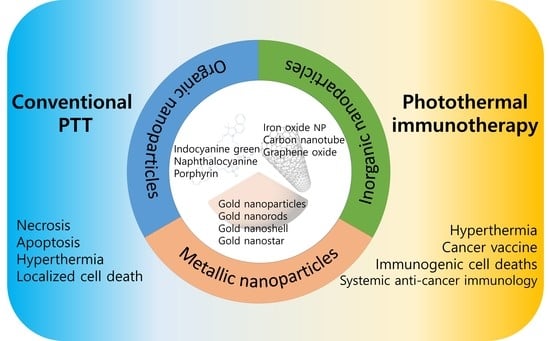
How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy?
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Nanoparticle-Mediated PTT
1.2. Cancer Immunotherapy with Nanoparticle-Mediated PTT
2. Photothermal Therapy with Organic-Dye Nanoparticles
3. Photothermal Therapy with Inorganic Nanoparticles
4. Photothermal Therapy with Metallic Nanoparticles
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Breasted, J.H. The Edwin Smith Surgical Papyrus; The University of Chicago Press: Chicago, IL, USA, 1930; p. 365. [Google Scholar]
- Mirza, A.N.; Fornage, B.D.; Sneige, N.; Kuerer, H.M.; Newman, L.A.; Ames, F.C.; Singletary, S.E. Radiofrequency ablation of solid tumors. Cancer J. 2001, 7, 95–102. [Google Scholar] [PubMed]
- Gazelle, G.S.; Goldberg, S.N.; Solbiati, L.; Livraghi, T. Tumor ablation with radio-frequency energy. Radiology 2000, 217, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Watanabe, Y.; Ueda, S.; Iseki, S.; Abe, Y.; Sato, N.; Kimura, S.; Okubo, K.; Onji, M. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology 1996, 110, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Wakabayashi, M.; Nakagawa, T.; Imamura, M.; Tamai, T.; Nishimura, A.; Yamashiki, N.; Okamura, A.; Inoue, K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: Comparison with percutaneous ethanol injection therapy. Cancer 1999, 85, 1694–1702. [Google Scholar] [CrossRef]
- Wu, F.; Chen, W.Z.; Bai, J.; Zou, J.Z.; Wang, Z.L.; Zhu, H.; Wang, Z.B. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med. Biol. 2001, 27, 1099–1106. [Google Scholar] [CrossRef]
- Marmor, J.B.; Pounds, D.; Postic, T.B.; Hahn, G.M. Treatment of superficial human neoplasms by local hyperthermia induced by ultrasound. Cancer 1979, 43, 188–197. [Google Scholar] [CrossRef]
- Castren-Persons, M.; Schroder, T.; Ramo, O.J.; Puolakkainen, P.; Lehtonen, E. Contact nd:Yag laser potentiates the tumor cell killing effect of hyperthermia. Lasers Surg. Med. 1991, 11, 595–600. [Google Scholar] [CrossRef]
- Waldow, S.M.; Morrison, P.R.; Grossweiner, L.I. Nd:Yag laser-induced hyperthermia in a mouse tumor model. Lasers Surg. Med. 1988, 8, 510–514. [Google Scholar] [CrossRef]
- Huang, X.H.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (pptt) using gold nanoparticles. Laser Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Yang, S.; Sun, I.C.; Hwang, H.S.; Shim, M.K.; Yoon, H.Y.; Kim, K. Rediscovery of nanoparticle-based therapeutics: Boosting immunogenic cell death for potential application in cancer immunotherapy. J. Mater. Chem. B 2021, 9, 3983–4001. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Laser Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodriguez, E.M.; Sole, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Laser Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]
- Zharov, V.P.; Kim, J.W.; Curiel, D.T.; Everts, M. Self- assembling nanoclusters in living systems: Application for integrated photothermal nanodiagnostics and nanotherapy. Nanomed. Nanotechnol. 2005, 1, 326–345. [Google Scholar] [CrossRef]
- Kotaidis, V.; Plech, A. Cavitation dynamics on the nanoscale. Appl. Phys. Lett. 2005, 87, 213102. [Google Scholar] [CrossRef]
