Simple Summary
Low-grade serous cancer (LGSC) accounts for approximately 5% of all ovarian cancers. It is characterized by its high resistance to chemotherapy. Cytoreductive surgery, therefore, is the primary treatment modality for this disease and previous studies have shown that complete removal of all visible tumor tissue should be achieved. In this study, 176 women with LGSC were included and most of them had advanced disease stages, where the disease had already spread. CA-125 is a biomarker that has been previously studied in ovarian cancer. We have found that CA-125 level following treatment of LGSC is an important and independent prognostic factor for progression-free and overall survival. It may be a better surrogate for the true amount of residual disease following treatment compared to the gross estimation of visible residual disease during surgery.
Abstract
Objective: Studies on low-grade serous ovarian cancer (LGSC) are limited by a low number of cases. The aim of this study was to define the prognostic significance of age, stage, and CA-125 levels on survival in a multi-institutional cohort of women with pathologically confirmed LGSC. Methods: Women with LGSC were identified from the collaborative Ovarian Cancer Association Consortium (OCAC). Cases of newly diagnosed primary LGSC were included if peri-operative CA-125 levels were available. Age at diagnosis, FIGO stage, pre- and post-treatment CA-125 levels, residual disease, adjuvant chemotherapy, disease recurrence, and vital status were collected by the participating institutions. Progression-free (PFS) and overall survival (OS) were calculated. Multivariable (MVA) Cox proportional hazard models were used and hazard ratios (HR) calculated. Results: A total of 176 women with LGSC were included in this study; 82% had stage III/IV disease. The median PFS was 2.3 years and the median OS was 6.4 years. Age at diagnosis was not significantly associated with worse PFS (p = 0.23) or OS (p = 0.3) (HR per year: 0.99; 95%CI, 0.96–1.01 and 0.98; 95%CI 0.95–1.01). FIGO stage III/IV was independently associated with PFS (HR 4.26, 95%CI 1.43–12.73) and OS (HR 1.69, 95%CI 0.56–5.05). Elevated CA-125 (≥35 U/mL) at diagnosis was not significantly associated with worse PFS (p = 0.87) or OS (p = 0.78) in MVA. Elevated CA-125 (≥35 U/mL) after completion of primary treatment was independently associated with worse PFS (HR 2.81, 95%CI 1.36–5.81) and OS (HR 6.62, 95%CI 2.45–17.92). In the MVA, residual disease was independently associated with PFS (0.022), but not OS (0.85). Conclusion: Advanced LGSC was associated with poor long-term prognosis. FIGO stage and abnormal post-treatment CA-125 level are key prognostic factors inversely associated with PFS and OS. Highlights: 1. Through a multi-center collaborative effort, data from 176 women with low-grade serous ovarian cancer were analyzed. 2. Although low-grade serous ovarian cancer is often considered indolent, the progression-free and overall survival are poor. 3. Elevated post-treatment CA-125 levels are independently associated with poor survival.
1. Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death from gynecologic malignancies in the United States, Canada, and Europe [1,2,3]. Serous carcinoma is the most common subtype of epithelial ovarian malignancies. A two-tier classification system distinguishes high-grade (HGSC) from low-grade serous ovarian carcinoma (LGSC) based on the degree of nuclear atypia [4,5]. Recent evidence suggests that the more common HGSC clearly differs from the less common LGSC with regard to cells of origin, molecular pathogenesis, clinical behavior, and pathologic characteristics. Thus, LGSC and HGSC are considered two distinct malignancies [6,7,8].
LGSC accounts for approximately 5% of serous ovarian malignancies and may arise from serous low malignant potential tumors of the ovary or de novo from the ovary or peritoneum [9,10]. While the tumor typically evolves slower, existing evidence suggests limited benefit of cytotoxic therapy given the relatively high resistance of LGSC to platinum-based chemotherapy [11,12]. A retrospective study from the German AGO registry found an objective response rate of 23% for women with suboptimal cytoreduction of advanced LGSC given adjuvant chemotherapy, as compared to a 90% response rate in a similar population of patients with HGSC [13]. Moreover, objective response rates of <5% have been reported for LGSC in the neoadjuvant and recurrent setting [14,15]. Surgery is therefore considered the cornerstone of treatment for LGSC; however, novel systemic and targeted treatment modalities are needed in conjunction with surgery given the poor chemosensitivity of these tumors.
Due to the indolent nature of LGSC, patients may experience either a protracted clinical course, or in the case of treatment-refractory disease, a more rapid progression where palliative care is typically required soon after treatment initiation [16]. The identification of prognostic factors can aid in clinical decision-making with regard to treatment [17]. Therefore, recent efforts have been directed towards identifying and contextualizing known, and novel, prognostic factors into clinical practice. For EOC in general, tumor stage and grade, physical status at time of diagnosis, body mass index (BMI), extent of surgical cytoreduction, heredity, immunological factors, and molecular biomarkers have been identified [18].
The aim of this study was to define the prognostic significance of age, stage, residual disease, and pre- and post-treatment CA-125 levels in terms of survival in a large multi-institutional cohort of women with pathologically confirmed LGSC.
2. Methods
2.1. Patient Population
Women with LGSC were identified from the international collaborative Ovarian Cancer Association Consortium (OCAC). The OCAC consortium was founded in 2005 to combine data from individual studies on ovarian cancer [19]. Clinical data were collected at each participating site and entered into a universal de-identified clinical database. Only women with newly diagnosed, primary LGSC who underwent primary surgical resection were included in this analysis. A pathology report by a specialized gynecologic oncology pathologist confirming the diagnosis of LGSC, the documented date of birth, and pre-operative, diagnostic CA-125 levels, prior to the commencement of any therapy and following completion of primary treatment, were also required.
