Magnetic Resonance-Guided Reirradiation for Local Recurrence within the Prostate or in the Prostate Bed: One-Year Clinical Results of a Prospective Registry Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Inclusion Criteria
2.2. Simulation
2.3. Treatment Planning
2.4. Daily Adaptive Treatment Workflow
2.5. Clinical Assessment, Dosimetric Evaluation and Endpoints
2.6. Statistical Analysis
3. Results
Initial Patient and Treatment Characteristics
4. Initial Treatment Plans
4.1. Dosimetric Benefits of Adaptive MRgRT
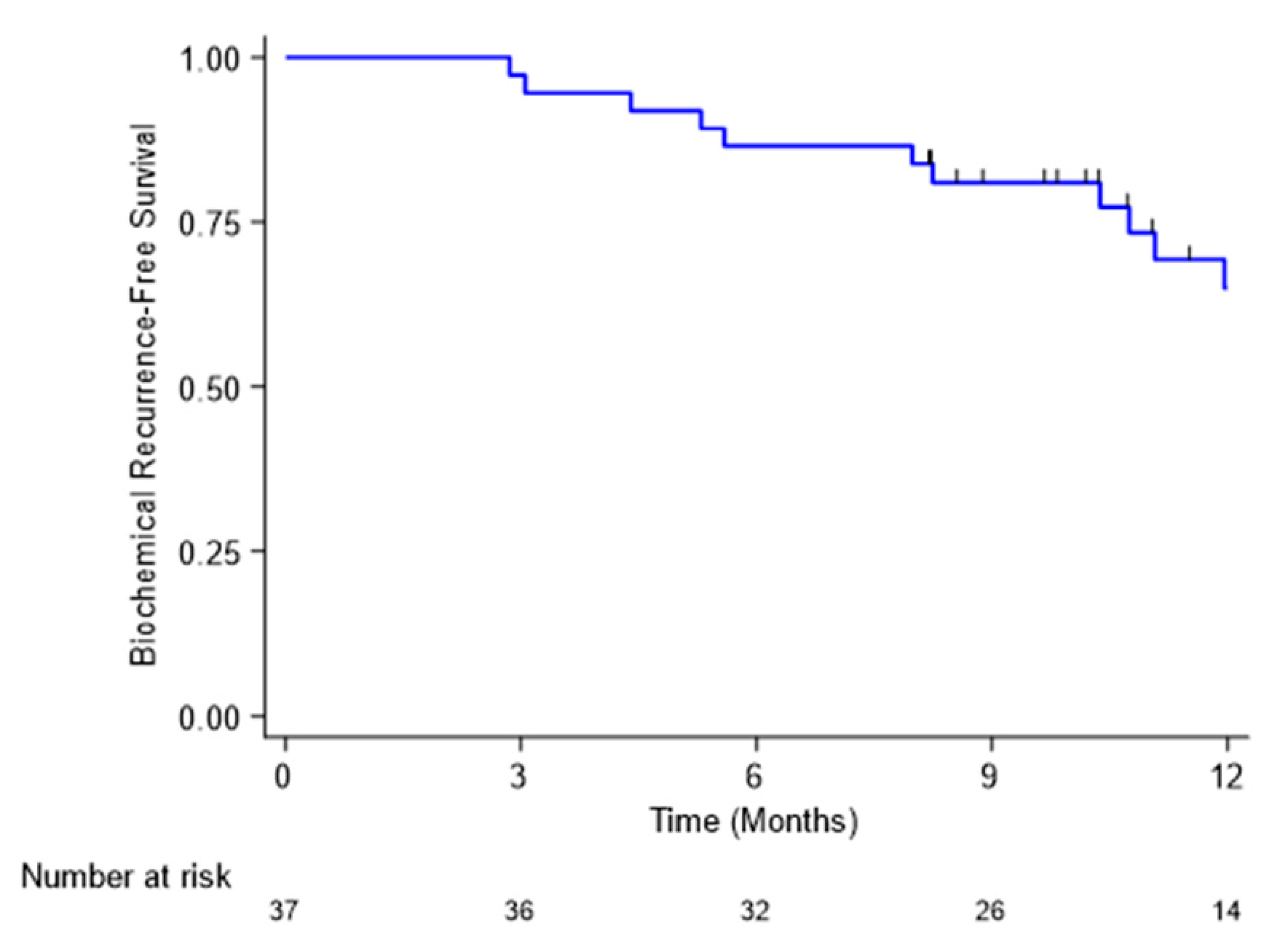
4.2. Biochemical Response
4.3. Acute Toxicities
5. Late Toxicities
Topography of Recurrences
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherzer, N.D.; DiBiase, Z.S.; Srivastav, S.K.; Thomas, R.; DiBiase, S.J. Regional Differences in the Treatment of Localized Prostate Cancer: An Analysis of Surgery and Radiation Utilization in the United States. Adv. Radiat. Oncol. 2019, 4, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nombre de patients traités par radiothérapie en fonction de la localisation [Internet]. 5 January 2022. Available online: http://lesdonnees.e-cancer.fr/Fiches-Indicateurs/Prise-en-charge/RadioT/Nombre-de-patients-traites-par-radiotherapie-en-fonction-de-la-localisation#sources (accessed on 25 February 2022).
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Sadetsky, N.; Konety, B.R.; Resnick, M.I.; Carroll, P.R. Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer 2008, 112, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.H.; van Leeuwen, P.J.; Wondergem, M.; van der Sluis, T.M.; Nieuwenhuijzen, J.A.; Knol, R.J.; van Moorselaar, R.J.; van der Poel, H.G.; Oprea-Lager, D.E.; Vis, A.N. Detection of Recurrent Prostate Cancer Using Prostate-specific Membrane Antigen Positron Emission Tomography in Patients not Meeting the Phoenix Criteria for Biochemical Recurrence After Curative Radiotherapy. Eur. Urol. Oncol. 2020, 4, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Duchesne, G.M.; Woo, H.H.; Bassett, J.K.; Bowe, S.; D’Este, C.; Frydenberg, M.; King, M.; Ledwich, L.; Loblaw, A.; Malone, S.; et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016, 17, 727–737. [Google Scholar] [CrossRef]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2020, 80, 280–292. [Google Scholar] [CrossRef]
- Chade, D.C.; Eastham, J.; Graefen, M.; Hu, J.C.; Karnes, R.J.; Klotz, L.; Montorsi, F.; van Poppel, H.; Scardino, P.T.; Shariat, S.F. Cancer Control and Functional Outcomes of Salvage Radical Prostatectomy for Radiation-recurrent Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. 2012, 61, 961–971. [Google Scholar] [CrossRef]