- Rau, K.R.; Quinto-Su, P.A.; Hellman, A.N.; Venugopalan, V. Pulsed laser microbeam-induced cell lysis: Time-resolved imaging and analysis of hydrodynamic effects. Biophys. J. 2006, 91, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, S.; Werner, D.; Uwada, T. Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication. J. Photochem. Photobiol. C 2012, 13, 28–54. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhan, X.L.; Xiong, J.; Peng, S.S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.L.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.V.P.; Dubey, S.K.; Tiwari, S.; Puri, A.; Hejmady, S.; Gorain, B.; Kesharwani, P. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int. J. Pharmaceut. 2021, 606, 120848. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H. Induction of apoptotic temperature in photothermal therapy under various heating conditions in multi-layered skin structure. Int. J. Mol. Sci. 2021, 22, 11091. [Google Scholar] [CrossRef]
- Yi, X.L.; Duan, Q.Y.; Wu, F.G. Low-temperature photothermal therapy: Strategies and applications. Research 2021, 2021, 9816594. [Google Scholar] [CrossRef]
- Roti, J.L.R. Cellular responses to hyperthermia (40–46 degrees c): Cell killing and molecular events. Int. J. Hyperther. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Lepock, J.R.; Frey, H.E.; Ritchie, K.P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat-shock. J. Cell Biol. 1993, 122, 1267–1276. [Google Scholar] [CrossRef]
- Henle, K.J.; Nagle, W.A. Inhibition of heat-shock protein-synthesis and protein glycosylation by stepdown heating. Exp. Cell Res. 1991, 196, 184–191. [Google Scholar] [CrossRef]
- Lepock, J.R. How do cells respond to their thermal environment? Int. J. Hyperther. 2005, 21, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Arancia, G.; Malorni, W.; Mariutti, G.; Trovalusci, P. Effect of hyperthermia on the plasma-membrane structure of chinese-hamster v79 fibroblasts—A quantitative freeze-fracture study. Radiat. Res. 1986, 106, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Skibba, J.L.; Gwartney, E.A. Liver hyperthermia and oxidative stress: Role of iron and aldehyde production. Int. J. Hyperther. 1997, 13, 215–226. [Google Scholar] [CrossRef]
- Harmon, B.V.; Corder, A.M.; Collins, R.J.; Gobe, G.C.; Allen, J.; Allan, D.J.; Kerr, J.F. Cell death induced in a murine mastocytoma by 42-47 degrees c heating in vitro: Evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int. J. Radiat. Biol. 1990, 58, 845–858. [Google Scholar] [CrossRef]
- Perez-Hernandez, M.; del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Galvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Li, T.Y.; Cheng, H.; Zhang, F.; Yang, X.Y.; Wang, S.H.; Zhou, J.P.; Ding, Y. Nanomedicine potentiates mild photothermal therapy for tumor ablation. Asian J. Pharm. Sci. 2021, 16, 738–761. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, W.Q.; Pei, Q.; Lord, M.S.; Yu, H.J. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 986–994. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Ng, C.W.; Li, J.C.; Pu, K.Y. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Sweeney, E.E.; Cano-Mejia, J.; Fernandes, R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small 2018, 14, e1800678. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017, 17, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and damps in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Serrano-del Valle, A.; Anel, A.; Naval, J.; Marzo, I. Immunogenic cell death and immunotherapy of multiple myeloma. Front. Cell Dev. Biol. 2019, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Valentinis, B.; Voll, R.E.; Herrmann, M.; Nawroth, P.P.; Arnold, B.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Release of high mobility group box 1 by dendritic cells controls t cell activation via the receptor for advanced glycation end products. J. Immunol. 2005, 174, 7506–7515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, X.; Pan, W.; Li, N.; Tang, B. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chem. Commun. 2020, 56, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Li, X.; Zhao, Y.; Li, M.; Jiang, W.; Tang, X.; Dou, J.; Lu, L.; Wang, F. Near-infrared ii phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano 2019, 13, 11967–11980. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [Green Version]
- Conniot, J.; Silva, J.M.; Fernandes, J.G.; Silva, L.C.; Gaspar, R.; Brocchini, S.; Florindo, H.F.; Barata, T.S. Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014, 2, 105. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-devd-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, M.K.; Yang, S.; Moon, Y.; Song, S.; Choi, J.; Kim, J.; Kim, K. Combination of cancer-specific prodrug nanoparticle with bcl-2 inhibitor to overcome acquired drug resistance. J. Control. Release 2020, 330, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.K.; Moon, Y.; Yang, S.; Kim, J.; Cho, H.; Lim, S.; Yoon, H.Y.; Seong, J.-K.; Kim, K. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin b-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 2020, 261, 120347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-step assembly of dox/icg loaded lipid polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, P.; Luo, Z.; Gong, P.; Zheng, C.; Zhang, P.; Yue, C.; Gao, D.; Ma, Y.; Cai, L. Robust icg theranostic nanoparticles for folate targeted cancer imaging and highly effective photothermal therapy. ACS Appl. Mater. Interfaces 2014, 6, 6709–6716. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef]
- Taratula, O.; Doddapaneni, B.S.; Schumann, C.; Li, X.; Bracha, S.; Milovancev, M.; Alani, A.W.G.; Taratula, O. Naphthalocyanine-based biodegradable polymeric nanoparticles for image-guided combinatorial phototherapy. Chem. Mater. 2015, 27, 6155–6165. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Sun, B.; Carter, K.; Qin, Y.; Wei, W.; Wang, D.; Jeon, M.; Geng, J.; Nickles, R.J.; et al. Surfactant-stripped naphthalocyanines for multimodal tumor theranostics with upconversion guidance cream. Nanoscale 2017, 9, 3391–3398. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Li, C.; Xu, N.; Watanabe, M.; Xue, R.; Xu, A.; Araki, M.; Sun, R.; Liu, C.; Nasu, Y.; et al. Dual-functional plga nanoparticles co-loaded with indocyanine green and resiquimod for prostate cancer treatment. Int. J. Nanomed. 