Patient demographics and clinical data included: age at diagnosis, International Federation of Obstetrics and Gynecology (FIGO) stage, CA-125 level at diagnosis, CA-125 level after treatment completion, residual disease-status following primary cytoreductive surgery (no macroscopic disease, macroscopic disease ≤ 1 cm, and macroscopic disease > 1 cm), first-line adjuvant chemotherapy, disease recurrence, and vital status. For CA-125, a cut-off of 35 was used as generally accepted. In addition, secondary analyses were performed comparing patients with CA-125 levels < 35, 35–500 and >500 to explore the difference between moderately increased and very high levels of CA-125. Research ethics board approval was obtained at the participating sites.
2.2. Statistical Analyses
Descriptive statistics were used to report demographic data. Chi square tests were used to compare categorical data. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of first progression or the date of death. Overall survival (OS) was calculated from the date of diagnosis to the date of death. Patients who remained alive were censored on the date of last follow-up. PFS and OS were estimated using the Kaplan–Meier method with log-rank tests to examine survival differences. Univariable (UVA) and multivariable (MVA) Cox proportional hazards models were fit to assess the association of age, FIGO stage, residual disease, and CA-125 with PFS and OS. The proportional hazards assumption for the multivariable model was verified; the models were stratified by study site; and p-values < 0.05 were considered statistically significant. All tests were two-sided. Statistical analyses were performed using R version 3.6.0, www.r-project.org accessed on 1 April 2022 and SPSS Version 26, IBM, Armonk, NY, USA.
3. Results
3.1. Patient Population
