How to Manage Patients with Lenalidomide-Refractory Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
| Family | Active Compound | Combination | Therapeutic Indication a | Ref. |
|---|---|---|---|---|
| Proteasome inhibitors (PI) | Bortezomib (V) | VRd | Not approved for RRMM | [19] |
| Vd | Adult patients with progressing MM who have received at least one previous regimen and have undergone HPCT or are not candidates for HPCT | [20] | ||
| VdT-PACE | Not approved for RRMM | [21] | ||
| Carfilzomib (K) | KRd | Adult patients with MM who have received at least one previous regimen | [7,19] | |
| Kd | Adult patients with MM who have received at least one previous regimen | [15] | ||
| KPd | Not approved for RRMM | [19,22,23] | ||
| KCd | Not approved for RRMM | [24] | ||
| Ixazomib (I) | IRd | Adult patients with MM who have received at least one previous regimen | [9,19,25] | |
| IPd | Not approved for RRMM | [19] | ||
| Immuno- modulatory agents (IMiD) | Iberdomide (cereblon E3 ligase modulators (CELMoDs)) | - | Not approved for RRMM | [26] |
| Lenalidomide (R) | Rd | Adult patients with MM who have received at least one previous regimen | [8,27,28] | |
| Pomalidomide (P) | PVd | Adult patients with MM who have received at least one previous regimen (including lenalidomide) | [18,29] | |
| Pd | Adult patients with RRMM who have received at least two previous regimens (including lenalidomide and bortezomib) and who have progressed on the latest therapy | [30,31,32] | ||
| PCd | Not approved for RRMM | [33] | ||
| Thalidomide (T) | VdT-PACE | Not approved for RRMM | [21] | |
| Anti- CD38 MoAb | Daratumumab (Dara) | DaraRd | Adult patients with MM who have received at least one previous regimen | [8,19,34] |
| DaraVd | Adult patients with MM who have received at least one previous regimen | [16,20] | ||
| DaraPd | In RRMM patients after disease has not improved with lenalidomide and PI or when treated with them separately | [19,35] | ||
| DaraKd | Adult patients with MM who have received at least one previous regimen | [36] | ||
| DaraCd | Not approved for RRMM | [37] | ||
| Isatuximab (Isa) | IsaKd | Adult patients with MM who have received at least one previous regimen | [38,39] | |
| IsaPd | Adult patients with RRMM who have received at least two previous regimens (including lenalidomide and PI) and who have shown disease progression on the last therapy | [40] | ||
| Anti SLAMF7 monoclonalantibody | Elotuzumab (Elo) | EloRd | Adult patients with MM who have received at least one previous regimen | [10,11,12,41] |
| EloPd | Adult patients with RRMM who have received at least two previous treatments (including lenalidomide and PI) and who have shown disease progression on the last therapy | [42] | ||
| Antibody-drug conjugate (ADC) | Belantamab mafodotin (belamaf) b | Bela monotherapy | Adult patients with MM who have received at least four prior therapies and whose illness is refractory to PI, IMiD and anti-CD38 MoAb, and who have shown disease progression on the last therapy | [43,44] |
| Bi-specific drugs (BiABs) | Teclistamab | - | Conditional marketing authorisation for adult patients with RRMM, who have received at least three prior therapies | [45,46] |
| CAR T-cell | Idecabtagene vicleucel (ide-cel) | - | Conditional marketing authorisation for adult patients with RRMM, who have received at least three prior therapies, including an IMiD, a PI and an anti-CD38 MoAb and whose disease has worsened since the last treatment | [47] |
