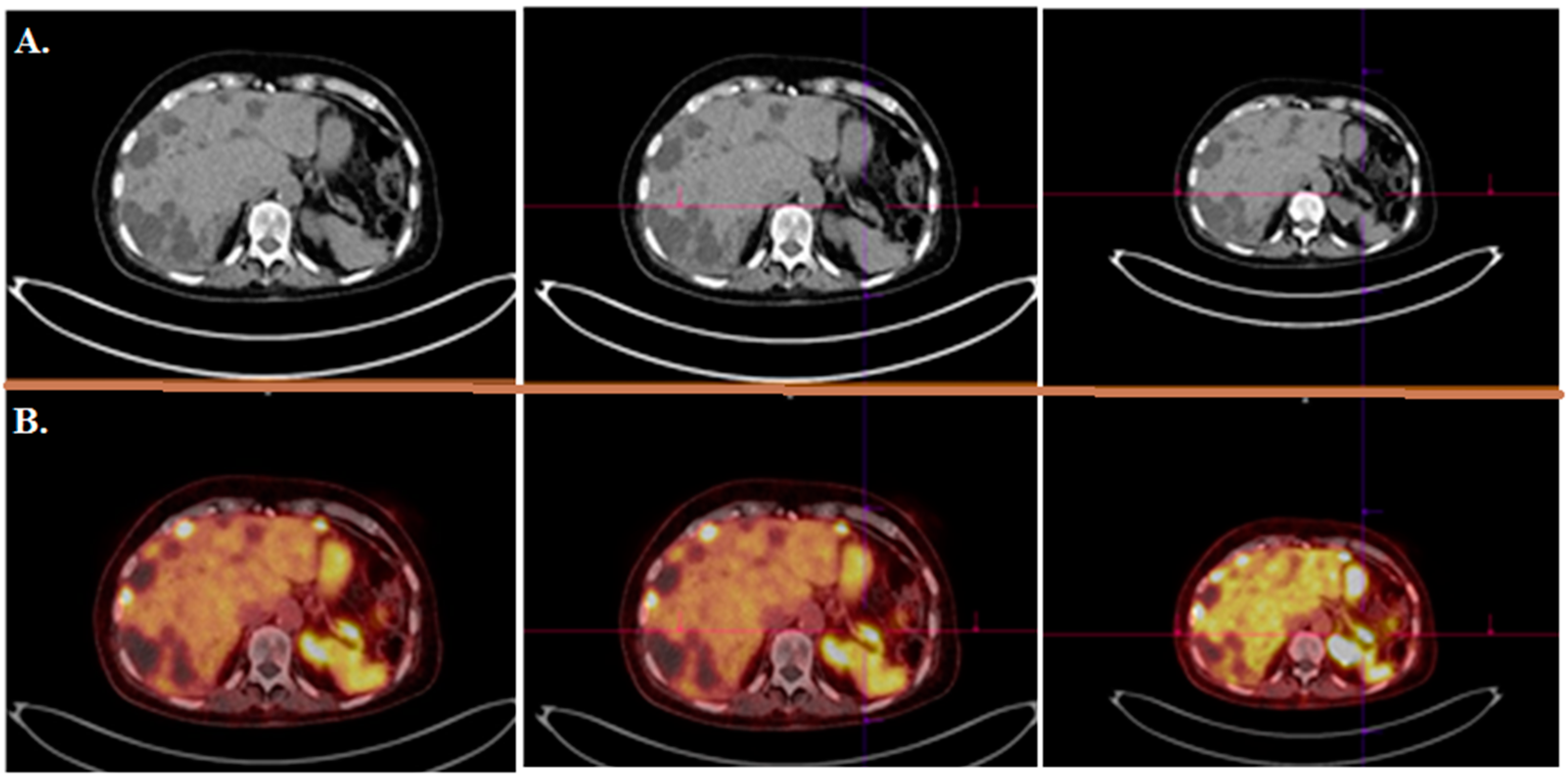
| Radionuclide/Physical Properties | Half-Life (t1/2) | Decay Mode | Energy (Kev) | Source | Application |
|---|---|---|---|---|---|
| 68Ga | 67.71 min | EC (10.49%) β+ (89.14%) | 1899 822 | Generator/Cyclotron | Imaging |
| 64Cu | 12.7 h | β+ (19%) γ (43%) β- (38.4%) | 657 511–1346 573 | Reactor | Imaging/Therapy |
| 225Ac | 9.9 days | Pure α | 5935.1 | Cyclotron | Therapy |
| 177Lu | 6.7 days | β- (82.6%) γ (17.4%) | 497–384–176 208–113 | Reactor | Therapy/Imaging |
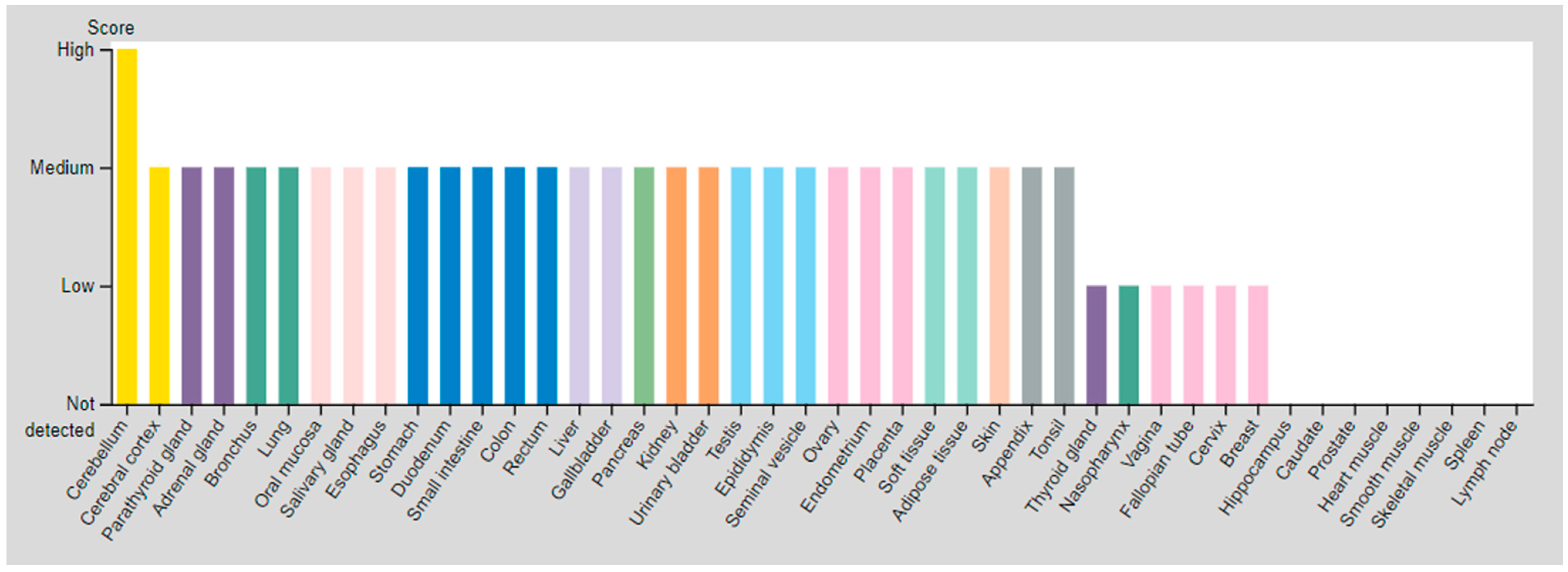
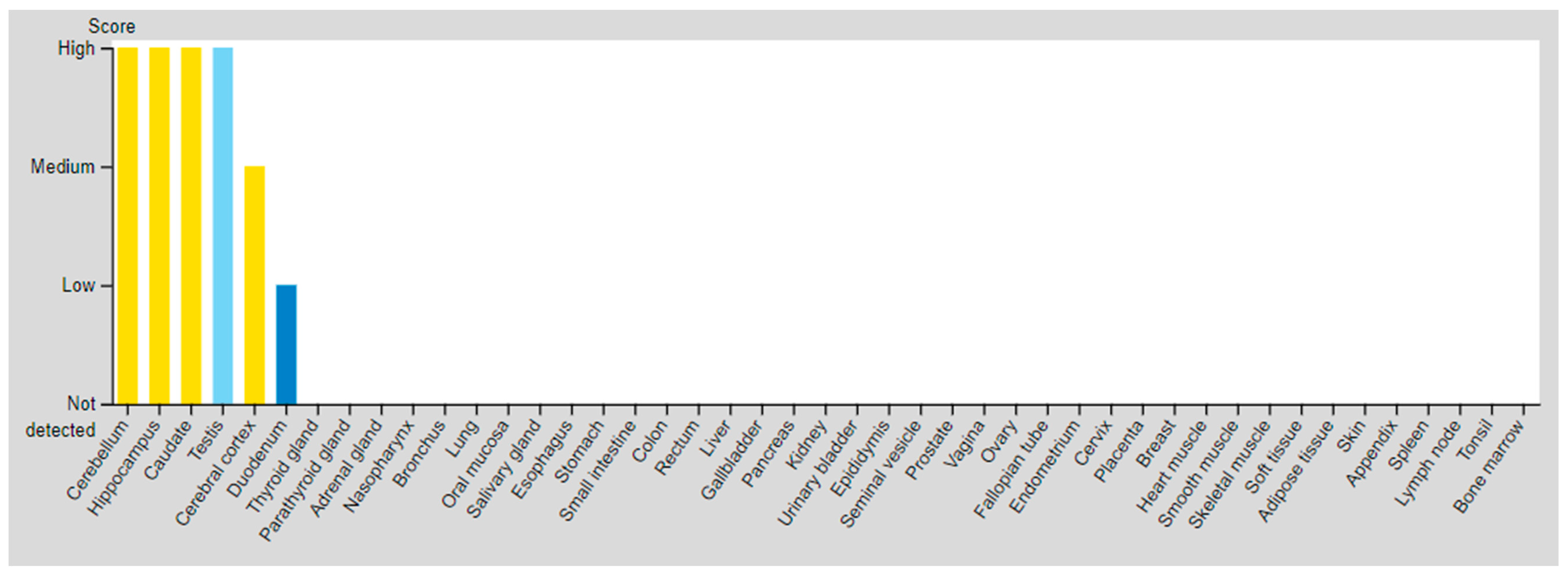
| SSTR | Critical Organs’ Tissue (High Expressed) | Critical Organs’ Tissue (Medium Expressed) | Critical Organs’ Tissue (Low Expressed) | |
|---|---|---|---|---|