A total of 176 women with LGSC from 15 cancer centers within the OCAC collaborative network were included in this study. The number of patients included from the individual study cohorts is shown in Supplementary Table S1. Patient demographics and clinical characteristics are shown in Table 1. The median age at diagnosis was 54 years (range 18–80 years). FIGO stage was reported in 172 patients (98%). The majority of patients were diagnosed with advanced disease, with the following disease distribution noted: 14 patients (8%) had stage I disease, 17 (10%) stage II, 125 (73%) stage III, and 16 (9%) stage IV disease. A total of 114 patients (65%) had information on cytoreductive surgical outcome. Complete cytoreduction to no macroscopic residual disease was achieved in 57% of patients, optimal cytoreduction to macroscopic residual disease ≤ 1 cm was achieved in 26% of patients, and suboptimal cytoreduction with macroscopic disease > 1 cm was performed in 17% of cases. There was a statistical trend towards higher rates of suboptimal cytoreduction in older women, where 57% of women younger than 54 years had complete cytoreduction to no macroscopic residual disease, 33% had optimal cytoreduction to macroscopic residual disease ≤ 1 cm, and 10% had suboptimal cytoreduction with macroscopic disease > 1 cm. In women older than 54 years, 57% had complete cytoreduction to no macroscopic residual disease, 20% had optimal cytoreduction to macroscopic residual disease ≤ 1 cm, and 23% had suboptimal cytoreduction with macroscopic disease > 1 cm (p = 0.096).

Table 1.
Demographic and clinical characteristics.
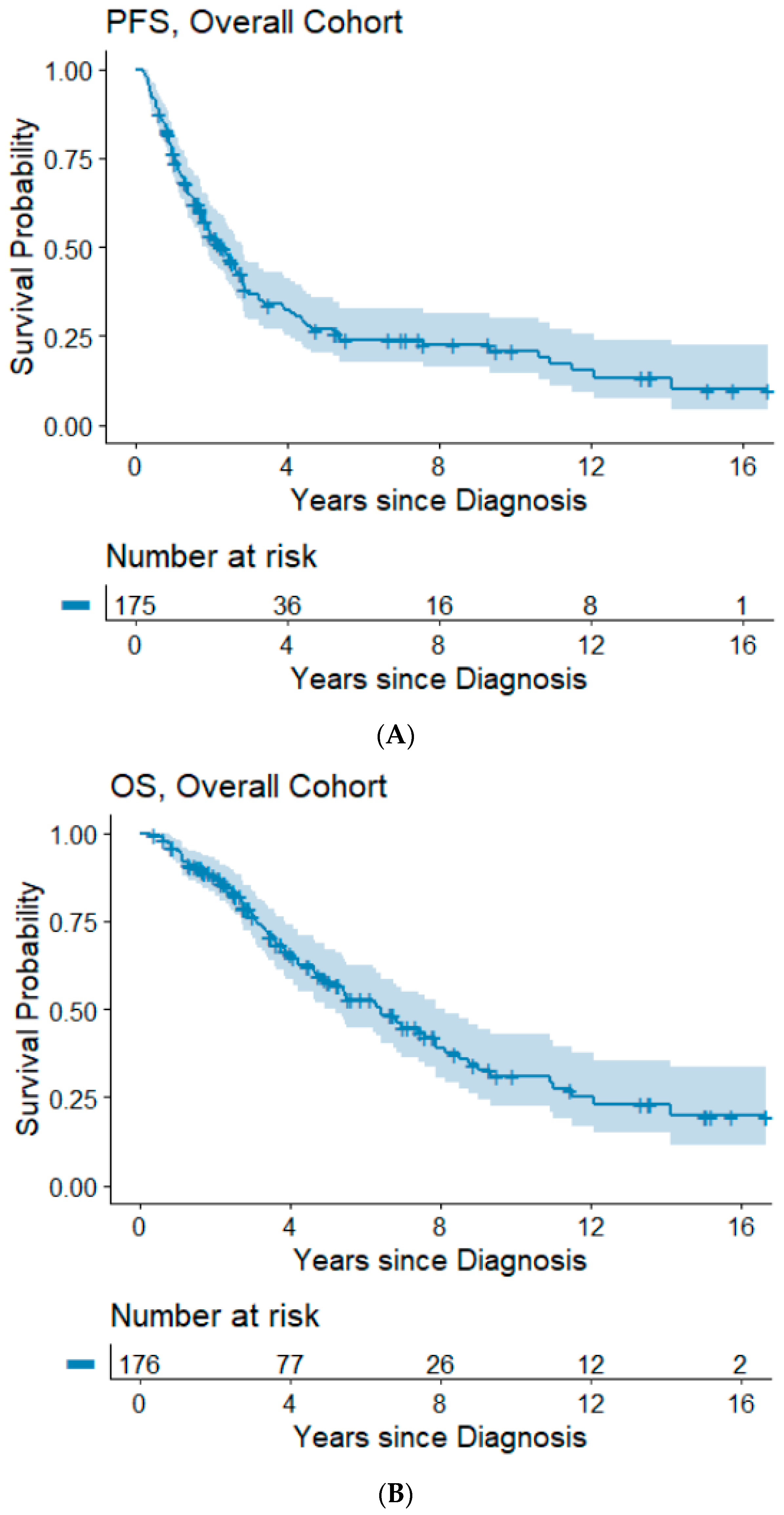
The median follow-up time was 3.5 years (range: 0.3–16.7 years). Information on progression/recurrence status was available for 175 patients, of which 120 (68%) experienced disease progression. The median time to progression was 2.3 years and the 5- and 10-year PFS rates were 26.8% and 20.9%. In our cohort, 85 (48%) patients had died. The median OS was 6.4 years and the 5- and 10-year OS rates were 57.8% and 31.1%, respectively. Kaplan–Meier survival plots for PFS and OS are shown in Figure 1A,B.
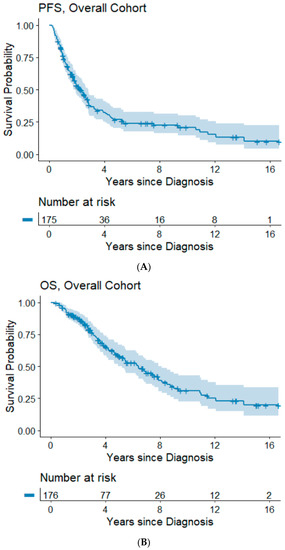
Figure 1.
(A,B) Progression-free and overall survival of the overall cohort. Abbreviations: OS, overall survival; PFS, progression-free survival.
