Diagnostic Challenges in the Cytology of Thymic Epithelial Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Thymoma
3. Thymic Carcinoma
4. Neuroendocrine Neoplasms
5. Challenging Differential Diagnosis: Other Anterior Mediastinal Tumors and Ectopic Thymus
6. Conclusions
Funding
Conflicts of Interest
References
- Thoracic Tumours WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 5.
- Carter, B.W.; Marom, E.M.; Detterbeck, F.C. Approaching the patient with an anterior mediastinal mass: A guide for clinicians. J. Thorac. Oncol. 2014, 9, S102–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, Z.; Chen, Y.; Tan, L.; Zeng, Z.; Ding, J.; Du, S. Induction Strategy for Locally Advanced Thymoma. Front. Oncol. 2021, 11, 704220. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Huang, J. Induction therapy for locally advanced thymoma. J. Thorac. Oncol. 2010, 5, S323–S326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendogni, P.; Toker, A.; Moser, B.; Trancho, F.H.; Aigner, C.; Bravio, I.G.; Novoa, N.M.; Molins, L.; Costardi, L.; Voltolini, L.; et al. Surgical resection of Masaoka stage III thymic epithelial tumours with great vessels involvement: A retrospective multicentric analysis from the European Society of Thoracic Surgeons thymic database. Eur. J. Cardiothorac. Surg. 2022. [Google Scholar] [CrossRef]
- Bilaceroglu, S. How to obtain adequate biopsy specimen in suspected thymic tumors. J. Thorac. Dis. 2020, 12, 7598–7606. [Google Scholar] [CrossRef]
- Kattach, H.; Hasan, S.; Clelland, C.; Pillai, R. Seeding of stage I thymoma into the chest wall 12 years after needle biopsy. Ann. Thorac. Surg. 2005, 79, 323–324. [Google Scholar] [CrossRef]
- Detterbeck, F.C. Does an anecdote substantiate dogma? Ann. Thorac. Surg. 2006, 81, 1182. [Google Scholar] [CrossRef]
- Padda, S.K.; Keijzers, M.; Wakelee, H.A. Pretreatment biopsy for thymic epithelial tumors-does histology subtype matter for treatment strategy? J. Thorac. Dis. 2016, 8, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kundu, U.; Gong, Y. Pitfalls of FNA diagnosis of thymic tumors. Cancer Cytopathol. 2020, 128, 57–67. [Google Scholar] [CrossRef]
- Assaad, M.W.; Pantanowitz, L.; Otis, C.N. Diagnostic accuracy of image-guided percutaneous fine needle aspiration biopsy of the mediastinum. Diagn. Cytopathol. 2007, 35, 705–709. [Google Scholar] [CrossRef]