- Williams, A.K.; Martínez, C.H.; Lu, C.; Ng, C.K.; Pautler, S.E.; Chin, J.L. Disease-Free Survival Following Salvage Cryotherapy for Biopsy-Proven Radio-Recurrent Prostate Cancer. Eur. Urol. 2010, 60, 405–410. [Google Scholar] [CrossRef]
- Crouzet, S.; Blana, A.; Murat, F.J.; Pasticier, G.; Brown, S.C.W.; Conti, G.N.; Ganzer, R.; Chapet, O.; Gelet, A.; Chaussy, C.G.; et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. Br. J. Urol. 2017, 119, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Tocco, B.R.; Kishan, A.U.; Ma, T.M.; Kerkmeijer, L.G.W.; Tree, A.C. MR-Guided Radiotherapy for Prostate Cancer. Front. Oncol. 2020, 10, 2763. [Google Scholar] [CrossRef]
- Michalet, M.; Riou, O.; Valdenaire, S.; Debuire, P.; Ailleres, N.; Draghici, R.; Charissoux, M.; Moscardo, C.L.; Farcy-Jacquet, M.-P.; Fenoglietto, P.; et al. Magnetic Resonance–Guided Reirradiation for Local Recurrence Within the Prostate or in the Prostate Bed: Preliminary Results of a Prospective Registry Study. Adv. Radiat. Oncol. 2021, 6, 100748. [Google Scholar] [CrossRef]
- Baty, M.; Créhange, G.; Pasquier, D.; Palard, X.; Deleuze, A.; Gnep, K.; Key, S.; Beuzit, L.; Castelli, J.; de Crevoisier, R. Salvage reirradiation for local prostate cancer recurrence after radiation therapy. For who? When? How? Cancer/Radiothérapie 2019, 23, 541–558. [Google Scholar] [CrossRef]
- Loi, M.; Di Cataldo, V.; Simontacchi, G.; Detti, B.; Bonomo, P.; Masi, L.; Desideri, I.; Greto, D.; Francolini, G.; Carfora, V.; et al. Robotic Stereotactic Retreatment for Biochemical Control in Previously Irradiated Patients Affected by Recurrent Prostate Cancer. Clin. Oncol. 2018, 30, 93–100. [Google Scholar] [CrossRef]
- Leroy, T.; Lacornerie, T.; Bogart, E.; Nickers, P.; Lartigau, E.; Pasquier, D. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: Preliminary results of the Oscar Lambret Center. Radiat. Oncol. 2017, 12, 95. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Rojas, D.P.; Zerini, D.; Fodor, C.I.; Viola, A.; Fanetti, G.; Volpe, S.; Luraschi, R.; Bazani, A.; Rondi, E.; et al. Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br. J. Radiol. 2019, 92, 20180494. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Slevin, F.; Scarsbrook, A.F.; Serra, M.; Choudhury, A.; Hoskin, P.J.; Brown, S.; Henry, A.M. Salvage Reirradiation Options for Locally Recurrent Prostate Cancer: A Systematic Review. Front. Oncol. 2021, 11, 681448. [Google Scholar] [CrossRef]
