A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiotherapy
2.2. Chemotherapy
2.3. Surgery
2.4. Follow-Up and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Eyck, B.M.; Van Lanschot, J.J.B.; Hulshof, M.C.C.M.; Van Der Wilk, B.J.; Shapiro, J.; Van Hagen, P.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J. Clin. Oncol. 2021, 39, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma. JAMA Surg. 2021, 156, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Xu, C.; Yang, J.; Komaki, R.; Lin, S.H. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother. Oncol. 2017, 125, 48–54. [Google Scholar] [CrossRef]
- Takakusagi, Y.; Yoshida, D.; Kusano, Y.; Kano, K.; Anno, W.; Tsuchida, K.; Mizoguchi, N.; Serizawa, I.; Katoh, H.; Imura, K.; et al. Dosimetric Comparison Between Carbon-ion Radiotherapy and Photon Radiotherapy for Stage I Esophageal Cancer. In Vivo 2021, 35, 447–452. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, K.L.; Guerrero, T.M.; McGuire, S.E.; Yaremko, B.; Komaki, R.; Cox, J.D.; Hui, Z.; Li, Y.; Newhauser, W.D.; et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Ling, T.C.; Slater, J.M.; Nookala, P.; Mifflin, R.; Grove, R.; Ly, A.M.; Patyal, B.; Slater, J.D.; Yang, G.Y. Analysis of Intensity-Modulated Radiation Therapy (IMRT), Proton and 3D Conformal Radiotherapy (3D-CRT) for Reducing Perioperative Cardiopulmonary Complications in Esophageal Cancer Patients. Cancers 2014, 6, 2356–2368. [Google Scholar] [CrossRef]
- Xu, C.; Jin, J.Y.; Zhang, M.; Liu, A.; Wang, J.; Mohan, R.; Kong, F.S.; Lin, S.H. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother. Oncol. 2020, 146, 180–186. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Fang, P.; Xu, C.; Song, J.; Krishnan, S.; Koay, E.J.; Mehran, R.J.; Hofstetter, W.L.; Blum-Murphy, M.; Ajani, J.A.; et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother. Oncol. 2018, 128, 154–160. [Google Scholar] [CrossRef]