3.2. The Impact of Clinical Parameters on Survival
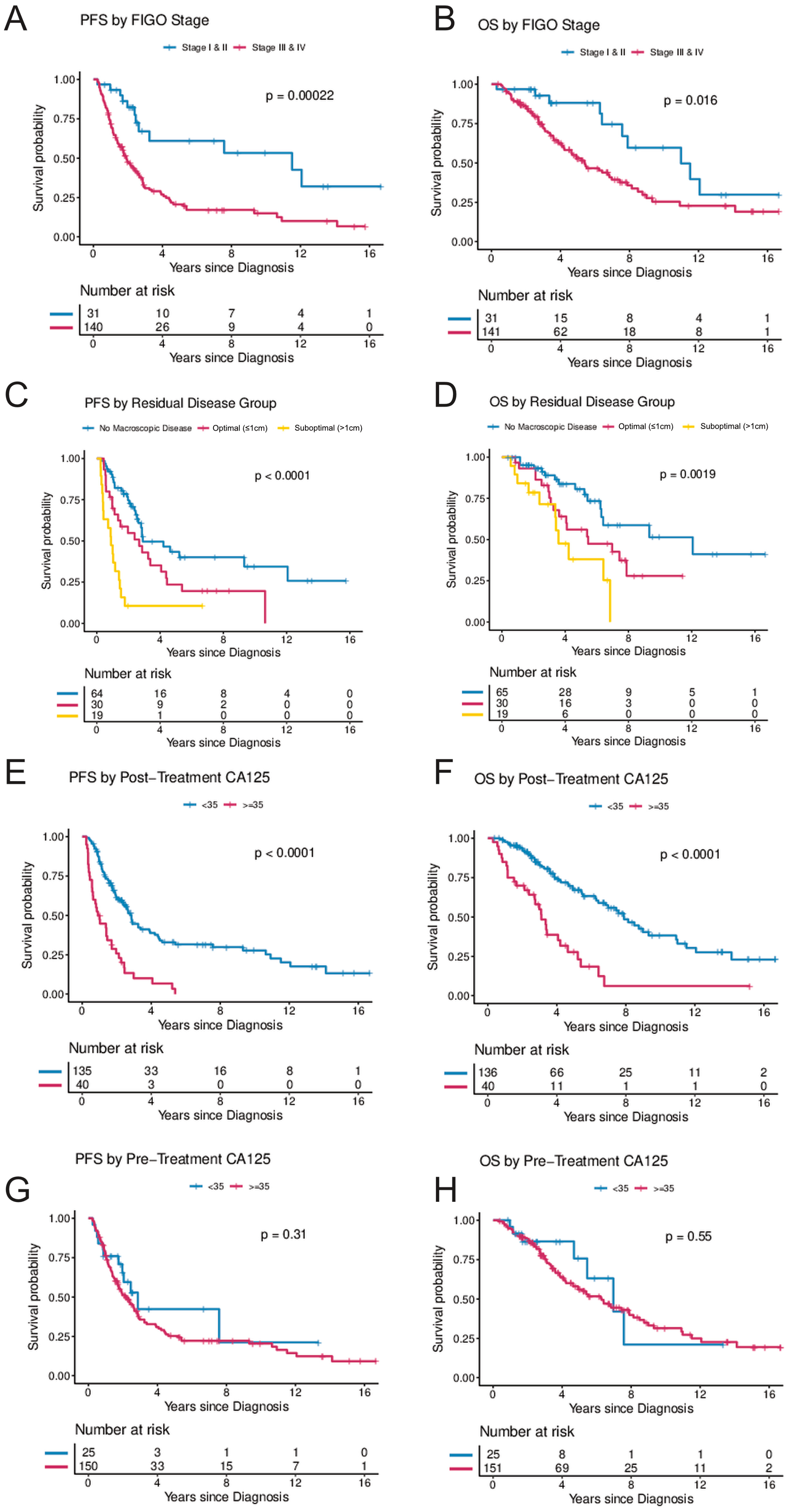
FIGO stage at diagnosis was significantly associated with PFS and OS (Table 2 and Figure 2A,B) in the UVA, and with PFS in the MVA. Of women with stage III/IV disease, 70.9% experienced disease progression during the observation period and the median time to progression was 1.7 years. In the MVA, stage III and IV were associated with a hazard ratio (HR) of 4.26 (95%CI, 1.43–12.73) for progression, and 1.69 (95%CI, 0.56–5.05) for death, respectively.

Table 2.
Univariate and multivariate cox proportional hazard model for progression-free and overall survival.
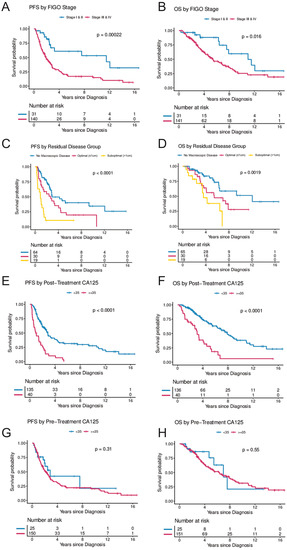
Figure 2.
(A–H) Progression-free and overall survival by FIGO stage, residual disease, and post-treatment CA-125 and pre-treatment CA-125. (A,B) illustrate progression-free and overall survival by FIGO stage (Stages I and II combined vs. Stages III and IV). (C,D) illustrate progression-free and overall survival by residual disease following cytoreductive surgery (no visible macroscopic disease vs. macroscopic disease ≤ 1 cm vs. macroscopic disease > 1 cm). (E,F) illustrate progression-free and overall survival by CA-125 levels following treatment (<35 vs. ≥35) and (G,H) illustrate progression-free and overall survival by CA-125 levels at diagnosis (<35 vs. ≥35). The Kaplan–Meier method with log-rank tests was used to examine survival differences. p-values < 0.05 were considered statistically significant. Individuals at risk are shown in the legends. Abbreviations: OS, overall survival; PFS, progression-free survival.
Residual disease after surgical cytoreduction was significantly associated with PFS. Suboptimal cytoreduction with macroscopic residual disease > 1 cm compared to complete cytoreduction with no macroscopic residual disease was associated with an HR of 2.93 (95%CI, 1.33–6.47) for progression and 1.06 (95%CI, 0.4–2.81) for death, respectively (Table 2 and Figure 2C,D).