- Fuller, D.; Wurzer, J.; Shirazi, R.; Bridge, S.; Law, J.; Crabtree, T.; Mardirossian, G. Retreatment for Local Recurrence of Prostatic Carcinoma After Prior Therapeutic Irradiation: Efficacy and Toxicity of HDR-Like SBRT. Int. J. Radiat. Oncol. 2019, 106, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Cuccia, F.; Nicosia, L.; Mazzola, R.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Vitale, C.; Corradini, S.; Ruggieri, R.; et al. Linac-based SBRT as a feasible salvage option for local recurrences in previously irradiated prostate cancer. Strahlenther. Onkol. 2020, 196, 628–636. [Google Scholar] [CrossRef]
- Matrone, F.; Revelant, A.; Fanetti, G.; Polesel, J.; Chiovati, P.; Avanzo, M.; De Renzi, F.; Colombo, C.B.; Arcicasa, M.; De Paoli, A.; et al. Partial prostate re-irradiation for the treatment of isolated local recurrence of prostate cancer in patients previously treated with primary external beam radiotherapy: Short-term results of a monocentric study. Neoplasma 2021, 68, 216–226. [Google Scholar] [CrossRef]
- Caroli, P.; Colangione, S.P.; De Giorgi, U.; Ghigi, G.; Celli, M.; Scarpi, E.; Monti, M.; Di Iorio, V.; Sarnelli, A.; Paganelli, G.; et al. 68Ga-PSMA-11 PET/CT-Guided Stereotactic Body Radiation Therapy Retreatment in Prostate Cancer Patients with PSA Failure after Salvage Radiotherapy. Biomedicines 2020, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, S.; Eade, T.; Kneebone, A.; Booth, J.; Hsiao, E.; Schembri, G.P.; Szymura, K.; Le, A.; Kwong, C.; Brown, C.; et al. Interim Results of a Prospective Prostate-Specific Membrane Antigen-Directed Focal Stereotactic Reirradiation Trial for Locally Recurrent Prostate Cancer. Int. J. Radiat. Oncol. 2020, 108, 1172–1178. [Google Scholar] [CrossRef]
- D’Agostino, G.R.; Di Brina, L.; Mancosu, P.; Franzese, C.; Iftode, C.; Franceschini, D.; Clerici, E.; Tozzi, A.; Navarria, P.; Scorsetti, M. Reirradiation of Locally Recurrent Prostate Cancer With Volumetric Modulated Arc Therapy. Int. J. Radiat. Oncol. 2019, 104, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, D.; Martinage, G.; Janoray, G.; Rojas, D.P.; Zerini, D.; Goupy, F.; De Crevoisier, R.; Bogart, E.; Calais, G.; Toledano, A.; et al. Salvage Stereotactic Body Radiation Therapy for Local Prostate Cancer Recurrence After Radiation Therapy: A Retrospective Multicenter Study of the GETUG. Int. J. Radiat. Oncol. 2019, 105, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Scher, N.; Bauduceau, O.; Bollet, M.; Lamallem, H.; Charas, T.; Garaud, P.; Foster, D.; Fawzi, M.; Labidi, M.; Toledano, A. Stereotactic prostate focal reirradiation therapy for local recurrence: Preliminary results of Hartmann Oncology Radiotherapy Group. BJR Open 2019, 1, 20180027. [Google Scholar] [CrossRef] [PubMed]