| Ciltacabtagene autoleucel (cilta-cel) | - | Conditional marketing authorisation for adult patients with RRMM, who have received at least three prior therapies, including an IMiD, a PI and an anti-CD38 MoAb and whose disease has worsened since the last treatment | [48] | |
| BCL-2 inhibitor | Venetoclax | - | Not approved for RRMM | [49] |
| Selective inhibitor of nuclear export | Selinexor (S) | SVd | Adult patients with MM who have received at least one previous regimen | [50] |
| Sd | Adult patients with MM who have received at least four previous therapies, whose illness is refractory to at least two PIs, two IMiDs and one anti-CD38 MoAb and has worsened since the last treatment | [51] | ||
| Alkylating agents | Carmustine | Dexa-BEAM | Secondary therapy and conditioning treatment prior to ASCT in malignant haematological diseases (non-Hodgkin’s lymphoma and Hodgkin’s diseases) | [52] |
| Cisplatin | VdT-PACE | Not approved for RRMM | [21] | |
| PACE | Not approved for RRMM | [53] | ||
| DCEP | Not approved for RRMM | [54] | ||
| Dexa-BEAM | Not approved for RRMM | [52] | ||
| VdT-PACE | Not approved for RRMM | [21] | ||
| KCd | Not approved for RRMM | [24] | ||
| Melphalan (M) | DaraMPV | Not approved for RRMM | [55] | |
| Bendamustine | - | Not approved for RRMM | [56] | |
| Anti-inflammatory steroids | Dexamethasone (d) | VdT-PACE | Not approved for RRMM | [21] |
| Macrolide antibiotic | Clarithromycin (Cla) | ClaPd | Not approved for RRMM | [57] |
| Alkaloid | Etoposide € | VdT-PACE | Not approved for RRMM | [21] |
| Cytotoxic antibiotics | Doxorubicin (A) | VdT-PACE | Not approved for RRMM | [21] |
| Antimetabolites | Cytarabine | Dexa-BEAM | Not approved for RRMM | [52] |
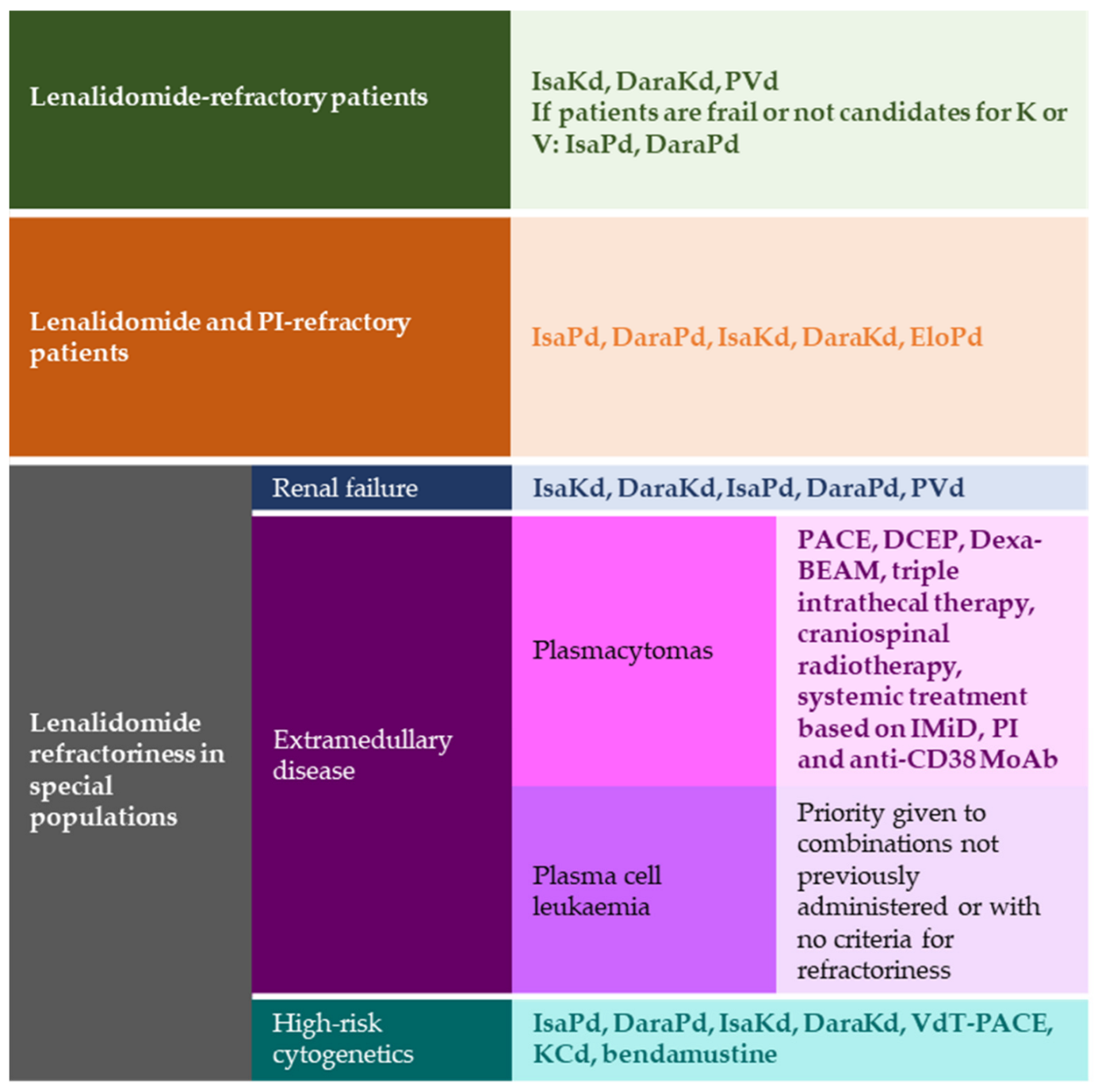
2. Lenalidomide-Refractory Patients
3. Multirefractory Patients
3.1. Double Refractory Patients
3.2. Triple Refractory Patients
3.2.1. New Therapies
4. Lenalidomide Relapse in Special Populations
4.1. Renal Failure
4.2. Extramedullary Disease
4.3. High-Risk Cytogenetics
5. Discussion and Expert Opinion
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerecke, C.; Fuhrmann, S.; Strifler, S.; Schmidt-Hieber, M.; Einsele, H.; Knop, S. The Diagnosis and Treatment of Multiple Myeloma. Dtsch. Arztebl. Int. 2016, 113, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Hemasphere 2021, 5, e528. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.J.; Americo, A.D. Treatment of relapsed/refractory multiple myeloma in the bortezomib and lenalidomide era: A systematic review and network meta-analysis. Ann. Hematol. 2021, 100, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Martino, E.A.; Conticello, C.; Mendicino, F.; Vigna, E.; Romano, A.; Palumbo, G.A.; Cerchione, C.; Martinelli, G.; Morabito, F.; et al. Treatment of Lenalidomide Exposed or Refractory Multiple Myeloma: Network Meta-Analysis of Lenalidomide-Sparing Regimens. Front. Oncol. 2021, 11, 643490. [Google Scholar] [CrossRef]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Harousseau, J.L.; Mohty, M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia 2019, 33, 2343–2357. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Bahlis, N.J.; Chng, W.J.; Masszi, T.; Viterbo, L.; Pour, L.; Ganly, P.; Palumbo, A.; Cavo, M.; Langer, C.; et al. Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patients. Blood 2017, 130, 2610–2618. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; Betts, K.A.; Chen, C.; Zichlin, M.L.; Brun, A.; Signorovitch, J.E.; Makenbaeva, D.; Mekan, S.; Sy, O.; et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer 2018, 124, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; San Miguel, J.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv52–iv61. [Google Scholar] [CrossRef]