- Deng, W.; Xu, C.; Liu, A.; van Rossum, P.S.N.; Deng, W.; Liao, Z.; Koong, A.C.; Mohan, R.; Lin, S.H. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother. Oncol. 2019, 133, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-F.; Liu, J.-S.; Huang, Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine 2014, 93, e257. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Jiang, W.; Davuluri, R.; Xu, C.; Krishnan, S.; Mohan, R.; Koong, A.C.; Hsu, C.C.; Lin, S.H. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother. Oncol. 2018, 128, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Hobbs, B.P.; Verma, V.; Tidwell, R.S.; Smith, G.L.; Lei, X.; Corsini, E.M.; Mok, I.; Wei, X.; Yao, L.; et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 1569–1579. [Google Scholar] [CrossRef]
- Wang, J.; Wei, C.; Tucker, S.L.; Myles, B.; Palmer, M.; Hofstetter, W.L.; Swisher, S.G.; Ajani, J.A.; Cox, J.D.; Komaki, R.; et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Davuluri, R.; Jiang, W.; Fang, P.; Xu, C.; Komaki, R.; Gomez, D.R.; Welsh, J.; Cox, J.D.; Crane, C.H.; Hsu, C.C.; et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 128–135. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; Henegouwen, M.I.v.B.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef]
- Welsh, J.; Gomez, D.; Palmer, M.B.; Riley, B.A.; Mayankkumar, A.V.; Komaki, R.; Dong, L.; Zhu, X.R.; Likhacheva, A.; Liao, Z.; et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Merrell, K.W.; Shen, J.; Verma, V.; Correa, A.M.; Wang, L.; Thall, P.F.; Bhooshan, N.; James, S.E.; Haddock, M.G.; et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother. Oncol. 2017, 123, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.G.; Bayasgalan, U.; Kim, H.T.; Lee, J.M.; Kim, M.S.; Lee, Y.; Lee, D.Y.; Lee, S.U.; Kim, T.H.; Moon, S.H. Photon Versus Proton Beam Therapy for T1-3 Squamous Cell Carcinoma of the Thoracic Esophagus Without Lymph Node Metastasis. Front. Oncol. 2021, 11, 699172. [Google Scholar] [CrossRef] [PubMed]