- Engels, E.A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. 2010, 5, S260–S265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.H.; Chan, J.K.; Yin, C.H.; Lee, C.C.; Chern, C.U.; Liao, C.I. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS ONE 2019, 14, e0227197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bhatnagar, V.; Ding, L.; Atay, S.M.; David, E.A.; McFadden, P.M.; Stamnes, S.; Lechtholz-Zey, E.; Wightman, S.C.; Detterbeck, F.C.; et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J. Thorac. Cardiovasc. Surg. 2020, 160, 306–314.e14. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Willcox, N.; Leite, M.I.; Chuang, W.Y.; Schalke, B.; Nix, W.; Strobel, P. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Nicholson, A.G.; Kondo, K.; Van Schil, P.; Moran, C. The Masaoka-Koga stage classification for thymic malignancies: Clarification and definition of terms. J. Thorac. Oncol. 2011, 6, S1710–S1716. [Google Scholar] [CrossRef] [Green Version]
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.A.; Giaccone, G.; Huang, J.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9, S65–S72. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, D.; Henley, J.D.; Chiriboga, L.; Yee, H. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am. J. Surg. Pathol. 2007, 31, 1038–1044. [Google Scholar] [CrossRef]
- Laury, A.R.; Perets, R.; Piao, H.; Krane, J.F.; Barletta, J.A.; French, C.; Chirieac, L.R.; Lis, R.; Loda, M.; Hornick, J.L.; et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol. 2011, 35, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Dotto, J.; Pelosi, G.; Rosai, J. Expression of p63 in thymomas and normal thymus. Am. J. Clin. Pathol. 2007, 127, 415–420. [Google Scholar] [CrossRef]
- Hishima, T.; Fukayama, M.; Fujisawa, M.; Hayashi, Y.; Arai, K.; Funata, N.; Koike, M. CD5 expression in thymic carcinoma. Am. J. Pathol. 1994, 145, 268–275. [Google Scholar]
- Wakely, P.E., Jr. Fine needle aspiration in the diagnosis of thymic epithelial neoplasms. Hematol. Oncol. Clin. N. Am. 2008, 22, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Wakely, P.E., Jr. Cytopathology of thymic epithelial neoplasms. Semin. Diagn. Pathol. 2005, 22, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, M.F.; Rosado-de-Christenson, M.L.; Sabloff, B.S.; Moran, C.A.; Swisher, S.G.; Marom, E.M. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics 2011, 31, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Benveniste, M.F.; Madan, R.; Godoy, M.C.; de Groot, P.M.; Truong, M.T.; Rosado-de-Christenson, M.L.; Marom, E.M. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017, 37, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Blum, T.G.; Misch, D.; Kollmeier, J.; Thiel, S.; Bauer, T.T. Autoimmune disorders and paraneoplastic syndromes in thymoma. J. Thorac. Dis. 2020, 12, 7571–7590. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Koh, J.S.; Park, S.; Kim, M.S.; Cho, S.Y.; Lee, S.S. Classification of thymoma by fine needle aspiration biopsy according to WHO classification: A cytological algorithm for stepwise analysis in the classification of thymoma. Acta Cytol. 2012, 56, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Illei, P.B.; Shyu, S. Fine needle aspiration of thymic epithelial neoplasms and non-neoplastic lesions. Semin. Diagn. Pathol. 2020, 37, 166–173. [Google Scholar] [CrossRef]
- Oramas, D.M.; Moran, C.A. Thymoma: Challenges and Pitfalls in Biopsy Interpretation. Adv. Anat. Pathol. 2021, 28, 291–297. [Google Scholar] [CrossRef]
- Zakowski, M.F.; Huang, J.; Bramlage, M.P. The role of fine needle aspiration cytology in the diagnosis and management of thymic neoplasia. J. Thorac. Oncol. 2010, 5, S281–S285. [Google Scholar] [CrossRef] [Green Version]
- Marcus, A.; Narula, N.; Kamel, M.K.; Koizumi, J.; Port, J.L.; Stiles, B.; Moreira, A.; Altorki, N.K.; Giorgadze, T. Sensitivity and specificity of fine needle aspiration for the diagnosis of mediastinal lesions. Ann. Diagn. Pathol. 2019, 39, 69–73. [Google Scholar] [CrossRef]
- Chhieng, D.C.; Rose, D.; Ludwig, M.E.; Zakowski, M.F. Cytology of thymomas: Emphasis on morphology and correlation with histologic subtypes. Cancer 2000, 90, 24–32. [Google Scholar] [CrossRef]
- Weissferdt, A.; Kalhor, N.; Bishop, J.A.; Jang, S.J.; Ro, J.; Petersson, F.; Wu, B.; Langman, G.; Bancroft, H.; Bi, Y.; et al. Thymoma: A clinicopathological correlation of 1470 cases. Hum. Pathol. 2018, 73, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Suster, D.; Suster, S. The role of needle core biopsies in the evaluation of thymic epithelial neoplasms. J. Am. Soc. Cytopathol. 2020, 9, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Bakhos, C.T.; Salami, A.C.; Kaiser, L.R.; Petrov, R.V.; Abbas, A.E. Thymic Neuroendocrine Tumors and Thymic Carcinoma: Demographics, Treatment, and Survival. Innovations 2020, 15, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.A.; Tiscornia-Wasserman, P.G.; Aziz, M. Diagnosis of thymic clear cell carcinoma by cytology. Case Rep. Pathol. 2013, 2013, 617810. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Suster, S. Thymoma with prominent cystic and hemorrhagic changes and areas of necrosis and infarction: A clinicopathologic study of 25 cases. Am. J. Surg. Pathol. 2001, 25, 1086–1090. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, D.; Tawfik, O. Thymoma with extensive necrosis: A case report and review of literature. Pathologica 2006, 98, 652–654. [Google Scholar]