- Kollmeier, M.A.; McBride, S.; Taggar, A.; Anderson, E.; Lin, M.; Pei, X.; Weiji, S.; Voros, L.; Cohen, G.; Yamada, Y.; et al. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: A comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy 2017, 16, 1091–1098. [Google Scholar] [CrossRef]
- Wojcieszek, P.; Szlag, M.; Głowacki, G.; Cholewka, A.; Gawkowska-Suwińska, M.; Kellas-Ślęczka, S.; Białas, B.; Fijałkowski, M. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 119, 405–410. [Google Scholar] [CrossRef]
- Łyczek, J.; Kawczyńska, M.M.; Garmol, D.; Kasprowicz, A.; Kulik, A.; Dąbkowski, M.; Czyżew, B.; Gruszczyńska, E.; Bijok, M.; Kowalik, Ł. HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J. Contemp. Brachytherapy 2009, 1, 105–108. [Google Scholar]
- Pasquier, D.; Le Deley, M.-C.; Tresch, E.; Cormier, L.; Duterque, M.; Nenan, S.; Lartigau, E. GETUG-AFU 31: A phase I/II multicentre study evaluating the safety and efficacy of salvage stereotactic radiation in patients with intraprostatic tumour recurrence after external radiation therapy—study protocol. BMJ Open 2019, 9, e026666. [Google Scholar] [CrossRef] [Green Version]
- Klüter, S. Technical design and concept of a 0.35 T MR-Linac. Clin. Transl. Radiat. Oncol. 2019, 18, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.P.; Weinberg, V.; Shinohara, K.; Roach, M.; Nash, M.; Gottschalk, A.; Chang, A.J.; Hsu, I.-C. Salvage HDR Brachytherapy for Recurrent Prostate Cancer After Previous Definitive Radiation Therapy: 5-Year Outcomes. Int. J. Radiat. Oncol. 2013, 86, 324–329. [Google Scholar] [CrossRef]
- Maenhout, M.; Peters, M.; van Vulpen, M.; Moerland, M.A.; Meijer, R.P.; Bosch, M.A.A.J.V.D.; Nguyen, P.L.; Frank, S.J.; Zyp, J.R.N.V.D.V.V. Focal MRI-Guided Salvage High-Dose-Rate Brachytherapy in Patients with Radiorecurrent Prostate Cancer. Technol. Cancer Res. Treat. 2017, 16, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Murgic, J.; Morton, G.; Loblaw, A.; D’Alimonte, L.; Ravi, A.; Wronski, M.; Davidson, M.; Haider, M.; Commisso, K.; Zhang, L.; et al. Focal Salvage High Dose-Rate Brachytherapy for Locally Recurrent Prostate Cancer After Primary Radiation Therapy Failure: Results from a Prospective Clinical Trial. Int. J. Radiat. Oncol. 2018, 102, 561–567. [Google Scholar] [CrossRef]