| SSTR2 | Cerebellum | Parathyroid gland Adrenal gland Bronchus Lung Oral mucosa Salivary gland Esophagus Stomach Duodenum Small intestine Colon Rectum Liver Gallbladder Pancreas Kidney Urinary bladder Testis | Epididymis Seminal vesicle Prostate Ovary Endometrium Placenta Adipose tissue Peripheral nerve Fibroblasts Keratinocytes Langerhans Melanocytes Epidermal cells Glandular cells Squamous epidermal cells | Cerebral cortex Thyroid gland Nasopharynx Vagina Fallopian tube Cervix, uterine Breast |
| SSTR3 | Testis (Pachytene spermatocytes/Round or early spermatids) | Cerebral cortex Hippocampal formation Basal ganglia Cerebellum Testis (Peritubular cells) | Duodenum Testis (Spermatogonia cells) | |
| [64Cu]Cu-DOTA-Octreotide Derivatives | Disease | Patients Included in the Study | Year | Result | Refs. |
|---|---|---|---|---|---|
| [64Cu]Cu-DOTA-TATE | NET | 12 patients divided into 3 dose groups | 2020 | This protocol was introduced as a safe imaging method provides high quality and accurate images using optimal dose of 148 MBq (4.0 mCi) injection | [92] |
| NET | 60 | 2015 | Potential role of 64Cu-DOTATATE in the assessment of atherosclerosis was confirmed | [23] | |
| NEN | 128 | 2020 | The study demonstrated prediction potency of [64Cu]Cu-DOTA-TATE in PFS | [24] | |
| NET | 112 | 2015 | Superiority of [64Cu]Cu-DOTA-TATE over [111In]In-DTPA-OC was proved | [5] | |
| NEN | 35 | 2020 | Excellent performance of [64Cu]Cu-DOTA-TATE PET/CT during 1–3 h after injection was clarified | [95] | |