- Shin, H.J.; Katz, R.L. Thymic neoplasia as represented by fine needle aspiration biopsy of anterior mediastinal masses. A practical approach to the differential diagnosis. Acta Cytol. 1998, 42, 855–864. [Google Scholar] [CrossRef]
- Kojika, M.; Ishii, G.; Yoshida, J.; Nishimura, M.; Hishida, T.; Ota, S.J.; Murata, Y.; Nagai, K.; Ochiai, A. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: Diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod. Pathol. 2009, 22, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Thomas de Montpreville, V.; Quilhot, P.; Chalabreysse, L.; De Muret, A.; Hofman, V.; Lantuejoul, S.; Parrens, M.; Payan, M.J.; Rouquette, I.; Secq, V.; et al. Glut-1 intensity and pattern of expression in thymic epithelial tumors are predictive of WHO subtypes. Pathol. Res. Pract. 2015, 211, 996–1002. [Google Scholar] [CrossRef]
- Jeong, J.H.; Pyo, J.S.; Kim, N.Y.; Kang, D.W. Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma. Diagnostics 2020, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.K.; Kang, C.H.; Kim, Y.T.; Jung, K.C.; Won, J.K. An immunohistochemical panel consisting of EZH2, C-KIT, and CD205 is useful for distinguishing thymic squamous cell carcinoma from type B3 thymoma. Pathol. Res. Pract. 2018, 214, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hirokawa, M.; Takada, N.; Higuchi, M.; Tanaka, A.; Hayashi, T.; Kuma, S.; Miyauchi, A. Utility of monoclonal PAX8 antibody for distinguishing intrathyroid thymic carcinoma from follicular cell-derived thyroid carcinoma. Endocr. J. 2018, 65, 1171–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissferdt, A.; Moran, C.A. Thymic carcinoma, part 1: A clinicopathologic and immunohistochemical study of 65 cases. Am. J. Clin. Pathol. 2012, 138, 103–114. [Google Scholar] [CrossRef]
- Toriyama, A.; Mori, T.; Sekine, S.; Yoshida, A.; Hino, O.; Tsuta, K. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: A comparison with polyclonal PAX8 antibody. Histopathology 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Weissferdt, A.; Moran, C.A. Pax8 expression in thymic epithelial neoplasms: An immunohistochemical analysis. Am. J. Surg. Pathol. 2011, 35, 1305–1310. [Google Scholar] [CrossRef]