- Routman, D.M.; Garant, A.; Lester, S.C.; Day, C.N.; Harmsen, W.S.; Sanheuza, C.T.; Yoon, H.H.; Neben-Wittich, M.A.; Martenson, J.A.; Haddock, M.G.; et al. A Comparison of Grade 4 Lymphopenia with Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv. Radiat. Oncol. 2019, 4, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharzai, L.; Verma, V.; Denniston, K.A.; Bhirud, A.R.; Bennion, N.R.; Lin, C. Radiation Therapy and Cardiac Death in Long-Term Survivors of Esophageal Cancer: An Analysis of the Surveillance, Epidemiology, and End Result Database. PLoS ONE 2016, 11, e0158916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Finn, C.F.; CIASLC Advanced Radiation Technology committee. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef]
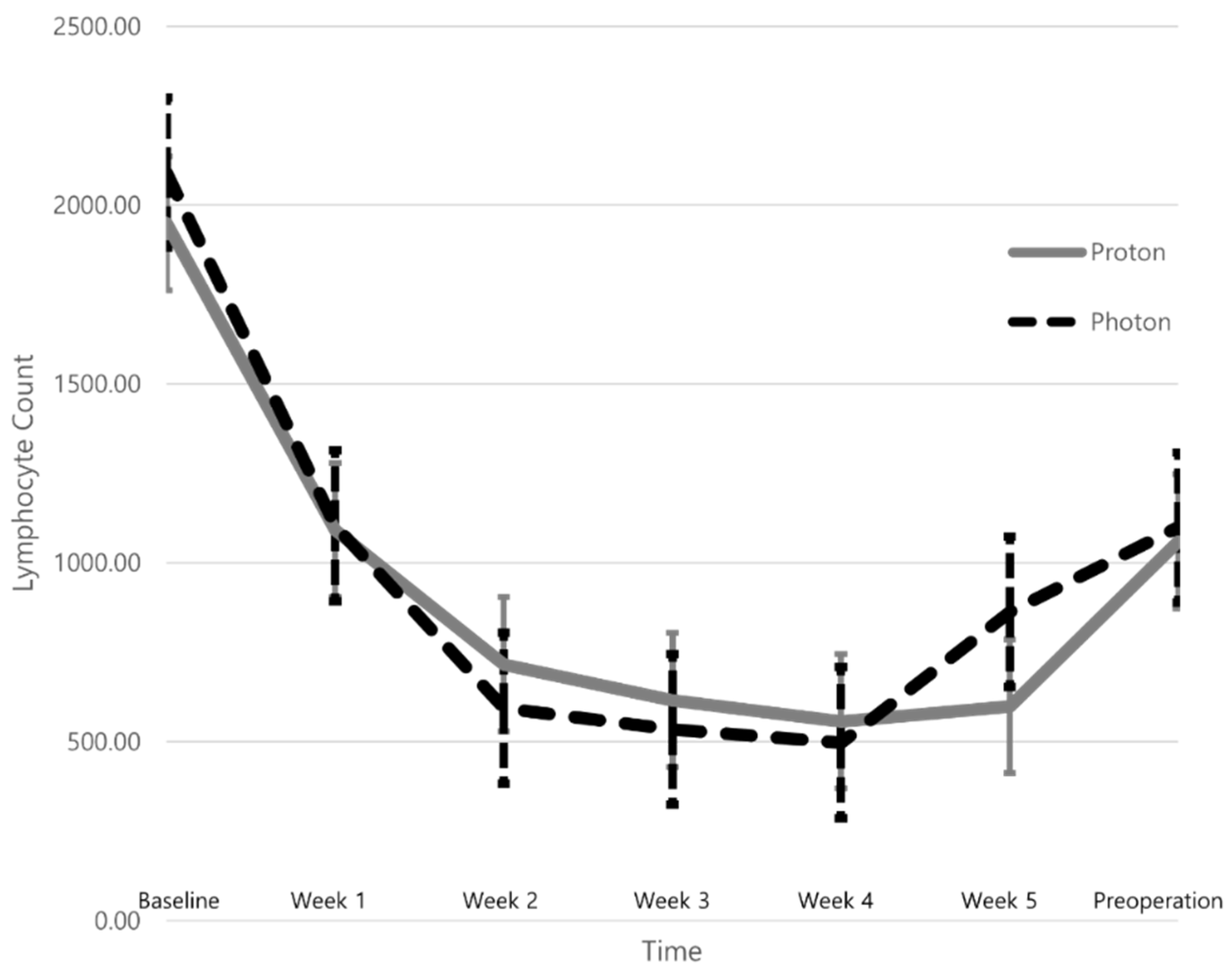
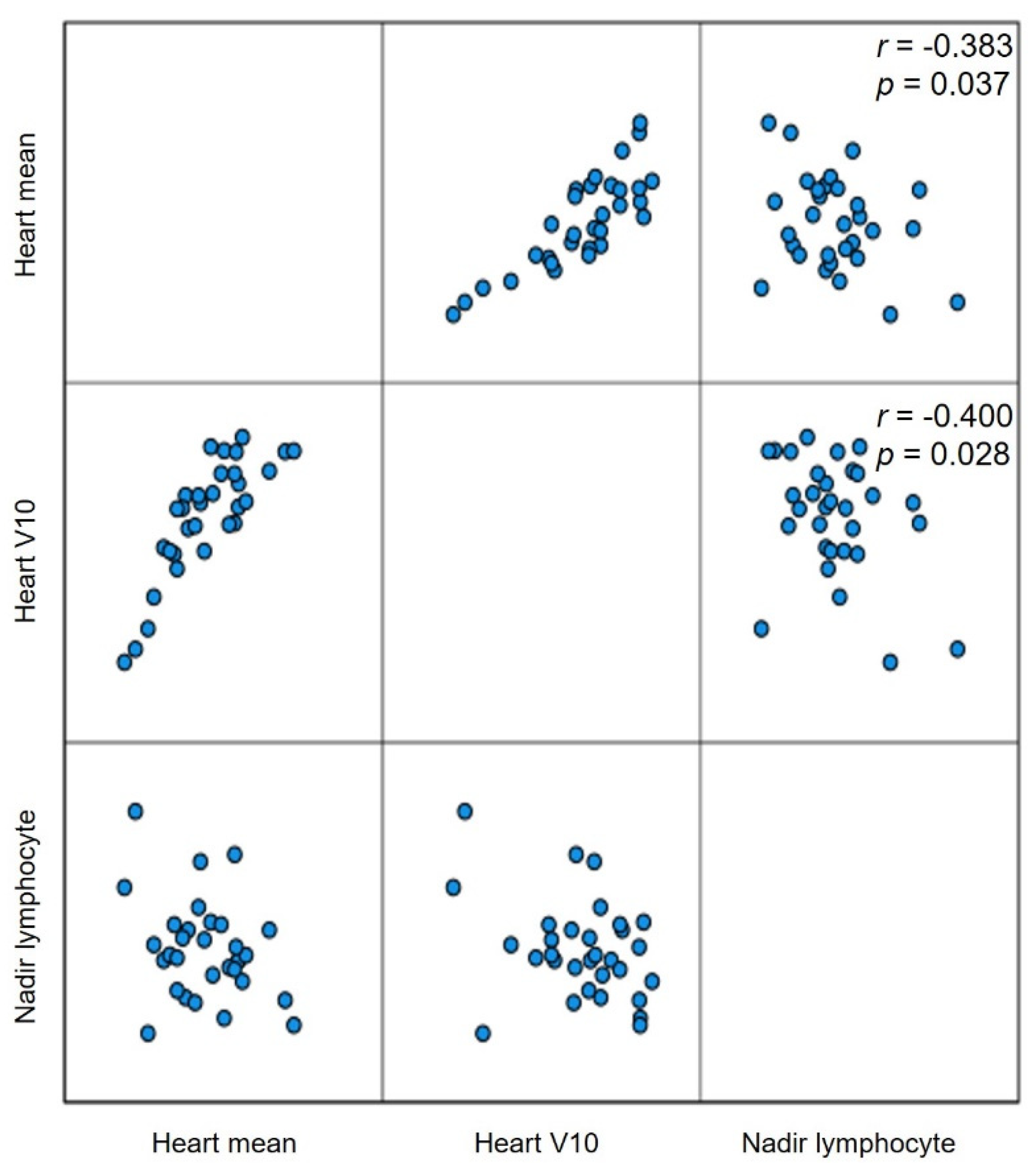

| Variables | Groupphoton (n = 16) | Groupproton (n = 15) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 59.38 ± 4.92 | 62.27 ± 7.26 | 0.202 |
| Sex (male, %) | 14 (87.5) | 13 (86.7) | 1.000 |
| Maximum SUV (mean ± SD) | 12.71 ± 4.54 1 | 13.18 ± 5.23 1 | 0.800 |
| ECOG PS (n, %) | 0.043 | ||
| 0 | 0 (0.0) | 4 (26.7) | |
| 1 | 16 (100.0) | 11 (73.3) | |
| Comorbidities | |||
| None | 10 (62.5) | 5 (33.3) | 0.104 |
| Other cancer | 1 (6.3) | 3 (20.0) | 0.333 |
| Cardiovascular disease | |||
| Hypertension | 3 (18.8) | 7 (46.7) | 0.135 |
| Arrhythmia | 1 (6.3) | 0 (0.0) | 1.000 |
| Coronary artery disease | 0 (0.0) | 1 (6.7) | 0.484 |
| Cerebrovascular disease | 1 (6.3) | 1 (6.7) | 1.000 |
| Asthma | 0 (0.0) | 1 (6.7) | 0.484 |
| Alcoholic liver cirrhosis | 1 (6.3) | 0 (0.0) | 1.000 |
| Diabetes | 4 (25.0) | 0 (0.0) | 0.101 |
| cT category (n, %) 2 | 0.326 | ||
| 1 | 1 (6.3) | 1 (6.7) | |
| 2 | 7 (43.8) | 3 (20.0) | |
| 3 | 7 (43.8) | 11 (73.3) | |
| 4 | 1 (6.3) | 0 (0.0) | |
| cN category (n, %) 2 | 0.978 | ||
| 1 | 7 (43.8) | 6 (40.0) | |