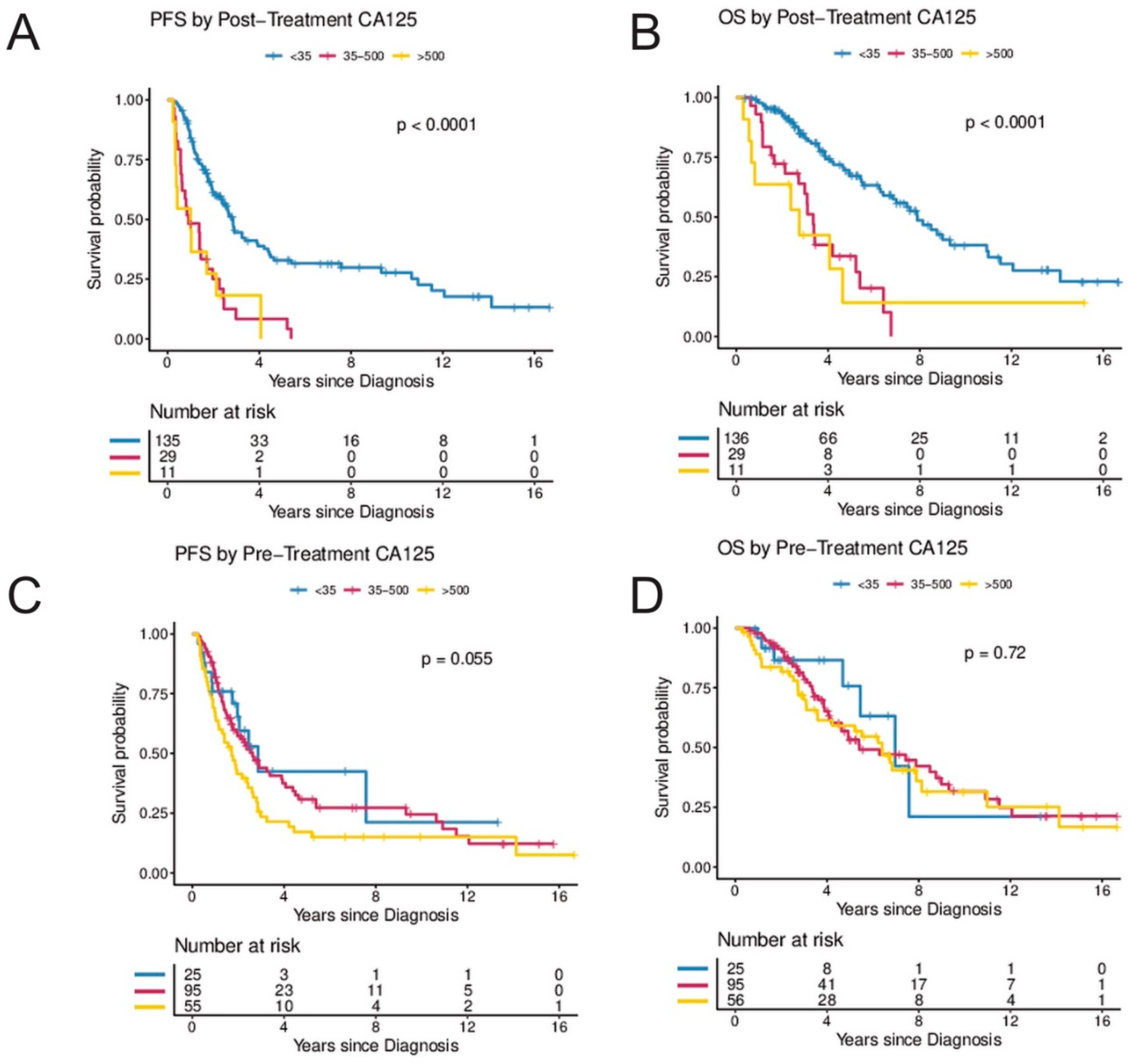
Elevated CA-125 levels at diagnosis prior to surgery were not associated with PFS or OS when compared to patients with normal CA-125 levels at diagnosis (Figure 2G,H). In contrast, women with abnormal post-treatment CA-125 (≥35 U/mL) had a significantly higher risk for progression and death. In the UVA, an elevated post-treatment CA-125 level was associated with an HR of 3.45 (95%CI, 2.23–5.33) for progression and 3.58 (95%CI, 2.19–5.87) for death, respectively. In the MVA (when controlling for age, stage, and residual disease), CA-125, following completion of treatment was independently associated with risk of progression (HR 2.93; 95%CI, 1.36–5.81; p = 0.005) and death (HR 6.62; 95%CI, 2.45–17.92; p < 0.001) (Table 2 and Figure 2E,F). In a secondary analysis, the range of CA-125 values at diagnosis were analyzed by comparing survival in women with baseline CA-125 < 35 to women with baseline CA-125 levels between 35 and 500 and to women with baseline CA-125 levels > 500. There was a trend towards improved PFS in women with pre-treatment CA-125 < 35 (p = 0.055) but no statistically significant difference in OS (p = 0.72) (Figure 3C,D). In contrast, women with normal post-treatment CA-125 < 35 had significantly better PFS (p < 0.0001) and OS (p < 0.0001) when compared to those with post-treatment CA-125 between 35–500 and those with post-treatment CA-125 > 500 (Figure 3A,B).
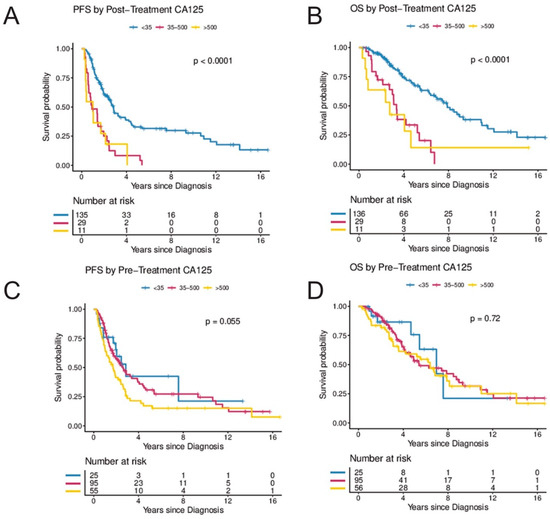
Figure 3.
(A–D) Secondary analysis of post-treatment and pre-treatment CA-125 by CA-125 level. (A,B) illustrate progression-free and overall survival by CA-125 levels following treatment (<35 vs. 35–500 vs. >500) and (C,D) illustrate progression-free and overall survival by CA-125 levels at diagnosis (<35 vs. 35–500 vs. >500). The Kaplan–Meier method with log-rank tests was used to examine survival differences. p-values < 0.05 were considered statistically significant. Individuals at risk are shown in the legends. Abbreviations: OS, overall survival; PFS, progression-free survival.