- Asirvatham, J.R.; Esposito, M.J.; Bhuiya, T.A. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 372–376. [Google Scholar] [CrossRef]
- Kriegsmann, M.; Muley, T.; Harms, A.; Tavernar, L.; Goldmann, T.; Dienemann, H.; Herpel, E.; Warth, A. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: A large scale study of 1465 non-small cell lung cancer cases. Diagn. Pathol. 2015, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Dinter, H.; Bohnenberger, H.; Beck, J.; Bornemann-Kolatzki, K.; Schutz, E.; Kuffer, S.; Klein, L.; Franks, T.J.; Roden, A.; Emmert, A.; et al. Molecular Classification of Neuroendocrine Tumors of the Thymus. J. Thorac. Oncol. 2019, 14, 1472–1483. [Google Scholar] [CrossRef] [Green Version]
- Strobel, P.; Zettl, A.; Shilo, K.; Chuang, W.Y.; Nicholson, A.G.; Matsuno, Y.; Gal, A.; Laeng, R.H.; Engel, P.; Capella, C.; et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: A multi-institutional clinicopathologic study. Genes Chromosom. Cancer 2014, 53, 738–749. [Google Scholar] [CrossRef]
- Moran, C.A.; Suster, S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am. J. Clin. Pathol. 2000, 114, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Suster, S. Thymic neuroendocrine carcinomas with combined features ranging from well-differentiated (carcinoid) to small cell carcinoma. A clinicopathologic and immunohistochemical study of 11 cases. Am. J. Clin. Pathol. 2000, 113, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renshaw, A.A.; Haja, J.C.; Neal, M.H.; Wilbur, D.C. Distinguishing carcinoid tumor of the mediastinum from thymoma: Correlating cytologic features and performance in the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytopathology. Arch. Pathol. Lab. Med. 2006, 130, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; La Rosa, S.; Volante, M.; Papotti, M. Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr. Pathol. 2018, 29, 150–168. [Google Scholar] [CrossRef]
- Staaf, J.; Tran, L.; Soderlund, L.; Nodin, B.; Jirstrom, K.; Vidarsdottir, H.; Planck, M.; Mattsson, J.S.M.; Botling, J.; Micke, P.; et al. Diagnostic Value of Insulinoma-Associated Protein 1 (INSM1) and Comparison With Established Neuroendocrine Markers in Pulmonary Cancers. Arch. Pathol. Lab. Med. 2020, 144, 1075–1085. [Google Scholar] [CrossRef] [Green Version]