- Kukiełka, A.; Hetnał, M.; Dąbrowski, T.; Walasek, T.; Brandys, P.; Nahajowski, D.; Kudzia, R.; Dybek, D.; Reinfuss, M. Salvage prostate HDR brachytherapy combined with interstitial hyperthermia for local recurrence after radiation therapy failure. Strahlenther. und Onkol. 2013, 190, 165–170. [Google Scholar] [CrossRef]
- Peters, M.; Maenhout, M.; Zyp, J.R.V.D.V.V.; Moerland, M.A.; Moman, M.R.; Steuten, L.M.; van Deursen, M.J.; van Vulpen, M. Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: A retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother. Oncol. 2014, 112, 77–82. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Marvaso, G.; Zaffaroni, M.; Gugliandolo, S.G.; Zerini, D.; Corso, F.; Gandini, S.; Alongi, F.; Bossi, A.; Cornford, P.; et al. Salvage stereotactic body radiotherapy (SBRT) for intraprostatic relapse after prostate cancer radiotherapy: An ESTRO ACROP Delphi consensus. Cancer Treat. Rev. 2021, 98, 102206. [Google Scholar] [CrossRef]

| Characteristics | N = 37 | % |
|---|---|---|
| Age | ||
| Median (range, in years) | 74.5 (56–93) | |
| ISUP group before the primary treatment | ||
| 1 | 10 | 27.8 |
| 2 | 7 | 19.4 |
| 3 | 10 | 27.8 |
| 4 | 6 | 16.7 |
| 5 | 3 | 8.3 |
| Unknown | 1 | |
| Primary treatment techniques | ||
| EBRT or EBRT + ADT | 25 | 67.5 |
| Brachytherapy | 2 | 5.4 |
| Prostatectomy + EBRT | 8 | 22.2 |
| Other (EBRT + HIFU or BT + EBRT) | 2 | 5.4 |
| Irradiation dose delivered during the primary treatment | ||
| Median (range, in Gy) | 74.0 (62.0–180) | |
| Median time between the primary treatment and the re-irradiation (range, in months) | 88.0 (21–240) | |
| WHO score before re-irradiation | ||
| 0 | 15 | 40.5 |
| 1 | 18 | 48.6 |
| 2 | 4 | 10.8 |
| PSA level (ng/mL) before re-irradiation | ||
| Median (range) | 3.36 (0.34–34.7) | |
| PSA doubling time (range, in months) | 7.20 (0.80–144.0) | |
| IPSS score and symptom groups before re-irradiation | ||
| Median score (range) | 6 (0–33) | |
| Mild (1–7) | 13 | 35.1 |
| Moderate (8–19) | 5 | 13.5 |
| Severe (20–35) | 3 | 8.1 |
| Unknown | 16 | 43.2 |
| Dose prescription | ||
| 27.5 Gy/5 fractions | 6 | 16.2 |
| 30 Gy/5 fractions | 28 | 75.6 |
| 30 Gy/6 fractions | 2 | 5.4 |
| 38.7 Gy/9 fractions | 1 | 2.7 |
| ADT sensitivity | ||
| Hormone sensitive | 31 | 83.8 |
| Castration resistant | 6 | 16.2 |
| Concomitant ADT during re-irradiation | ||
| Yes | 8 | 21 |
| No | 30 | 79 |
| Adaptive treatment | ||
| Yes | 25 | 67.6 |
| No | 12 | 32.4 |
| Treatment duration by fraction | ||
| Median (range, in minutes) | 42 (30–95) | |
| Total treatment duration | ||
| Median (in days) | 11 (9–31) | |