- Short, K.D.; Rajkumar, S.V.; Larson, D.; Buadi, F.; Hayman, S.; Dispenzieri, A.; Gertz, M.; Kumar, S.; Mikhael, J.; Roy, V.; et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 2011, 25, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hajek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Touzeau, C.; Quignot, N.; Meng, J.; Jiang, H.; Khachatryan, A.; Singh, M.; Taieb, V.; Chauny, J.V.; Desamericq, G. Survival and treatment patterns of patients with relapsed or refractory multiple myeloma in France—A cohort study using the French National Healthcare database (SNDS). Ann. Hematol. 2021, 100, 1825–1836. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
- Davies, F.; Rifkin, R.; Costello, C.; Morgan, G.; Usmani, S.; Abonour, R.; Palumbo, A.; Romanus, D.; Hajek, R.; Terpos, E.; et al. Real-world comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in the US. Ann. Hematol. 2021, 100, 2325–2337. [Google Scholar] [CrossRef]
- Mateos, M.V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2020, 20, 509–518. [Google Scholar] [CrossRef]
- Lakshman, A.; Singh, P.P.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Dingli, D.; Hwa, Y.L.; Fonder, A.L.; et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am. J. Hematol. 2018, 93, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.J.; Stadtmauer, E.A.; Abonour, R.; Cohen, A.D.; Bensinger, W.I.; Gasparetto, C.; Kaufman, J.L.; Lentzsch, S.; Vogl, D.T.; Gomes, C.L.; et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 2015, 126, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Bringhen, S.; Mina, R.; Cafro, A.M.; Liberati, A.M.; Spada, S.; Belotti, A.; Gaidano, G.; Patriarca, F.; Troia, R.; Fanin, R.; et al. Once-weekly carfilzomib, pomalidomide, and low-dose dexamethasone for relapsed/refractory myeloma: A phase I/II study. Leukemia 2018, 32, 1803–1807. [Google Scholar] [CrossRef]
- Mateos, M.V.; Ocio, E.M.; Balari, A.S.; Oriol, A.; González Garcia, E.; Moreno, M.J.; Granell, M.; Escalante, F.; Gonzalez De La Calle, V.; Rosinol Dachs, L.; et al. Randomized Phase 2 Study of Weekly Carfilzomib 70 Mg/m2 and Dexamethasone Plus/Minus Cyclophosphamide in Relapsed and/or Refractory Multiple Myeloma (RRMM) Patients (GEM-KyCyDex) Presented at Myeloma/amyloidosis: Therapy, excluding transplantation. Am. Soc. Hematol. 2020, 136, 8–9. [Google Scholar]
- Richardson, P.G.; Kumar, S.K.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; et al. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; van de Donk, N.; Popat, R.; Zonder, J.; Minnema, M.C.; Larsen, J.; Nguyen, T.V.; Chen, M.S.; Bensmaine, A.; Cota, M.; et al. First clinical (phase 1b/2a) study of iberdomide (CC-220; IBER), a CELMoD, in combination with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2019, 37, 8006. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Spencer, A.; Attal, M.; Prince, H.M.; Harousseau, J.L.; Dmoszynska, A.; San Miguel, J.; Hellmann, A.; Facon, T.; Foa, R.; et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2007, 357, 2123–2132. [Google Scholar] [CrossRef]
- Weber, D.M.; Chen, C.; Niesvizky, R.; Wang, M.; Belch, A.; Stadtmauer, E.A.; Siegel, D.; Borrello, I.; Rajkumar, S.V.; Chanan-Khan, A.A.; et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 2007, 357, 2133–2142. [Google Scholar] [CrossRef]