- Liau, J.Y.; Tsai, J.H.; Jeng, Y.M.; Kuo, K.T.; Huang, H.Y.; Liang, C.W.; Yang, C.Y. The Diagnostic Utility of PAX8 for Neuroendocrine Tumors: An Immunohistochemical Reappraisal. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 57–63. [Google Scholar] [CrossRef]
- Ozcan, A.; Shen, S.S.; Hamilton, C.; Anjana, K.; Coffey, D.; Krishnan, B.; Truong, L.D. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: A comprehensive immunohistochemical study. Mod. Pathol. 2011, 24, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Du, E.Z.; Goldstraw, P.; Zacharias, J.; Tiffet, O.; Craig, P.J.; Nicholson, A.G.; Weidner, N.; Yi, E.S. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum. Pathol. 2004, 35, 825–831. [Google Scholar] [CrossRef]
- Viale, G.; Doglioni, C.; Gambacorta, M.; Zamboni, G.; Coggi, G.; Bordi, C. Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 1992, 70, 2268–2277. [Google Scholar] [CrossRef]
- Jaffee, I.M.; Rahmani, M.; Singhal, M.G.; Younes, M. Expression of the intestinal transcription factor CDX2 in carcinoid tumors is a marker of midgut origin. Arch. Pathol. Lab. Med. 2006, 130, 1522–1526. [Google Scholar] [CrossRef]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, F. Application of immunohistochemistry in thyroid pathology. Arch. Pathol. Lab. Med. 2015, 139, 67–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, Z.W.; LiVolsi, V.A. Neuroendocrine tumors of the thyroid gland. Am. J. Clin. Pathol. 2001, 115, S56–S67. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Yasukawa, M.; Uchiyama, T.; Kawaguchi, T.; Sawabata, N.; Ohbayashi, C.; Taniguchi, S. A case of atypical thymic carcinoid mimicking a paraganglioma. Int. J. Surg. Case Rep. 2020, 66, 408–411. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Dermawan, J.K.; Mukhopadhyay, S.; Shah, A.A. Frequency and extent of cytokeratin expression in paraganglioma: An immunohistochemical study of 60 cases from 5 anatomic sites and review of the literature. Hum. Pathol. 2019, 93, 16–22. [Google Scholar] [CrossRef]
- Ferolla, P.; Falchetti, A.; Filosso, P.; Tomassetti, P.; Tamburrano, G.; Avenia, N.; Daddi, G.; Puma, F.; Ribacchi, R.; Santeusanio, F.; et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: The Italian series. J. Clin. Endocrinol. Metab. 2005, 90, 2603–2609. [Google Scholar] [CrossRef] [Green Version]
- Temes, R.; Chavez, T.; Mapel, D.; Ketai, L.; Crowell, R.; Key, C.; Follis, F.; Pett, S.; Wernly, J. Primary mediastinal malignancies: Findings in 219 patients. West. J. Med. 1999, 170, 161–166. [Google Scholar]