2021, 16, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Xing, R.; Yuan, C.; Xue, H.; Yan, X. Supramolecular nanofibrils formed by coassembly of clinically approved drugs for tumor photothermal immunotherapy. Adv. Mater. 2021, 33, e2100595. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Duong, T.; Taylor, K.L.; Taratula, O. Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale 2015, 7, 3888–3902. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Hu, D.H.; Xue, M.M.; He, M.; Gong, P.; Cai, L.T. Indocyanine green nanoparticles for theranostic applications. Nano-Micro Lett. 2013, 5, 145–150. [Google Scholar] [CrossRef]
- Gomes, A.J.; Lunardi, L.O.; Marchetti, J.M.; Lunardi, C.N.; Tedesco, A.C. Indocyanine green nanoparticles useful for photomedicine. Photomed. Laser Surg. 2006, 24, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci. Rep. 2017, 7, 13958. [Google Scholar] [CrossRef]
- West, C.L.; Doughty, A.C.V.; Liu, K.; Chen, W.R. Monitoring tissue temperature during photothermal therapy for cancer. J. Bio-X Res. 2019, 2, 159–168. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Wu, A.G.; Chen, X.Y. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol. Pharmaceut. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Hooshmand, S.; Hayat, S.M.G.; Ghorbani, A.; Khatami, M.; Pakravanan, K.; Darroudi, M. Preparation and applications of superparamagnetic iron oxide nanoparticles in novel drug delivery systems: An overview. Curr. Med. Chem. 2021, 28, 777–799. [Google Scholar] [CrossRef]
- Zeinoun, M.; Domingo-Diez, J.; Rodriguez-Garcia, M.; Garcia, O.; Vasic, M.; Ramos, M.; Olmedo, J.J.S. Enhancing magnetic hyperthermia nanoparticle heating efficiency with non-sinusoidal alternating magnetic field waveforms. Nanomaterials 2021, 11, 3240. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Luo, L.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Yang, J.; Zhu, C.; Du, Y.; et al. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 16581–16593. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, H.; Yin, S.; Cheng, L.; Wang, C.; Li, Z.; Li, Y.; Wang, X.; Liu, G.; Liu, Z. Ultra-small iron oxide doped polypyrrole nanoparticles for in vivo multimodal imaging guided photothermal therapy. Adv. Funct. Mater. 2014, 24, 1194–1201. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An innovative mwcnts/dox/tc nanosystem for chemo-photothermal combination therapy of cancer. Nanomedicine 2017, 13, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Piranfar, A.; Rostami, S. Ph-sensitive loading/releasing of doxorubicin using single-walled carbon nanotube and multi-walled carbon nanotube: A molecular dynamics study. Comput. Methods Programs Biomed. 2020, 186, 105210. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-embedded indocyanine green-hyaluronic acid nanoparticles for photoacoustic-imaging-guided phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Guo, Z.Y.; Huang, D.Q.; Liu, Z.M.; Guo, X.; Zhong, H.Q. Synergistic effect of chemo-photothermal therapy using pegylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-activatable fe3o4 superparticle nanodrug carriers with pd-l1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Song, J.; Liu, Y.; Liu, M.; Zhang, L.; Sheng, D.; Deng, L.; Yi, H.; Wu, M.; Zheng, Y.; et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-pd1 antibody and synergizes with antitumor immunotherapy for melanoma. J. Control. Release 2019, 306, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Jing, H.; Dong, X.; Leng, X. Mwcnt-mediated combinatorial photothermal ablation and chemo-immunotherapy strategy for the treatment of melanoma. J. Mater. Chem. B 2020, 8, 4245–4258. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.M.; Liu, Y.J.; Zhu, X.H.; Wang, X.L.; Liu, L.X.; Sun, H.F.; Wang, C.; Kong, D.L.; Ma, G.L. Nanoscale reduced graphene oxide-mediated photothermal therapy together with ido inhibition and pd-l1 blockade synergistically promote antitumor immunity. ACS Appl. Mater. Interfaces 2019, 11, 1876–1885. [Google Scholar] [CrossRef]
- Jabeen, F.; Najam-ul-Haq, M.; Javeed, R.; Huck, C.W.; Bonn, G.K. Au-nanomaterials as a superior choice for near-infrared photothermal therapy. Molecules 2014, 19, 20580–20593. [Google Scholar] [CrossRef] [Green Version]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.C.; Eun, D.K.; Koo, H.; Ko, C.Y.; Kim, H.S.; Yi, D.K.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. Tumor-targeting gold particles for dual computed tomography/optical cancer imaging. Angew. Chem. Int. Ed. 2011, 50, 9348–9351. [Google Scholar] [CrossRef]
- Sun, I.C.; Eun, D.K.; Na, J.H.; Lee, S.; Kim, I.J.; Youn, I.C.; Ko, C.Y.; Kim, H.S.; Lim, D.; Choi, K.; et al. Heparin-coated gold nanoparticles for liver-specific ct imaging. Chem. Eur. J. 2009, 15, 13341–13347. [Google Scholar] [CrossRef]
- Sun, I.C.; Lee, S.; Koo, H.; Kwon, I.C.; Choi, K.; Ahn, C.H.; Kim, K. Caspase sensitive gold nanoparticle for apoptosis imaging in live cells. Bioconjugate Chem. 2010, 21, 1939–1942. [Google Scholar] [CrossRef]
- Sun, I.C.; Na, J.H.; Jeong, S.Y.; Kim, D.E.; Kwon, I.C.; Choi, K.; Ahn, C.H.; Kim, K. Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting ct imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser photothermal melting and fragmentation of gold nanorods: Energy and laser pulse-width dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Huang, X.H.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yang, J.; Jang, E.; Suh, J.-S.; Huh, Y.-M.; Lee, K.; Haam, S. Gold nanostructures as photothermal therapy agent for cancer. Anti-Cancer Agents Med. Chem. 2011, 11, 953–964. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-egfr antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Finlayson, L.; Barnard, I.R.M.; McMillan, L.; Ibbotson, S.H.; Brown, C.T.A.; Eadie, E.; Wood, K. Depth penetration of light into skin as a function of wavelength from 200 to 1000 nm. Photochem. Photobiol. 2021. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. Ph-induced aggregation of gold nanoparticles for photothermal cancer therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J.; Ryu, S.; Kwon, Y.; Kim, K.H.; Jeong, D.H.; Paik, S.R.; Kim, B.S. Gold nanoparticle/graphene oxide hybrid sheets attached on mesenchymal stem cells for effective photothermal cancer therapy. Chem. Mater. 2017, 29, 3461–3476. [Google Scholar] [CrossRef]