4. Discussion
In this study, the prognostic significance of age, FIGO stage, residual disease, and pre- and post-treatment CA-125 levels were analyzed from a comprehensive multi-center cohort of 176 women with newly diagnosed and pathologically confirmed LGSC. Age at diagnosis was not an independent predictor for progression or death. FIGO stage was found to be an independent prognostic factor for disease progression. Importantly, LGSC is relatively chemoresistant and is primarily treated with surgical cytoreduction. We have observed that women with suboptimal cytoreduction have a significantly worse prognosis, which is in agreement with previous findings from a subgroup analysis of GOG-182 study which found that women with LGSC who had suboptimal cytoreduction with >1 cm residual disease have a worse outcome [8]. Women in this cohort with complete cytoreduction with no macroscopic residual disease had improved outcomes, followed by women with optimal cytoreduction with macroscopic residual disease ≤ 1 cm as compared to women with suboptimal cytoreduction with macroscopic residual disease > 1 cm. Unlike HGSC, medical treatment options for LGSC are limited. A study by the AGO found a limited benefit to systemic chemotherapy with poor response rates in LGSC when compared to HGSC [13]. In a recent phase 2/3 clinical trial assessing trametinib versus standard of care regimens (intravenous paclitaxel 80 mg/m2 by body surface area on days 1, 8, and 15 of every 28-day cycle; intravenous pegylated liposomal doxorubicin 40–50 mg/m2 by body surface area once every 4 weeks; intravenous topotecan 4 mg/m2 by body surface area on days 1, 8, and 15 of every 28-day cycle; oral letrozole 2·5 mg once daily; or oral tamoxifen 20 mg twice daily), trametinib was associated with improved median progression-free survival (13.0 months (95%CI 9.9–15.0) compared with 7.2 months (5.6–9.9) in the standard-of-care group (hazard ratio 0.48 [95%CI 0.36–0.64]; p < 0.0001) [20]. However, surgery remains the primary treatment modality for LGSC. Therefore, careful evaluation and referral to a gynecologic oncologist for consideration of primary surgery with the aim for complete or optimal surgical cytoreduction is paramount for women with newly diagnosed LGSC including those presenting at older age.
CA-125 is a large membrane glycoprotein encoded by the gene MUC16 that is overexpressed in 85% of serous ovarian cancers [21,22,23]. Interestingly, in our cohort, elevated CA-125 at diagnosis was not associated with worse PFS or OS. However, we found a significant association of post-treatment CA-125 levels with the risk of progression and death in this cohort. Previous studies have shown that the nadir of CA-125 levels are associated with PFS and OS in high-grade serous ovarian cancer [24,25]. In the MVA, after controlling for age and FIGO stage, an elevated post-treatment CA-125 level remained an independent risk factor for disease progression and death. This may aid in identifying women at higher risk of relapse after completing primary treatment and may guide additional treatments, monitoring and follow-up. Importantly, the value of post-treatment CA-125 appears to be a better predictor of OS than residual disease. One possible explanation is that the post-treatment CA-125 level may be a better indicator of the true amount of residual disease following treatment, completion of surgery, and systemic therapy than gross estimation of visible residual disease.
Our study provides data from a well characterized, international multi-center cohort of women with LGSC. All original pathology reports were generated by subspecialized gynecologic pathologists. The strengths of this work include the relatively large number of patients, especially in this rare disease. The study is limited by its retrospective nature and the lack of central pathology review.
5. Conclusions
In summary, advanced LGSC was associated with poor prognosis and low long-term survival in this international cohort of patients. FIGO stage at diagnosis and the amount of residual disease following surgical cytoreduction are important prognostic factors. Age and elevated CA-125 levels at presentation were not associated with worse survival outcomes. Abnormal CA-125 level after completing treatment was an independent predictor of recurrence and worse survival.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14081954/s1, Table S1: OCAC study sites with the number of included cases (n = 176).
Author Contributions
Conceptualization: T.M.; Methodology: C.W., K.L. and T.M.; Statistical Analysis and Data curation: K.L.; Provision and Review of Data: S.K.K., A.J., R.G., P.R., A.D., S.E.J., P.M.W., F.M., D.L., J.M.S., A.B., L.C.V.T., L.B., E.H., C.K.H., E.L.G., S.J.W., K.M., B.Y.K., J.L., M.T.G., P.J.T., T.P., M.J.R., A.T. and T.M.; Writing: C.W. and T.M. Critical review of the manuscript: C.W., V.D., S.K.K., A.J., R.G., P.R., A.D., S.E.J., P.M.W., F.M., D.L., J.M.S., A.B., L.C.V.T., L.B., E.H., C.K.H., E.L.G., S.J.W., K.M., B.Y.K., J.L., M.T.G., P.J.T., T.P., M.J.R., K.L., A.T. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07). The scientific development and funding for this project were in part supported by the US National Cancer Institute GAME-ON Post-GWAS Initiative (U19-CA148112). This study made use of data generated by the Wellcome Trust Case Control consortium that was funded by the Wellcome Trust under award 076113. The results published here are in part based upon data generated by The Cancer Genome Atlas Pilot Project established by the National Cancer Institute and National Human Genome Research Institute (dbGap accession number phs000178.v8.p7). The OCAC OncoArray genotyping project was funded through grants from the U.S. National Institutes of Health (CA1X01HG007491-01 (C.I.A.), U19-CA148112 (T.A.S.), R01-CA149429 (C.M.P.) and R01-CA058598 (M.T.G.); Canadian Institutes of Health Research (MOP-86727 (L.E.K.) and the Ovarian Cancer Research Fund (A.B.). The COGS project was funded through a European Commission’s Seventh Framework Programme grant (agreement number 223175—HEALTH-F2-2009-223175). Funding for individual studies: AUS: The Australian Ovarian Cancer Study (AOCS) was supported by the U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729), National Health & Medical Research Council of Australia (199600, 400413 and 400281), Cancer Councils of New South Wales, Victoria, Queensland, South Australia, and Tasmania and Cancer Foundation of Western Australia (Multi-State Applications 191, 211, and 182). AOCS gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Foundation; BEL: National Kankerplan; JPN: Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare; MAL: Funding for this study was provided by research grant R01- CA61107 from the National Cancer Institute, Bethesda, MD, research grant 94 222 52 from the Danish Cancer Society, Copenhagen, Denmark; and the Mermaid I project; MAY: National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; NCO: National Institutes of Health (R01-CA76016) and the Department of Defense (DAMD17-02-1-0666); NOR: Helse Vest, The Norwegian Cancer Society, The Research Council of Norway; OPL: National Health and Medical Research Council (NHMRC) of Australia (APP1025142, APP1120431) and Brisbane Women’s Club; ORE: Sherie Hildreth Ovarian Cancer (SHOC) Foundation; SRO: Cancer Research UK (C536/A13086, C536/A6689) and Imperial Experimental Cancer Research Centre (C1312/A15589); UHN: Princess Margaret Cancer Centre Foundation-Bridge for the Cure.