- Paludo, J.; Mikhael, J.R.; LaPlant, B.R.; Halvorson, A.E.; Kumar, S.; Gertz, M.A.; Hayman, S.R.; Buadi, F.K.; Dispenzieri, A.; Lust, J.A.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed lenalidomide-refractory multiple myeloma. Blood 2017, 130, 1198–1204. [Google Scholar] [CrossRef]
- Moreau, P.; Weisel, K.C.; Song, K.W.; Gibson, C.J.; Saunders, O.; Sternas, L.A.; Hong, K.; Zaki, M.H.; Dimopoulos, M.A. Relationship of response and survival in patients with relapsed and refractory multiple myeloma treated with pomalidomide plus low-dose dexamethasone in the MM-003 trial randomized phase III trial (NIMBUS). Leuk. Lymphoma 2016, 57, 2839–2847. [Google Scholar] [CrossRef]
- Weisel, K.; Dimopoulos, M.; Song, K.W.; Moreau, P.; Palumbo, A.; Belch, A.; Schey, S.; Sonneveld, P.; Sternas, L.; Yu, X.; et al. Pomalidomide and Low-Dose Dexamethasone Improves Health-Related Quality of Life and Prolongs Time to Worsening in Relapsed/Refractory Patients With Multiple Myeloma Enrolled in the MM-003 Randomized Phase III Trial. Clin. Lymphoma Myeloma Leuk. 2015, 15, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Schiller, G.J.; Song, K.W.; Agajanian, R.; Stockerl-Goldstein, K.; Kaya, H.; Sebag, M.; Samaras, C.; Malek, E.; Talamo, G.; et al. Pomalidomide plus low-dose dexamethasone in relapsed refractory multiple myeloma after lenalidomide treatment failure. Br. J. Haematol. 2020, 188, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Garderet, L.; Kuhnowski, F.; Berge, B.; Roussel, M.; Escoffre-Barbe, M.; Lafon, I.; Facon, T.; Leleu, X.; Karlin, L.; Perrot, A.; et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed multiple myeloma. Blood 2018, 132, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Sebag, M.; Bahlis, N.; Venner, C.P.; McCurdy, A.; Kouroukis, C.T.; Shustik, J.; Kotb, R.; White, D.; Stakiw, J.; Laferriere, N.B.; et al. A Randomized Phase II, Open Label, Study of Daratumumab, Weekly Low-Dose Oral Dexamethasone and Cyclophosphamide with or without Pomalidomide in Patients with Relapsed and Refractory Multiple Myeloma. Blood 2019, 134, 3121. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Yong, K.; Mikhael, J.; Risse, M.L.; Asset, G.; Martin, T. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA Phase III study design. Future Oncol. 2020, 16, 4347–4358. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Spicka, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Palumbo, A.; San-Miguel, J.; Shpilberg, O.; Anderson, K.; Grosicki, S.; Spicka, I.; et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br. J. Haematol. 2017, 178, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.; Chari, A.; Abdallah, A.O.; Callander, N.S.; Sborov, D.; Suvannasankha, A.; et al. Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs). J. Clin. Oncol. 2020, 38, 8536. [Google Scholar]
- Verkleij, C.P.M.; Frerichs, K.A.; Broekmans, M.; Absalah, S.; Maas-Bosman, P.W.C.; Kruyswijk, S.; Nijhof, I.S.; Mutis, T.; Zweegman, S.; van de Donk, N. T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget 2020, 11, 4076–4081. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; et al. Updated Results from CARTITUDE-1: Phase 1b/2Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy, in Patients With Relapsed/Refractory Multiple Myeloma. In Proceedings of the 2021 American Society of Hematology (ASH) Annual Meeting & Exposition, Atlanta, Georgia, 11–13 December 2021. Abstract 549. [Google Scholar]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Rasche, L.; Strifler, S.; Duell, J.; Rosenwald, A.; Buck, A.; Maeder, U.; Einsele, H.; Knop, S. The lymphoma-like polychemotherapy regimen “Dexa-BEAM” in advanced and extramedullary multiple myeloma. Ann. Hematol. 