| Parameters | Median (Range) |
|---|---|
| PTV V100% (%) | 64 (50–100) |
| PTV V95% (%) | 96 (92–99) |
| Rectum V27 (cm3) | 0.20 (0.00–1.83) |
| Rectum V12 (%) | 13 (0–20) |
| Bladder V27 (cm3) | 0.01 (0.00–4.97) |
| Bladder V12 (%) | 3 (0–15) |
| Parameters | Predicted Plans: Median (Range) | Delivered Plans: Median (Range) | Difference (p-Value) |
|---|---|---|---|
| PTV V100% (%) | 63 (31–1.00) | 68 (54–100) | 0.002 |
| PTV V95% (%) | 94 (89-97) | 96 (93–98) | < 0.001 |
| Rectum V27 (cm3) | 0.51 (0.00–1.43) | 0.46 (0.00–1.42) | 0.893 |
| Rectum V12 (%) | 14 (0–21) | 14 (0–19) | 0.403 |
| Bladder V27 (cm3) | 0.17 (0.00–3.58) | 0.26 (0.00–3.84) | 0.468 |
| Bladder V12 (%) | 5 (0–17) | 5 (0–14) | 0.614 |
| Toxicity | Before MRgRT (Number of Patients) | Last Day of MRgRT (Number of Patients) | Three Months After MRgRT (Number of Patients) | Six Months After MRgRT (Number of Patients) |
|---|---|---|---|---|
| Dysuria | ||||
| g0 | 32 (86%) | 30 (81%) | 25 (83%) | 29 (80%) |
| g1 | 3 (8%) | 3 (8%) | 5 (17%) | 6 (17%) |
| g2 | 2 (5%) | 4 (11%) | 0 (0%) | 1 (3%) |
| Missing data | 0 | 0 | 7 | 7 |
| Hematuria | ||||
| g0 | 35 (95%) | 34 (92%) | 28 (94%) | 33 (91%) |
| g1 | 2 (5%) | 2 (5%) | 1 (3%) | 1 (3%) |
| g2 | 0 (%) | 1 (3%) | 1 (3%) | 1 (3%) |
| g3 | 0 (%) | 0 (%) | 0 (%) | 1 (3%) |
| Missing data | 0 | 0 | 7 | 1 |
| Urinary incontinence | ||||
| g0 | 30 (81%) | 30 (81%) | 27 (90%) | 30 (83%) |
| g1 | 5 (14%) | 4 (11%) | 3 (10%) | 5 (14%) |
| g2 | 2 (5%) | 3 (8%) | 0 (0%) | 1 (3%) |
| Missing data | 0 | 0 | 7 | 1 |
| Polyuria | ||||
| g0 | 28 (76%) | 23 (62%) | 24 (80%) | 22 (61%) |
| g1 | 7 (19%) | 9 (24%) | 5 (17%) | 13 (36%) |
| g2 | 2 (5%) | 5 (14%) | 1 (3%) | 1 (3%) |
| Missing data | 0 | 0 | 7 | 1 |
| Urinary pain | ||||
| g0 | 37 (100%) | 35 (95%) | 30 (100%) | 34 (94%) |
| g1 | 0 (0%) | 2 (5%) | 0 (0%) | 1 (3%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) |
| Missing data | 0 | 0 | 7 | 1 |
| Diarrhea | ||||
| g0 | 35 (95%) | 33 (89%) | 30 (100%) | 18 (90%) |
| g1 | 2 (5%) | 3 (8%) | 0 (0%) | 2 (10%) |
| g2 | 0 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
| Missing data | 0 | 0 | 7 | 1 |
| Rectal bleeding | ||||
| g0 | 35 (95%) | 37 (100%) | 30 (100%) | 36 (100%) |
| g1 | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing data | 0 | 0 | 7 | 1 |
| Rectal pain | ||||
| g0 | 37 (100%) | 30 (100%) | 36 (100%) | |
| g1 | 0 (0%) | 37 (100%) | 0 (0%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing data | 0 | 0 (0%) | 7 | 1 |