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.Y.; Sun, Y.G.; Lu, X.M.; Au, L.; Cobley, C.M.; Xia, Y.N. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, H.; Yue, X.; Wang, J.; Xing, S.; Zhang, Q.; Dai, Z.; Tian, J.; Wang, S.; Jin, Y. Gold nanoshelled liquid perfluorocarbon nanocapsules for combined dual modal ultrasound/ct imaging and photothermal therapy of cancer. Small 2014, 10, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold nanocages as photothermal transducers for cancer treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, F.; Nehl, C.L.; Hafner, J.H.; Nordlander, P. Plasmon resonances of a gold nanostar. Nano Lett. 2007, 7, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.S.; Shih, C.W.; Chen, C.D.; Lai, W.C.; Wang, C.R.C. The shape transition of gold nanorods. Langmuir 1999, 15, 701–709. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability enhancement of silica-coated gold nanostars for photoacoustic imaging guided photothermal therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M. Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J. Phys. Chem. B 2005, 109, 10531–10532. [Google Scholar] [CrossRef] [Green Version]
- Yi, D.K.; Sun, I.-C.; Ryu, J.H.; Koo, H.; Park, C.W.; Youn, I.-C.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.-H. Matrix metalloproteinase sensitive gold nanorod for simultaneous bioimaging and photothermal therapy of cancer. Bioconjugate Chem. 2010, 21, 2173–2177. [Google Scholar] [CrossRef]
- Bear, A.S.; Kennedy, L.C.; Young, J.K.; Perna, S.K.; Mattos Almeida, J.P.; Lin, A.Y.; Eckels, P.C.; Drezek, R.A.; Foster, A.E. Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive t cell transfer. PLoS ONE 2013, 8, e69073. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Song, Y.; Cao, K.X.; Zhang, L.X.; Fang, X.D.; Chen, F.F.; Feng, S.H.; Yan, F. Photothermal therapy mediated by gold nanocages composed of anti-pdl1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021, 134, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.J.; Liu, L.L.; He, H.M.; Chen, Z.K.; Han, Z.Q.; Luo, Z.Y.; Wu, Z.H.; Zheng, M.B.; Ma, Y.F.; Cai, L.T. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chang, Y.; Luo, H.; Jiang, W.; Xu, L.; Chen, T.; Zhu, X. Designing immunogenic nanotherapeutics for photothermal-triggered immunotherapy involving reprogramming immunosuppression and activating systemic antitumor responses. Biomaterials 2020, 255, 120153. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wang, X.; Lin, S.; Liu, Y.; Lin, J.; Jiang, B.; Zhao, X.; Wei, H. Combining photothermal therapy-induced immunogenic cell death and hypoxia relief-benefited m1-phenotype macrophage polarization for cancer immunotherapy. Adv. Ther. 2021, 4, 2000191. [Google Scholar] [CrossRef]
- Xu, P.; Liang, F. Nanomaterial-based tumor photothermal immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef]
- Deng, J.J.; Xun, X.J.; Zheng, W.J.; Su, Y.F.; Zheng, L.Y.; Wang, C.F.; Su, M. Sequential delivery of bismuth nanoparticles and doxorubicin by injectable macroporous hydrogels for combined anticancer kilovoltage x-ray radio- and chemo-therapy. J. Mater. Chem. B 2018, 6, 7966–7973. [Google Scholar] [CrossRef]