2014, 93, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Isola, I.; Granell, M.; Martí, J.M.; Gironella, M.; García-Guiñón, A.; López-Pardo, J.; Muntañola, A.; Abella, E.; Motlló, C.; Escoda, L.; et al. PACE as salvage therapy for relapsed or refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2015, 15, e300–e301. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.J.; Jung, C.W.; Jang, J.H.; Kim, S.J.; Kim, W.S.; Kim, K. DCEP for relapsed or refractory multiple myeloma after therapy with novel agents. Ann. Hematol. 2014, 93, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Ludwig, H.; Kasparu, H.; Leitgeb, C.; Rauch, E.; Linkesch, W.; Zojer, N.; Greil, R.; Seebacher, A.; Pour, L.; Weißmann, A.; et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood 2014, 123, 985–991. [Google Scholar] [CrossRef]
- Mark, T.M.; Forsberg, P.A.; Rossi, A.C.; Pearse, R.N.; Pekle, K.A.; Perry, A.; Boyer, A.; Tegnestam, L.; Jayabalan, D.; Coleman, M.; et al. Phase 2 study of clarithromycin, pomalidomide, and dexamethasone in relapsed or refractory multiple myeloma. Blood Adv. 2019, 3, 603–611. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Corso, A.; Mugge, L.O.; Shen, Z.X.; Desjardins, P.; Stoppa, A.M.; Decaux, O.; de Revel, T.; Granell, M.; Marit, G.; et al. Benefit of continuous treatment for responders with newly diagnosed multiple myeloma in the randomized FIRST trial. Leukemia 2017, 31, 2435–2442. [Google Scholar] [CrossRef][Green Version]
- Moreau, P.; Zamagni, E.; Mateos, M.V. Treatment of patients with multiple myeloma progressing on frontline-therapy with lenalidomide. Blood Cancer J. 2019, 9, 38. [Google Scholar] [CrossRef]
- Mohyuddin, G.R.; Hampton, J.; Aziz, M.; Khuder, S.; Malik, S.; McClune, B.; Abdallah, A.O. A Systematic Review and Network Meta-analysis of Randomized Data on Efficacy of Novel Therapy Combinations in Patients with Lenalidomide-refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 489–496. [Google Scholar] [CrossRef]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Spicka, I.; Casca, F.; Mace, S.; et al. Updated progression-free survival (PFS) and depth of response in IKEMA, a randomized phase III trial of isatuximab, carfilzomib and dexamethasone (Isa-Kd) vs. Kd in relapsed multiple myeloma (MM). Hematol. Transfus. Cell Ther. 2022, 44, S249. [Google Scholar]
- Capra, M.; Martin, T.; Moreau, P.; Baker, R.; Pour, L.; Min, C.K.; Leleu, X.; Mohty, M.; Segura, M.R.; Turgut, M.; et al. Isatuximab plus carfilzomib and dexamethasone versus carfilzomib and dexamethasone in relapsed multiple myeloma patients with renal impairment: IKEMA subgroup analysis. Haematologica 2022, 107, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Augustson, B.; Castro, N.; Pika, T.; Delimpasi, S.; De la Rubia, J.; Maiolino, A.; Reiman, T.; Martinez-Lopez, J.; et al. Isatuximab plus carfilzomib and dexamethasone in patients with relapsed multiple myeloma based on prior lines of treatment and refractory status: IKEMA subgroup analysis. Am. J. Hematol. 2023, 98, E15–E19. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Gavriatopoulou, M.; Oriol, A.; Rabin, N.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol. 2022, 23, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Weisel, K.; Zahlten-Kumeli, A.; Medhekar, R.; Ding, B.; Leleu, X. Health-related quality of life outcomes from the CANDOR study in patients with relapsed or refractory multiple myeloma. Leuk. Lymphoma 2021, 62, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Nooka, A.; Samoylova, O.; Venner, C.P.; Kim, K.; Facon, T.; Spencer, A.; Usmani, S.Z.; Grosicki, S.; Suzuki, K.; et al. Carfilzomib, dexamethasone and daratumumab in relapsed or refractory multiple myeloma: Results of the phase III study CANDOR by prior lines of therapy. Br. J. Haematol. 2021, 194, 784–788. [Google Scholar] [CrossRef]