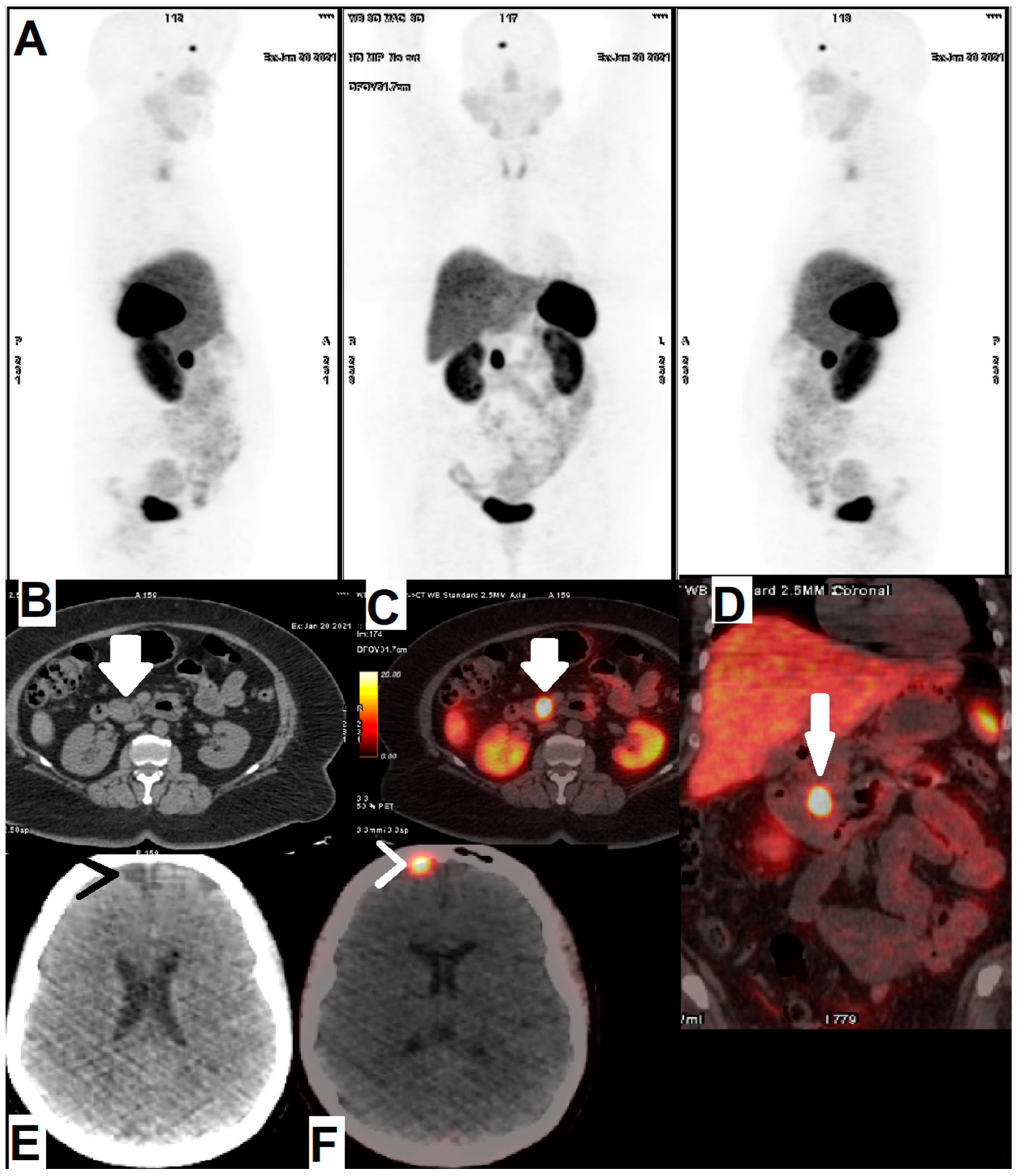
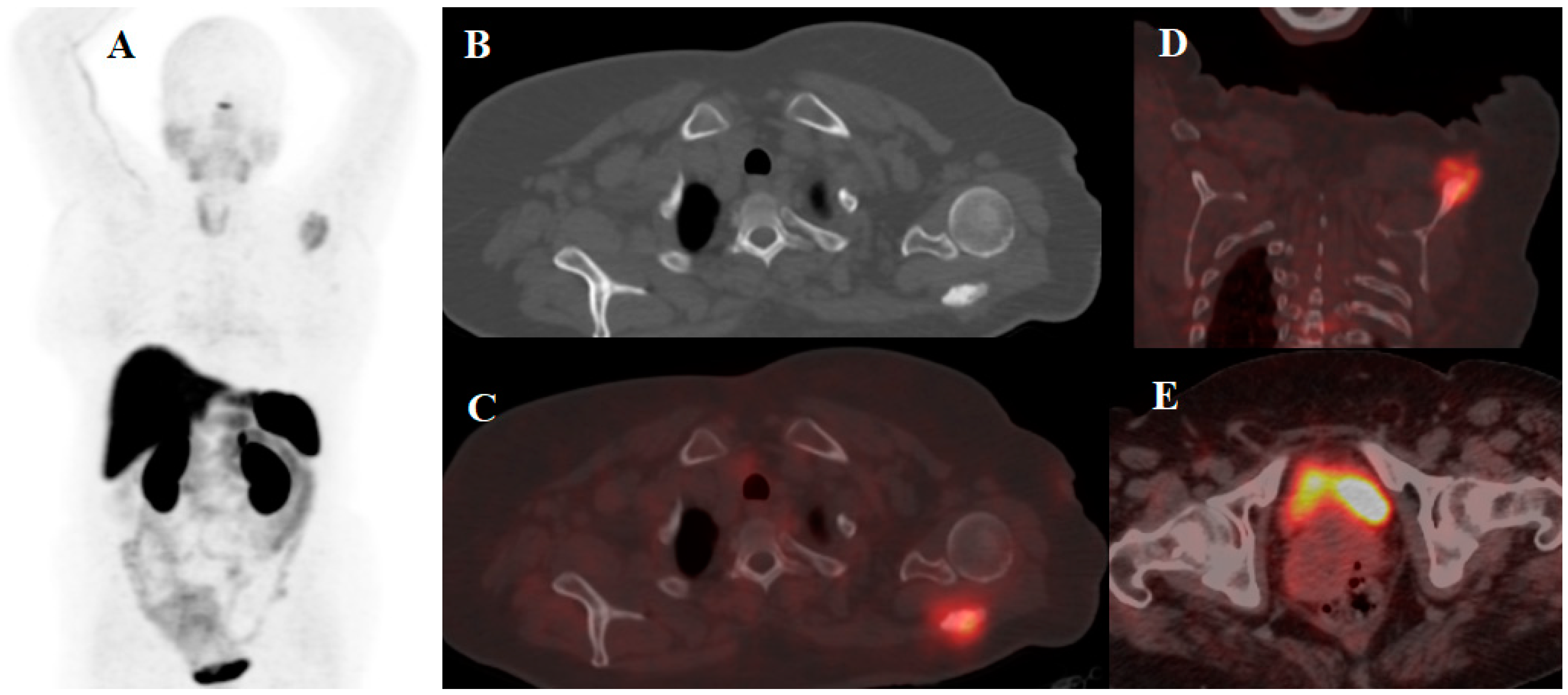
| [64Cu]Cu-DOTA-TOC | NET | 33 | 2019 | High detection rate and high target to background ratio in images raised [64Cu]Cu- DOTA-TATE as a promising and safe radiolabeled SST derivative for NET detection | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahidfar, N.; Farzanehfar, S.; Abbasi, M.; Mirzaei, S.; Delpassand, E.S.; Abbaspour, F.; Salehi, Y.; Biersack, H.J.; Ahmadzadehfar, H. Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC. Cancers 2022, 14, 1914. https://doi.org/10.3390/cancers14081914
Vahidfar N, Farzanehfar S, Abbasi M, Mirzaei S, Delpassand ES, Abbaspour F, Salehi Y, Biersack HJ, Ahmadzadehfar H. Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC. Cancers. 2022; 14(8):1914. https://doi.org/10.3390/cancers14081914
Chicago/Turabian StyleVahidfar, Nasim, Saeed Farzanehfar, Mehrshad Abbasi, Siroos Mirzaei, Ebrahim S. Delpassand, Farzad Abbaspour, Yalda Salehi, Hans Jürgen Biersack, and Hojjat Ahmadzadehfar. 2022. "Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC" Cancers 14, no. 8: 1914. https://doi.org/10.3390/cancers14081914
APA StyleVahidfar, N., Farzanehfar, S., Abbasi, M., Mirzaei, S., Delpassand, E. S., Abbaspour, F., Salehi, Y., Biersack, H. J., & Ahmadzadehfar, H. (2022). Diagnostic Value of Radiolabelled Somatostatin Analogues for Neuroendocrine Tumour Diagnosis: The Benefits and Drawbacks of [64Cu]Cu-DOTA-TOC. Cancers, 14(8), 1914. https://doi.org/10.3390/cancers14081914