- Liu, Y.J.; Bhattarai, P.; Dai, Z.F.; Chen, X.Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Jiang, R.M.; Dai, J.; Dong, X.Q.; Wang, Q.; Meng, Z.J.; Guo, J.J.; Yu, Y.J.; Wang, S.X.; Xia, F.; Zhao, Z.J.; et al. Improving image-guided surgical and immunological tumor treatment efficacy by photothermal and photodynamic therapies based on a multifunctional nir aiegen. Adv. Mater. 2021, 33, 2101158. [Google Scholar] [CrossRef]
- Wang, S.J.; Ma, X.Q.; Hong, X.H.; Cheng, Y.X.; Tian, Y.; Zhao, S.; Liu, W.F.; Tang, Y.X.; Zhao, R.Z.; Song, L.; et al. Adjuvant photothermal therapy inhibits local recurrences after breast-conserving surgery with little skin damage. ACS Nano 2018, 12, 662–670. [Google Scholar] [CrossRef]
- Simon, M.; Jorgensen, J.T.; Melander, F.; Andresen, T.L.; Christensen, A.; Kjaer, A. Photothermal therapy as adjuvant to surgery in an orthotopic mouse model of human fibrosarcoma. Cancers 2021, 13, 5820. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Lovell, J.F.; Yoon, J.; Chen, X.Y. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.P.; Zhang, Y.M.; Chitgupi, U.; Geng, J.M.; Wang, Y.H.; Zhang, Y.Z.; Cook, T.R.; Xia, J.; Lovell, J.F. A phosphorus phthalocyanine formulation with intense absorbance at 1000 nm for deep optical imaging. Theranostics 2016, 6, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Barbora, A.; Bohar, O.; Sivan, A.A.; Magory, E.; Nause, A.; Minnes, R. Higher pulse frequency of near-infrared laser irradiation increases penetration depth for novel biomedical applications. PLoS ONE 2021, 16, e0245350. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.G.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Deshpande, R.P.; Sharma, S.; Watabe, K. The confounders of cancer immunotherapy: Roles of lifestyle, metabolic disorders and sociological factors. Cancers 2020, 12, 2983. [Google Scholar] [CrossRef]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef]




| PTT Agent (Formulation or Modification) | Properties | Treatment Temp. | Laser Wavelength, Duration | Tumor Model | Therapeutic Effect | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Absorption Max. (nm) | |||||||
| Organic dye nanoparticles | ICG (PLGA, lipid, doxorubicin) | 90 | 815 nm | 53 °C | 808 nm, 5 min | MCF-7 | Apoptosis | [56] |
| ICG (cholesterol, lipid, folic acid) | 20~40 | 810 nm | 50 °C | 808 nm, 5 min | MCF-7 | Necrosis | [57] | |
| ICG (human serum albumin) | 80 | 816 nm | 57 °C | 785 nm, 5 min | 4T1 | Necrosis | [58] | |
| Naphthalocyanine (PEG-PCL, Si) | 40 | 785 nm | 47 °C | 785 nm, 10 min | A2780/AD | Apoptosis | [59] | |
| Naphthalocyanine (F127) | 30 | 860 nm | 60 °C | 860 nm, 10 min | 4T1 | Photothermal ablation | [60] | |
| Porphyrin (Dendrimer form + PEG) | 20 | 650–690 | 57 °C | 690 nm, 2 min | SKOV3 | Necrosis | [61] | |
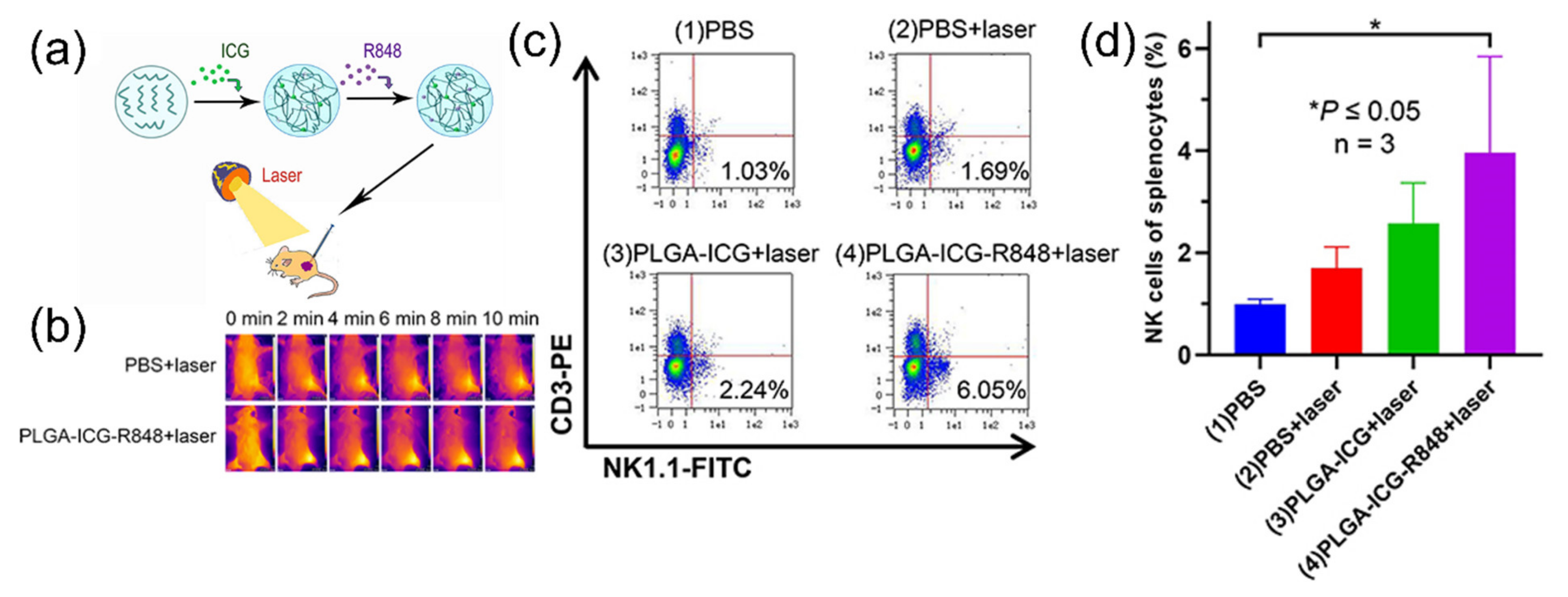
| ICG (PLGA, R848) | 160 | 780 nm | 50 °C | 808 nm, 10 min | RM9 | Immune response | [62] | |
| ICG (thymopentin) | 30 | broad | 47 °C | 808 nm, 10 min | Pan02 | Immune response | [63] | |
| Inorganic nanoparticles | Iron oxide nanoparticle | 20 | broad | 56 °C | 808 nm, 3 min | A549 | Apoptosis | [72] |
| Iron oxide nanoparticle (doxorubicin) | 10–310 | 480 nm (DOX) | 57 °C | 808 nm, 3 min | MCF-7 S180 | Apoptosis and necrosis | [73] | |
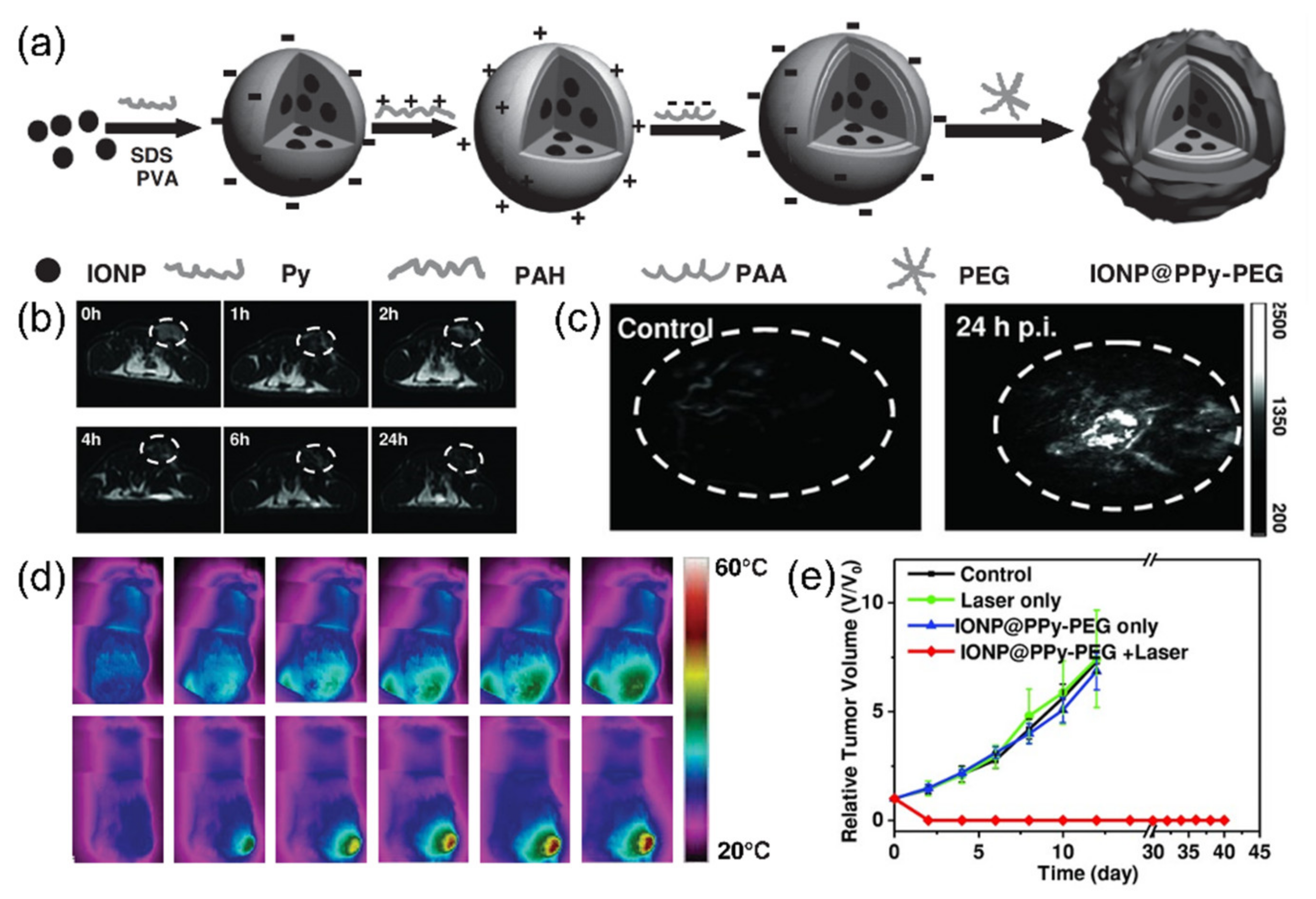
| Iron oxide nanoparticle (polypyrrole) | 100 | broad | 58 °C | 808 nm, 5 min | 4T1 | Photothermal ablation | [74] | |