- Reynolds, J.P.; Liu, S. Fine needle aspiration of mediastinal germ cell tumors. Semin. Diagn. Pathol. 2020, 37, 174–178. [Google Scholar] [CrossRef]
- Motoyama, T.; Yamamoto, O.; Iwamoto, H.; Watanabe, H. Fine needle aspiration cytology of primary mediastinal germ cell tumors. Acta Cytol. 1995, 39, 725–732. [Google Scholar] [PubMed]
- Pina-Oviedo, S.; Moran, C.A. Primary Mediastinal Classical Hodgkin Lymphoma. Adv. Anat. Pathol. 2016, 23, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Juco, J.; Mann, K.P.; Holden, J.T. Flow cytometry in the differential diagnosis of lymphocyte-rich thymoma from precursor T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma. Am. J. Clin. Pathol. 2004, 121, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, W.; Tugulea, S.; Liu, Z.; Li, X.; Wong, J.Y.; Weisberger, J. Flow cytometry in the diagnosis of mediastinal tumors with emphasis on differentiating thymocytes from precursor T-lymphoblastic lymphoma/leukemia. Leuk. Lymphoma 2004, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Bhaker, P.; Das, A.; Rajwanshi, A.; Gautam, U.; Trehan, A.; Bansal, D.; Varma, N.; Srinivasan, R. Precursor T-lymphoblastic lymphoma: Speedy diagnosis in FNA and effusion cytology by morphology, immunochemistry, and flow cytometry. Cancer Cytopathol. 2015, 123, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, N.; Moran, C.A. Primary thymic adenocarcinomas: A clinicopathological and immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation. Hum. Pathol. 2018, 74, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Regnard, J.F.; Magdeleinat, P.; Dromer, C.; Dulmet, E.; de Montpreville, V.; Levi, J.F.; Levasseur, P. Prognostic factors and long-term results after thymoma resection: A series of 307 patients. J. Thorac. Cardiovasc. Surg. 1996, 112, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Ruffini, E.; Mancuso, M.; Oliaro, A.; Casadio, C.; Cavallo, A.; Cianci, R.; Filosso, P.L.; Molinatti, M.; Porrello, C.; Cappello, N.; et al. Recurrence of thymoma: Analysis of clinicopathologic features, treatment, and outcome. J. Thorac. Cardiovasc. Surg. 1997, 113, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.; Okumura, M.; Asamura, H.; Yoshida, K.; Niwa, H.; Kondo, K.; Horio, H.; Matsumura, A.; Yokoi, K.; Japanese Association for Research on Thymus. Surgical management of recurrent thymic epithelial tumors: A retrospective analysis based on the Japanese nationwide database. J. Thorac. Oncol. 2015, 10, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Bille, A.; Sachidananda, S.; Moreira, A.L.; Rizk, N.P. Unusual late presentation of metastatic extrathoracic thymoma to gastrohepatic lymph node treated by surgical resection. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 130–132. [Google Scholar] [CrossRef]