| 2 | 8 (50.0) | 8 (53.3) | |
| 3 | 1 (6.3) | 1 (6.7) | |
| Location (n, %) | 0.412 | ||
| Upper thoracic | 5 (31.3) | 4 (26.7) | |
| Middle thoracic | 5 (31.3) | 8 (53.3) | |
| Lower thoracic | 6 (37.5) | 3 (20.0) |
| Variables | Groupphoton (n = 16) | Groupproton (n = 15) | p-Value |
|---|---|---|---|
| Total dose, Gy (median, range) | 41.4 Gy (26.0–50.4) | 41.4 Gy (37.8–50.0) | 0.705 |
| Lung mean, cGy (mean ± SD) | 816.00 ± 251.63 | 431.27 ± 189.35 | <0.001 |
| Lung V10, % (mean ± SD) | 23.65 ± 10.23 | 14.23 ± 6.21 | 0.005 |
| Lung V20, % (mean ± SD) | 13.31 ± 5.53 | 9.07 ± 3.87 | 0.02 |
| Heart mean, cGy (mean ± SD) | 2774.88 ± 651.92 | 1410.80 ± 615.92 | <0.001 |
| Heart V10, % (mean ± SD) | 80.46 ± 13.68 | 55.11 ± 24.54 | 0.001 |
| Heart V30, % (mean ± SD) | 51.89 ± 21.31 | 18.35 ± 8.89 | <0.001 |
| Heart V40, % (mean ± SD) | 35.43 ± 25.85 | 12.55 ± 6.40 | 0.003 |
| Grade 4 lymphopenia (n, %) | 2 (12.5%) | 3 (20.0) | 0.654 |
| Lymphocyte count nadir (mean ± SD) | 396.49 ± 156.48 | 388.02 ± 239.22 | 0.907 |
| Variables | Groupphoton (n = 14) 1 | Groupproton (n = 14) 2 | p-Value |
|---|---|---|---|
| Minimal invasive surgery (n, %) | 6 (42.9) | 4 (28.6) | 0.430 |
| Intrathoracic anastomosis (n, %) | 11 (78.6) | 9 (64.3) | 0.339 |
| Stomach conduit (n, %) | 14 (100.0) | 13 (92.9) | 1.000 |
| LND | |||
| 3-field LND (n, %) 3 | 6 (42.9) | 6 (42.9) | 1.000 |
| Resected LNs (median, range) | 0 (0–44) | 34 (0–66) | 0.001 |
| R0 resection | 13 (92.9) | 13 (92.9) | 1.000 |
| Tumor size, cm (median, range) | 0.2 (0.0–13.0) | 0.5 (0.0–3.1) | 0.867 |
| Pathologic stage (n, %) 4 | |||
| ypT0 | 5 (35.7) | 4 (28.6) | 0.544 |
| ypTis | 0 (0.0) | 1 (7.1) | |
| ypT1 | 2 (14.3) | 2 (14.3) | |
| ypT2 | 3 (21.4) | 4 (28.6) | |
| ypT3 | 4 (28.6) | 3 (21.4) | |
| ypN0 | 8 (57.1) | 7 (50.0) | 0.497 |
| ypN1 | 5 (35.7) | 7 (50.0) | |
| ypN2 | 1 (7.1) | 0 (0.0) | |
| ypT0N0 | 5 (35.7) | 4 (28.6) | 1.000 |
| ypM1 | 1 (7.1) | 0 (0.0) | 1.000 |