Institutional Review Board Statement
The individual studies used for the analysis were approved by QIMR Berghofer-HREC (ethics approval numbers P287 and P1424), Medical Ethics Committee of the University Hospitals KU Leuven (ethics approval number S52397), Institutional ethics committee of Aichi Cancer Center (ethics approval numbers 24 February 2020 and 25 February 2020), European Commission Directorate-General Justice (ethics approval number KF 01-224/96), Mayo Foundation Institutional Review Boards (ethics approval number IRB 1367-05), Duke Health Institutional Review Board (ethics approval number Pro00014425), Regional Committee for Medical and Health Research Ethics, Western-Norway (ethics approval number 15501), Oregon Health & Science University Research Integrity Office (ethics approval number IRB00008009, Multicenter ethics approval (ethics approval number Tax-GMA-601), and University Health Network Research Ethics Board (ethics approval number 13-6051-TE).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study by the participating centers.
Data Availability Statement
Data are available on request from the corresponding author.
Acknowledgments
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. The OncoArray and COGS genotyping projects would not have been possible without the contributions of the following: Per Hall (COGS); Douglas F. Easton, Kyriaki Michailidou, Manjeet K. Bolla, Qin Wang (BCAC), Rosalind A. Eeles, Douglas F. Easton, Ali Amin Al Olama, Zsofia Kote-Jarai, Sara Benlloch (PRACTICAL), Antonis Antoniou, Lesley McGuffog, Fergus Couch and Ken Offit (CIMBA), Joe Dennis, Jonathan P. Tyrer, Siddhartha Kar, Alison M. Dunning, Andrew Lee, and Ed Dicks, Craig Luccarini and the staff of the Centre for Genetic Epidemiology Laboratory, Javier Benitez, Anna Gonzalez-Neira and the staff of the CNIO genotyping unit, Jacques Simard and Daniel C. Tessier, Francois Bacot, Daniel Vincent, Sylvie LaBoissière and Frederic Robidoux and the staff of the McGill University and Génome Québec Innovation Centre, Stig E. Bojesen, Sune F. Nielsen, Borge G. Nordestgaard, and the staff of the Copenhagen DNA laboratory, and Julie M. Cunningham, Sharon A. Windebank, Christopher A. Hilker, Jeffrey Meyer and the staff of Mayo Clinic Genotyping Core Facility. We pay special tribute to the contribution of Professor Brian Henderson to the GAME-ON consortium; to Olga M. Sinilnikova for her contribution to CIMBA and for her part in the initiation and coordination of GEMO until she sadly passed away on 30 June 2014 and to Catherine M. Phelan for her contribution to OCAC and coordination of the OncoArray until she passed away on 22 September 2017. We thank the study participants, doctors, nurses, clinical and scientific collaborators, health care providers, and health information sources who have contributed to the many studies contributing to this manuscript. Acknowledgements for individual studies: AUS: The AOCS also acknowledges the cooperation of the participating institutions in Australia, and the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete AOCS Study Group can be found at www.aocstudy.org, accessed on 1 April 2022. We would like to thank all of the women who participated in this research program; BEL: We would like to thank Gilian Peuteman, Thomas Van Brussel, Annick Van den Broeck and Joke De Roover for technical assistance; OPL: Members of the OPAL Study Group (http://opalstudy.qimrberghofer.edu.au, accessed on 1 April 2022); SRO: We would like to thank all members of the Scottish Gynaecological Clinical Trials group and SCOTROC1 investigators; UHN: Princess Margaret Cancer Centre Foundation-Bridge for the Cure.
Conflicts of Interest
Penelope M. Webb and Anna DeFazio received grant funding and a speaker’s fee from AstraZeneca for an unrelated study of ovarian cancer. Supporting sources had no involvement in the decision to publish. The other authors report no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canadian Government. Canadian Cancer Statistics 2019. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 6 June 2021).
- European Commission. ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 6 June 2021).