- de la Rubia, J.; Mateos, M.V.; Bladé, J.; Lahuerta, J.J.; San Miguel, J.; Aguado, B.; Alegre, A.; Blanchart, M.J.; Cedena, T.; Cejalvo, M.J.; et al. Guía de Mieloma Múltiple. Con el Aval Científico de: Grupo Español de Mieloma. 2021. Available online: https://www.sehh.es/images/stories/recursos/2021/06/15/Guia-Mieloma-Multiple-21-04-2021.pdf (accessed on 21 December 2022).
- Facon, T.; Moreau, P.; Martin, T.G.; Spicka, I.; Oriol, A.; Koh, Y.; Lim, A.; Mikala, G.; Rosinol, L.; Yagci, M.; et al. Isatuximab plus carfilzomib and dexamethasone versus carfilzomib and dexamethasone in elderly patients with relapsed multiple myeloma: IKEMA subgroup analysis. Hematol. Oncol. 2022, 40, 1020–1029. [Google Scholar] [CrossRef]
- Schjesvold, F.H.; Richardson, P.G.; Facon, T.; Alegre, A.; Spencer, A.; Jurczyszyn, A.; Sunami, K.; Frenzel, L.; Min, C.K.; Guillonneau, S.; et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis. Haematologica 2021, 106, 1182–1187. [Google Scholar] [CrossRef]
- Bringhen, S.; Pour, L.; Vorobyev, V.; Vural, F.; Warzocha, K.; Benboubker, L.; Koh, Y.; Maisnar, V.; Karlin, L.; Pavic, M.; et al. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma according to prior lines of treatment and refractory status: ICARIA-MM subgroup analysis. Leuk. Res. 2021, 104, 106576. [Google Scholar] [CrossRef]
- Schjesvold, F.; Richardson, P.G.; Facon, T.; Alegre, A.; Spencer, A.; Jurczyszyn, A.; Sunami, K.; Frenzel, L.; Min, C.K.; Guillonneau, S.; et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis. Haematologica 2022, 107, 774–775. [Google Scholar] [CrossRef]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Joseph, N.S.; Kaufman, J.L.; Heffner, L.T.; Gupta, V.A.; Gleason, C.; Boise, L.H.; Lonial, S. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: Utility of re-treatment with daratumumab among refractory patients. Cancer 2019, 125, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.; Al Hadidi, S.; Usmani, S.; Yee, A.J.; Raje, N.; Davies, F.E. How to Treat High-Risk Myeloma at Diagnosis and Relapse. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 291–309. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Mateos, M.; Weisel, K.C.; De Stefano, V.; Perrot, A.; van de Donk, N.; Goldschmidt, H.; Kaiser, M.; Vij, R.; Gay, F.; Broijl, A.; et al. LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed/refractory multiple myeloma (RRMM) receiving ≥3 prior lines of therapy. J. Clin. Oncol. 2021, 39, 8041. [Google Scholar] [CrossRef]
- Mateos, M.V.; Weisel, K.; De Stefano, V.; Goldschmidt, H.; Delforge, M.; Mohty, M.; Cavo, M.; Vij, R.; Lindsey-Hill, J.; Dytfeld, D.; et al. LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 2022, 36, 1371–1376. [Google Scholar] [CrossRef]
- van Bueren, J.; Jakobs, D.; Kaldenhoven, N.; Roza, M.; Hiddingh, S.; Meesters, J.; Voorhorst, M.; Gresnigt, E.; Wiegman, L.; Ortiz Buijsse, A.; et al. Direct in vitro Comparison of Daratumumab with Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood 2014, 124, 3474. [Google Scholar] [CrossRef]