| Carbon nanotube, MW (chitosan, doxorubicin) | 250 | broad | 67 °C | 808 nm, 5 min | Bel-7402 | Photothermal ablation | [76] | |
| Carbon nanotube, SW (PEG) | - | borad | 55 °C | 808 nm, 10 min | 4T1 | Photothermal ablation | [79] | |
| Carbon nanotube, SW (hyaluronic acid, cholanic acid, PEG, ICG) | 390 | broad | 55 °C | 808 nm, 10 min | SCC-7 | Necrosis | [80] | |
| Graphene oxide (PEG) | 10~50 | broad | 50 °C | 808 nm, 5 min | 4T1 | Photothermal ablation | [82] | |
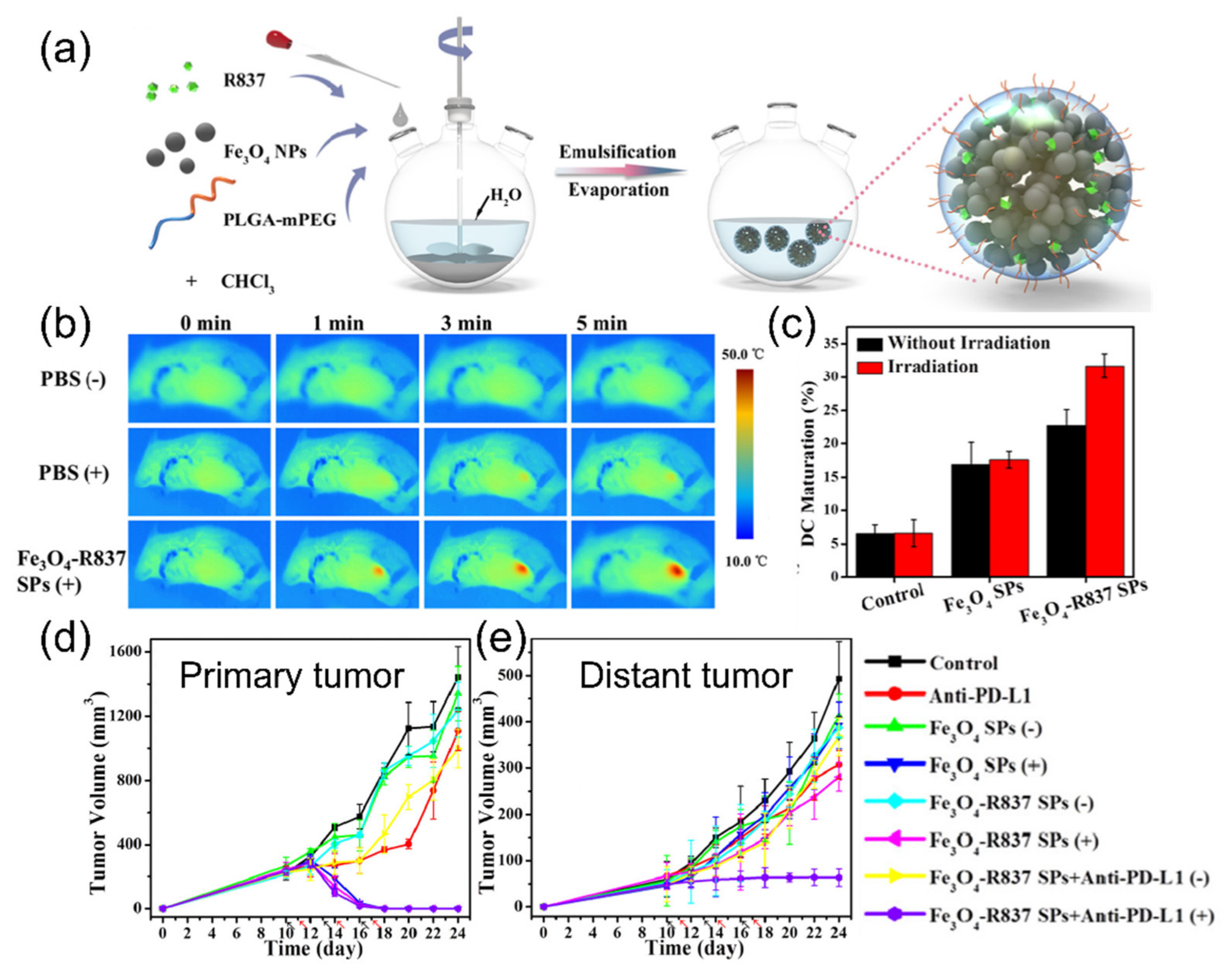
| Iron oxide nanoparticle (PLGA-PEG, R837,) | 150 | 320 nm | <50 °C | 808 nm, 10 min | 4T1 | Immune respones | [83] | |
| Iron oxide nanoparticle (PLGA-PEG, pentafluoropentane, anti-PD-1) | 220 | 700 nm | 45 °C | 660 nm, 10 min | B16F10 | ICD | [84] | |
| Carbon nanotube, MW (CpG or doxorubicin) | 200 | 480 nm (DOX) | 46 °C | 808 nm, 3 min | B16 | ICD | [85] | |
| Graphene oxide (IDO inhibitor, anti-PD-L1) | 200 | broad | 53 °C | 808 nm, 8 min | CT26 | Immune response | [86] | |
| Metallic nanoparticles | Gold nanosphere (anti-EGFR antibody) | 40 | 530 nm | - | 514 nm, 4 min | HSC 313 HSC 3 | Photothermal ablation | [97] |
| Gold nanoshell (PEG) | 300 | 550 nm | 60 °C | 808 nm, 10 min | U-87 MG | Necrosis | [103] | |
| Gold nanocage (PEG) | 90 | 800 nm | 54 °C | 808 nm, 10 min | U87MGwtEGFR | Necrosis | [104] | |
| Gold nanostar (PEG) | 30 | 945 nm | 50 °C | 980 nm, 10 min | Sarcoma | Necrosis | [106] | |
| Gold nanorod (peptide, Cy5.5) | - | 670 nm | 45 °C | 670 nm, 4 min | SCC-7 | Photothermal ablation | [110] | |
| Gold nanosphere (liposome) | 100 | 964 nm | 50 °C | 1064 nm, 10 min | 4T1 | ICD | [49] | |
| Gold nanoshell (PEG) | 40 | 808 nm | - | 808 nm, 3 min | B16-F10 | ICD | [111] | |
| Gold nanocage (anti-PDL1, galunisertib) | 60 | 800 nm | 45 °C | 808 nm, 10 min | CT26 | ICD | [112] | |
| Gold nanocage (MnO2) | 90 | 740 nm | 50 °C | 808 nm, 3 min | 4T1 | ICD | [113] | |
| Gold nanostar (selenium) | 120 | 850 nm | 52 °C | 808 nm, 10 min | U14 | ICD | [114] | |
| Gold nanostar (doxorubicin) | 150 | 775 nm | <50 °C | 808 nm, 5 min | CT26 | ICD | [115] | |
| Gold nanorod (MnO2) | 80 × 20 | 808 nm | 50 °C | 808 nm, 5 min | 4T1 | ICD | [116] | |
| Advantages | Disadvantages | ||
|---|---|---|---|
| Organic dye |
|
| |
| Inorganic Metallic | nanoparticles |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, W.S.; Park, J.-H.; Lim, D.-K.; Ahn, C.-H.; Sun, I.-C.; Kim, K. How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy? Cancers 2022, 14, 2044. https://doi.org/10.3390/cancers14082044
Yun WS, Park J-H, Lim D-K, Ahn C-H, Sun I-C, Kim K. How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy? Cancers. 2022; 14(8):2044. https://doi.org/10.3390/cancers14082044
Chicago/Turabian StyleYun, Wan Su, Ji-Ho Park, Dong-Kwon Lim, Cheol-Hee Ahn, In-Cheol Sun, and Kwangmeyung Kim. 2022. "How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy?" Cancers 14, no. 8: 2044. https://doi.org/10.3390/cancers14082044
APA StyleYun, W. S., Park, J.-H., Lim, D.-K., Ahn, C.-H., Sun, I.-C., & Kim, K. (2022). How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy? Cancers, 14(8), 2044. https://doi.org/10.3390/cancers14082044