| Toxicity | Before MRgRT (Number of Patients) | 12 Months After MRgRT (Number of Patients) |
|---|---|---|
| Dysuria | ||
| g0 | 32 (86%) | 20 (54%) |
| g1 | 3 (8%) | 12 (32%) |
| g2 | 2 (5%) | 5 (14%) |
| Hematuria | ||
| g0 | 35 (95%) | 36 (97%) |
| g1 | 2 (5%) | 1 (3%) |
| g2 | 0 (%) | 0 (0%) |
| Urinary incontinence | ||
| g0 | 30 (81%) | 31 (84%) |
| g1 | 5 (14%) | 4 (11%) |
| g2 | 2 (5%) | 2 (5%) |
| Polyuria | ||
| g0 | 28 (76%) | 9 (24%) |
| g1 | 7 (19%) | 27 (73%) |
| g2 | 2 (5%) | 1 (3%) |
| Urinary pain | ||
| g0 | 37 (100%) | 35 (94%) |
| g1 | 0 (0%) | 1 (3%) |
| g2 | 0 (0%) | 1 (3%) |
| Diarrhea | ||
| g0 | 35 (95%) | 37 (100%) |
| g1 | 2 (5%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) |
| Rectal bleeding | ||
| g0 | 35 (95%) | 37 (100%) |
| g1 | 2 (5%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) |
| Rectal pain | ||
| g0 | 37 (100%) | 37 (100%) |
| g1 | 0 (0%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalet, M.; Riou, O.; Cottet-Moine, J.; Castan, F.; Gourgou, S.; Valdenaire, S.; Debuire, P.; Ailleres, N.; Draghici, R.; Charissoux, M.; et al. Magnetic Resonance-Guided Reirradiation for Local Recurrence within the Prostate or in the Prostate Bed: One-Year Clinical Results of a Prospective Registry Study. Cancers 2022, 14, 1943. https://doi.org/10.3390/cancers14081943
Michalet M, Riou O, Cottet-Moine J, Castan F, Gourgou S, Valdenaire S, Debuire P, Ailleres N, Draghici R, Charissoux M, et al. Magnetic Resonance-Guided Reirradiation for Local Recurrence within the Prostate or in the Prostate Bed: One-Year Clinical Results of a Prospective Registry Study. Cancers. 2022; 14(8):1943. https://doi.org/10.3390/cancers14081943
Chicago/Turabian StyleMichalet, Morgan, Olivier Riou, Jeremy Cottet-Moine, Florence Castan, Sophie Gourgou, Simon Valdenaire, Pierre Debuire, Norbert Ailleres, Roxana Draghici, Marie Charissoux, and et al. 2022. "Magnetic Resonance-Guided Reirradiation for Local Recurrence within the Prostate or in the Prostate Bed: One-Year Clinical Results of a Prospective Registry Study" Cancers 14, no. 8: 1943. https://doi.org/10.3390/cancers14081943
APA StyleMichalet, M., Riou, O., Cottet-Moine, J., Castan, F., Gourgou, S., Valdenaire, S., Debuire, P., Ailleres, N., Draghici, R., Charissoux, M., Llacer Moscardo, C., Farcy-Jacquet, M.-P., Fenoglietto, P., & Azria, D. (2022). Magnetic Resonance-Guided Reirradiation for Local Recurrence within the Prostate or in the Prostate Bed: One-Year Clinical Results of a Prospective Registry Study. Cancers, 14(8), 1943. https://doi.org/10.3390/cancers14081943