- Mikhael, J.; Belhadj-Merzoug, K.; Hulin, C.; Vincent, L.; Moreau, P.; Gasparetto, C.; Pour, L.; Spicka, I.; Vij, R.; Zonder, J.; et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J. 2021, 11, 89. [Google Scholar] [CrossRef]
- Usmani, S.; Karanes, C.; Bensinger, W.I.; D’Souza, A.; Raje, N.; Tuchman, S.; Sborov, D.; Kanagavel, D.; Saleem, R.; Dubin, F.; et al. Isatuximab short-duration fixed-volume infusion combination therapy for relapsed/refractory multiple myeloma: Final results of a phase 1b feasibility/safety study. Clin. Lymphoma Myeloma Leuk. 2019, 19, e283. [Google Scholar] [CrossRef]
- Becnel, M.R.; Horowitz, S.B.; Thomas, S.K.; Iyer, S.; Patel, K.K.; Manasanch, E.E.; Weber, D.M.; Kaufman, G.P.; Lee, H.C.; Orlowski, R.Z. Descriptive Analysis of Isatuximab Use Following Prior Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 20–21. [Google Scholar] [CrossRef]
- Djebbari, F.; Poynton, M.; Sangha, G.; Anderson, L.; Maddams, R.; Eyre, T.A.; Vallance, G.; Basu, S.; Ramasamy, K. Outcomes of anti-CD38 isatuximab plus pomalidomide and dexamethasone in five relapsed myeloma patients with prior exposure to anti-C38 daratumumab: Case series. Hematology 2022, 27, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Becnel, M.R.; Shah, U.A.; Dong, H.; Gundarlapalli, S.; Peterson, T.; Orozco, J.S.; Horowitz, S.; Chhabra, S.; Dhakal, B.; et al. Clinical efficacy of sequencing CD38 targeting monoclonal antibodies in relapsed refractory multiple myeloma: A multi-institutional experience. Am. J. Hematol. 2022, 97, E276–E280. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Perrot, A.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; Huang, J.S.; et al. Isatuximab Plus Pomalidomide/Low-Dose Dexamethasone Versus Pomalidomide/Low-Dose Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma (ICARIA-MM): Characterization of Subsequent Antimyeloma Therapies. In Proceedings of the 2022 American Society of Hematology (ASH) Annual Meeting & Exposition, New Orleans, Luisiana, 10–13 December 2022. Abstract 247. [Google Scholar]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Kronke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef]
- Roex, G.; Timmers, M.; Wouters, K.; Campillo-Davo, D.; Flumens, D.; Schroyens, W.; Chu, Y.; Berneman, Z.N.; Lion, E.; Luo, F.; et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J. Hematol. Oncol. 2020, 13, 164. [Google Scholar] [CrossRef]
- Martino, M.; Canale, F.A.; Alati, C.; Vincelli, I.D.; Moscato, T.; Porto, G.; Loteta, B.; Naso, V.; Mazza, M.; Nicolini, F.; et al. CART-Cell Therapy: Recent Advances and New Evidence in Multiple Myeloma. Cancers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Hansen, D.K.; Sidana, S.; Peres, L.; Shune, L.; Sborov, D.W.; Hashmi, H.; Kocoglu, M.H.; Atrash, S.; Simmons, G.; Kalariya, N.; et al. Idecabtagene vicleucel (Ide-cel) chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory multiple myeloma (RRMM): Real-world experience. J. Clin. Oncol. 2022, 40, 8042. [Google Scholar] [CrossRef]
- Sgherza, N.; Curci, P.; Rizzi, R.; Musto, P. Novel Approaches Outside the Setting of Immunotherapy for the Treatment of Multiple Myeloma: The Case of Melflufen, Venetoclax, and Selinexor. Front. Oncol. 2021, 11, 716751. [Google Scholar] [CrossRef]