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading Ovarian Serous Carcinoma Using a Two-Tier System. Am. J. Surg. Pathol. 2004, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Malpica, A.; Deavers, M.T.; Tornos, C.; Kurman, R.J.; Soslow, R.; Seidman, J.D.; Munsell, M.F.; Gaertner, E.; Frishberg, D.; Silva, E.G. Interobserver and Intraobserver Variability of a Two-tier System for Grading Ovarian Serous Carcinoma. Am. J. Surg. Pathol. 2007, 31, 1168–1174. [Google Scholar] [CrossRef]
- O’Neill, C.J.; Deavers, M.T.; Malpica, A.; Foster, H.; McCluggage, W.G. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: Significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am. J. Surg. Pathol. 2005, 29, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.; Nelson, G.; Ghatage, P.; McIntyre, J.B.; Capper, D.; Chu, P.; Nation, J.G.; Karnezis, A.N.; Han, G.; Kalloger, S.E.; et al. The diagnostic utility of TP53 and CDKN2A to distinguish ovarian high-grade serous carcinoma from low-grade serous ovarian tumors. Mod. Pathol. 2013, 26, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Fader, A.N.; Java, J.; Ueda, S.; Bristow, R.E.; Armstrong, D.K.; Bookman, M.A.; Gershenson, D.M. Survival in Women With Grade 1 Serous Ovarian Carcinoma. Obstet. Gynecol. 2013, 122, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Machida, H.; Grubbs, B.H.; Sood, A.K.; Gershenson, D.M. Trends of low-grade serous ovarian carcinoma in the United States. J. Gynecol. Oncol. 2018, 29, e15. [Google Scholar] [CrossRef] [Green Version]
- Shvartsman, H.S.; Sun, C.C.; Bodurka, D.C.; Mahajan, V.; Crispens, M.; Lu, K.H.; Deavers, M.T.; Malpica, A.; Silva, E.G.; Gershenson, D.M. Comparison of the clinical behavior of newly diagnosed stages II–IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol. Oncol. 2007, 105, 625–629. [Google Scholar] [CrossRef]
- Santillan, A.; Kim, Y.W.; Zahurak, M.L.; Gardner, G.J.; Giuntoli, R.L.; Shih, I.M.; Bristow, R.E. Differences of chemoresistance assay between invasive micropapillary/low-grade serous ovarian carcinoma and high-grade serous ovarian carcinoma. Int. J. Gynecol. Cancer 2007, 17, 601–606. [Google Scholar] [CrossRef]
- Pavlidis, N.; Rassy, E.; Vermorken, J.B.; Assi, T.; Kattan, J.; Boussios, S.; Smith-Gagen, J. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021, 75, 102045. [Google Scholar] [CrossRef]
- Grabowski, J.P.; Harter, P.; Heitz, F.; Pujade-Lauraine, E.; Reuss, A.; Kristensen, G.; Ray-Coquard, I.; Heitz, J.; Traut, A.; Pfisterer, J.; et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol. Oncol. 2016, 140, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Sun, C.C.; Bodurka, D.C.; Deavers, M.T.; Malpica, A.; Coleman, R.L.; Ramirez, P.T.; Gershenson, D.M. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2008, 108, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Sun, C.C.; Bodurka, D.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Deavers, M.; Malpica, A.L.; Kavanagh, J.J. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009, 114, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M. Low-grade serous carcinoma of the ovary or peritoneum. Ann. Oncol. 2016, 27 (Suppl. 1), i45–i49. [Google Scholar] [CrossRef] [Green Version]
- Plaxe, S.C. Epidemiology of low-grade serous ovarian cancer. Am. J. Obstet. Gynecol. 2008, 198, 459.e1–459.e9. [Google Scholar] [CrossRef]
- Ezzati, M.; Abdullah, A.; Shariftabrizi, A.; Hou, J.; Kopf, M.; Stedman, J.K.; Samuelson, R.; Shahabi, S. Recent Advancements in Prognostic Factors of Epithelial Ovarian Carcinoma. Int. Sch. Res. Not. 2014, 2014, 953509. [Google Scholar] [CrossRef]
- Ramus, S.; Vierkant, R.; Johnatty, S.E.; Pike, M.C.; Berg, D.J.V.D.; Wu, A.H.; Pearce, C.L.; Menon, U.; Gentry-Maharaj, A.; Gayther, S.A.; et al. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int. J. Cancer 2008, 123, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Gershenson, D.M.; Miller, A.; Brady, W.E.; Paul, J.; Carty, K.; Rodgers, W.; Millan, D.; Coleman, R.L.; Moore, K.N.; Banerjee, S.; et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): An international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 2022, 399, 541–553. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Klug, T.L.; St. John, E.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A Radioimmunoassay Using a Monoclonal Antibody to Monitor the Course of Epithelial Ovarian Cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef]
- Høgdall, E.; Christensen, L.; Kjaer, S.; Blaakaer, J.; Kjærbye-Thygesen, A.; Gayther, S.; Jacobs, I.; Høgdall, C. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients: From The Danish “MALOVA” Ovarian Cancer Study. Gynecol. Oncol. 2007, 104, 508–515. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Piatek, S.; Panek, G.; Lewandowski, Z.; Piatek, D.; Kosinski, P.; Bidzinski, M. Nadir CA-125 has prognostic value for recurrence, but not for survival in patients with ovarian cancer. Sci. Rep. 2021, 11, 18190. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, J.M.; Wafflart, J.; Ricolleau, G.; Eche, N.; Larbre, H.; Basuyau, J.P.; Dalifard, I.; Hacene, K.; Pichon, M.F. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: Results of a French multicentric study. Ann. Oncol. 2006, 17, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).