| Operative complications (n, %) | |||
| Anastomosis or graft failure | 0.704 | ||
| None | 10 (71.4) | 9 (64.3) | |
| Delayed anastomosis heal 5 | 1 (7.1) | 2 (14.3) | |
| Endoscopic or surgical intervention 6 | 3 (21.4) | 1 (7.1) | |
| Life-threatening mediastinitis 7 | 0 (0.0) | 1 (7.1) | |
| Graft necrosis 8 | 0 (0.0) | 1 (7.1) | |
| Respiratory complication | 0.515 | ||
| None | 7 (50.0) | 9 (64.3) | |
| Atelectasis requiring toileting BFS 6 | 6 (42.9) | 5 (35.7%) | |
| ARDS 7 | 1 (7.1) | 0 (0.0) | |
| Postoperative arrhythmia | 1 (7.1) | 3 (21.4) | 0.596 |
| Vocal cord palsy | 5 (35.7%) | 4 (28.6) | 1.000 |
| Chyle leak | 0 (0.0) | 2 (14.3) | 0.481 |
| Wound dehiscence | 2 (14.3) | 1 (7.1) | 1.000 |
| Operative mortality (n, %) | 0 (0.0) | 1 (7.1) 9 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Lee, J.M.; Kim, M.S.; Lee, Y.; Suh, Y.-G.; Lee, S.U.; Lee, D.Y.; Oh, E.S.; Kim, T.h.; Moon, S.H. A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute. Cancers 2022, 14, 2033. https://doi.org/10.3390/cancers14082033
Choi J-H, Lee JM, Kim MS, Lee Y, Suh Y-G, Lee SU, Lee DY, Oh ES, Kim Th, Moon SH. A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute. Cancers. 2022; 14(8):2033. https://doi.org/10.3390/cancers14082033
Chicago/Turabian StyleChoi, Jin-Ho, Jong Mog Lee, Moon Soo Kim, Youngjoo Lee, Yang-Gun Suh, Sung Uk Lee, Doo Yeul Lee, Eun Sang Oh, Tae hyun Kim, and Sung Ho Moon. 2022. "A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute" Cancers 14, no. 8: 2033. https://doi.org/10.3390/cancers14082033
APA StyleChoi, J.-H., Lee, J. M., Kim, M. S., Lee, Y., Suh, Y.-G., Lee, S. U., Lee, D. Y., Oh, E. S., Kim, T. h., & Moon, S. H. (2022). A Comparative Analysis of Photon versus Proton Beam Therapy in Neoadjuvant Concurrent Chemoradiotherapy for Intrathoracic Squamous Cell Carcinoma of the Esophagus at a Single Institute. Cancers, 14(8), 2033. https://doi.org/10.3390/cancers14082033