- Taweevisit, M.; Sampatanukul, P.; Thorner, P.S. Ectopic thymoma can mimic benign and malignant thyroid lesions on fine needle aspiration cytology: A case report and literature review. Acta Cytol. 2013, 57, 213–220. [Google Scholar] [CrossRef] [PubMed]
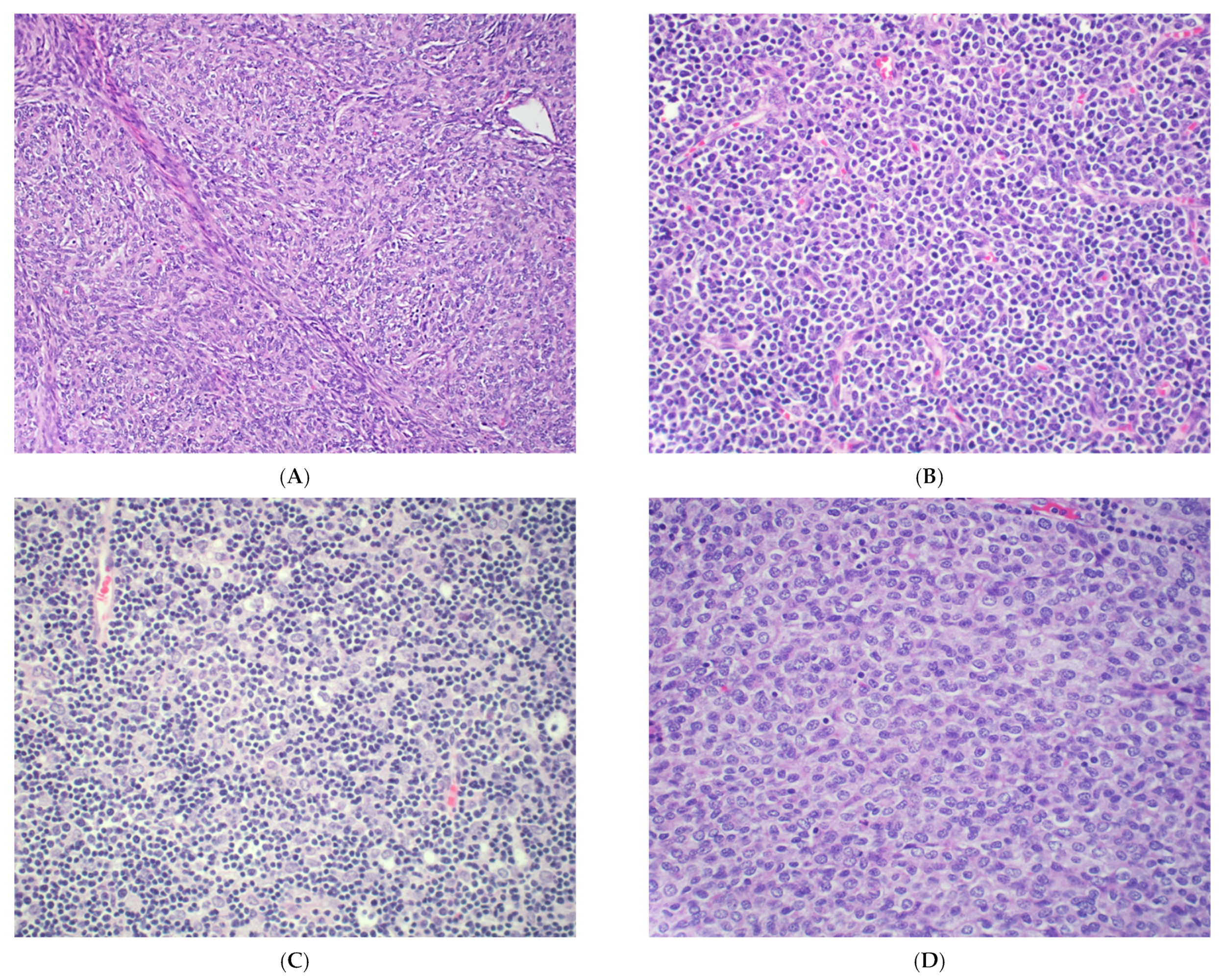









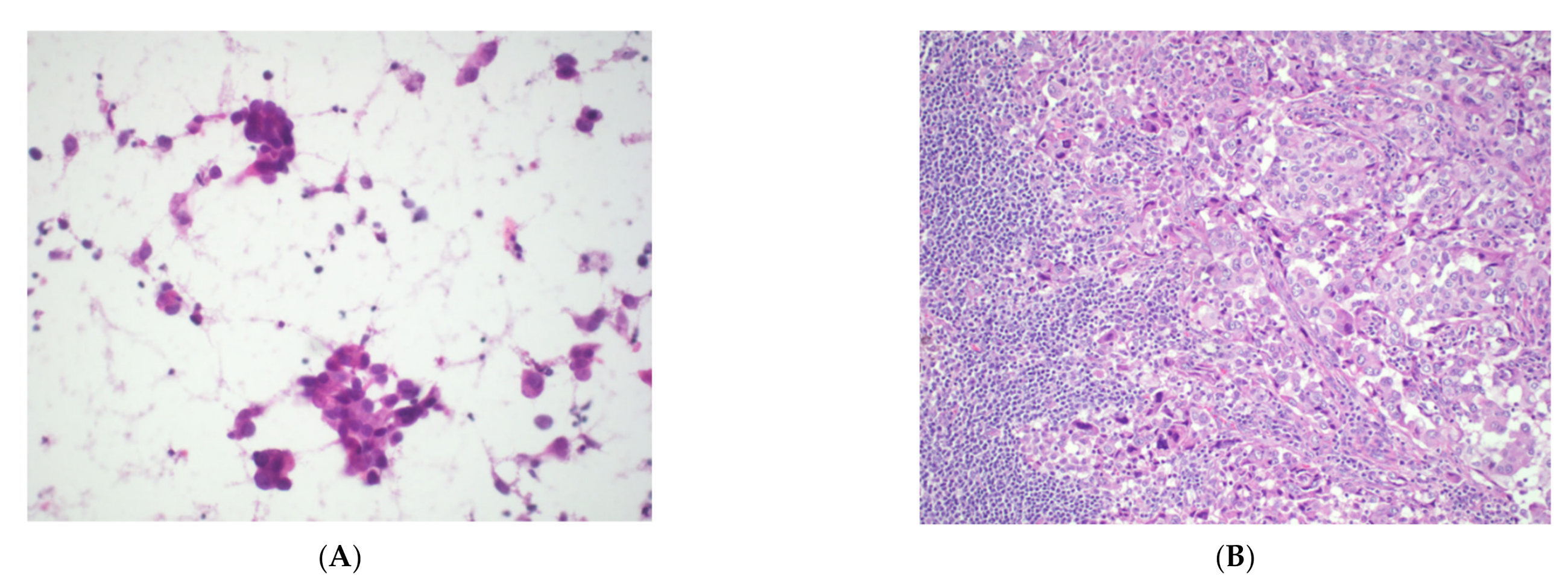
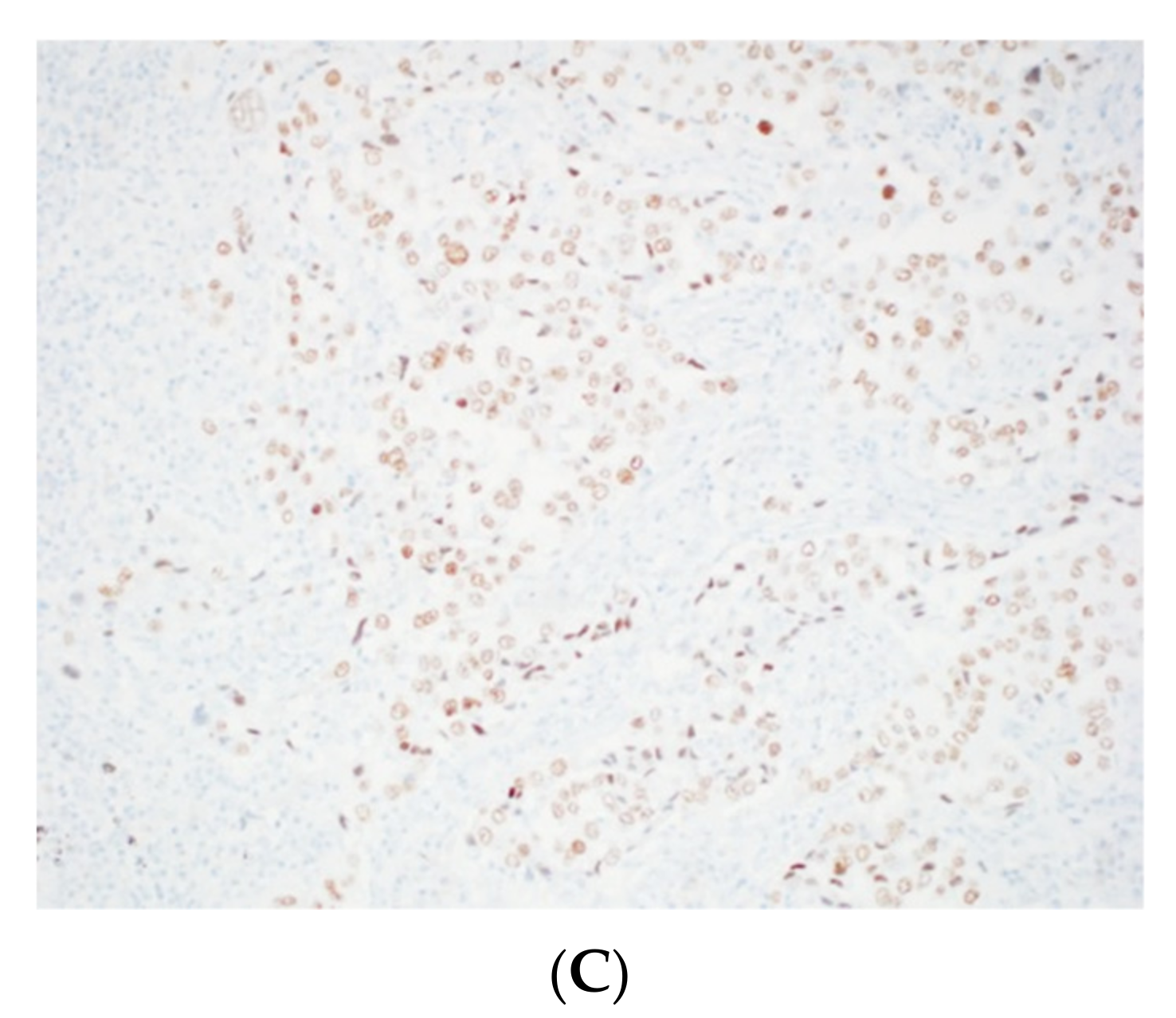
| WHO Classification | Cytological Features | Differential Diagnosis |
|---|---|---|
| Type A (including atypical subtype) |
|
|
| Type AB |
|
|
| Type B1 |
|
|
| Types B2 & B3 |
|
|
| Metaplastic | Not described | Not described |
| Micronodular | Not described | Not described |
| WHO Classification | Cytological Features |
|---|---|
| Squamous cell carcinoma |
|
| Basaloid carcinoma |
|
| Lymphoepithelial carcinoma |
|
| NUT carcinoma |
|
| Clear cell carcinoma |
|
| Low-grade papillary adenocarcinoma | Not described |
| Mucoepidermoid carcinoma |
|
| Thymic carcinoma with adenoid cystic carcinoma-like features | Not described |
| Enteric-type adenocarcinoma |
|
| Adenocarcinoma NOS | Not described |
| Adenosquamous carcinoma | Not described |
| Sarcomatoid carcinoma | Not described |
| Undifferentiated carcinoma | Not described |
| Thymic carcinoma NOS | Not described |
| WHO Classification | Cytological Features |
|---|---|
| Carcinoid tumor |
|
| Small cell carcinoma |
|
| Large cell neuroendocrine carcinoma |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willner, J.; Zhou, F.; Moreira, A.L. Diagnostic Challenges in the Cytology of Thymic Epithelial Neoplasms. Cancers 2022, 14, 2013. https://doi.org/10.3390/cancers14082013
Willner J, Zhou F, Moreira AL. Diagnostic Challenges in the Cytology of Thymic Epithelial Neoplasms. Cancers. 2022; 14(8):2013. https://doi.org/10.3390/cancers14082013
Chicago/Turabian StyleWillner, Jonathan, Fang Zhou, and Andre L. Moreira. 2022. "Diagnostic Challenges in the Cytology of Thymic Epithelial Neoplasms" Cancers 14, no. 8: 2013. https://doi.org/10.3390/cancers14082013
APA StyleWillner, J., Zhou, F., & Moreira, A. L. (2022). Diagnostic Challenges in the Cytology of Thymic Epithelial Neoplasms. Cancers, 14(8), 2013. https://doi.org/10.3390/cancers14082013