- Bobin, A.; Gruchet, C.; Guidez, S.; Gardeney, H.; Nsiala Makunza, L.; Vonfeld, M.; Lévy, A.; Cailly, L.; Sabirou, F.; Systchenko, T.; et al. Novel Non-Immunologic Agents for Relapsed and Refractory Multiple Myeloma: A Review Article. Cancers 2021, 13, 5210. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Trudel, S.; Quach, H.; Popat, R.; Lonial, S.; Orlowski, R.Z.; Kim, K.; Mateos, M.-V.; Pawlyn, C.; Ramasamy, K.; et al. Mezigdomide (CC-92480), a Potent, Novel Cereblon E3 Ligase Modulator (CELMoD), Combined with Dexamethasone (DEX) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Results from the Dose-Expansion Phase of the CC-92480-MM-001 Trial. In Proceedings of the 2022 American Society of Hematology (ASH) Annual Meeting & Exposition, New Orleans, Luisiana, 10–13 December 2022. Abstract 568. [Google Scholar]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Schjesvold, F.; Weisel, K.; Moreau, P.; Anderson, L.D., Jr.; White, D.; Rodriguez-Otero, P.; Sonneveld, P.; Engelhardt, M.; Jenner, M.; et al. Pomalidomide, bortezomib, and dexamethasone at first relapse in lenalidomide-pretreated myeloma: A subanalysis of OPTIMISMM by clinical characteristics. Eur. J. Haematol. 2022, 108, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bozic, B.; Rutner, J.; Zheng, C.; Ruckser, R.; Selimi, F.; Racz, K.; Köcher, M.; Tatzreiter, G.; Sebesta, C. Advances in the Treatment of Relapsed and Refractory Multiple Myeloma in Patients with Renal Insufficiency: Novel Agents, Immunotherapies and Beyond. Cancers 2021, 13, 5036. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Leleu, X.; Moreau, P.; Richardson, P.G.; Liberati, A.M.; Harrison, S.J.; Miles Prince, H.; Ocio, E.M.; Assadourian, S.; Campana, F.; et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia 2021, 35, 562–572. [Google Scholar] [CrossRef]
- Mizuno, S.; Kitayama, C.; Yamaguchi, K.; Sanada, S.; Sato, T. Successful management of hemodialysis-dependent refractory myeloma with modified daratumumab, bortezomib and dexamethasone regimen. Int. J. Hematol. 2020, 112, 860–863. [Google Scholar] [CrossRef]
- Richter, J.; Biran, N.; Duma, N.; Vesole, D.H.; Siegel, D. Safety and tolerability of pomalidomide-based regimens (pomalidomide-carfilzomib-dexamethasone with or without cyclophosphamide) in relapsed/refractory multiple myeloma and severe renal dysfunction: A case series. Hematol. Oncol. 2017, 35, 246–251. [Google Scholar] [CrossRef]
- Takakuwa, T.; Ohta, K.; Sogabe, N.; Nishimoto, M.; Kuno, M.; Makuuchi, Y.; Okamura, H.; Nakashima, Y.; Koh, H.; Nakamae, H.; et al. Isatuximab plus Pomalidomide and Dexamethasone in a Patient with Dialysis-Dependent Multiple Myeloma. Chemotherapy 2021, 66, 192–195. [Google Scholar] [CrossRef]
- Rosiñol, L.; Beksac, M.; Zamagni, E.; Van de Donk, N.; Anderson, K.C.; Badros, A.; Caers, J.; Cavo, M.; Dimopoulos, M.A.; Dispenzieri, A.; et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: Definition, disease assessment and treatment considerations. Br. J. Haematol. 2021, 194, 496–507. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Hájek, R.; Jelinek, T.; Moreau, P.; Martin, T.; Pour, L.; Mikala, G.; Symeonidis, A.; Bringhen, S.; Rawlings, A.; Risse, M.; et al. Isatuximab plus Carfilzomib and Dexamethasone in patients with relapsed Multiple Myeloma and soft-tissue Plasmacytomas: IKEMA subgroup analysis. Hematol. Transfus. Cell Ther. 2021, 43, S197–S198. [Google Scholar] [CrossRef]
- Beksac, M.; Richardson, P.; Unal, A.; Corradini, P.; Delimpasi, S.; Gulbas, Z.; Kerridge, I.A.; Mikala, G.; Neylon, A.; Symeonidis, A.; et al. Isatuximab Plus Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma and Soft-Tissue Plasmacytomas: Icaria-MM Subgroup Analysis. In Proceedings of the 62nd ASH Annual Meeting and Exposition, Washington, DC, USA, 5–8 December 2020. [Google Scholar]
- Ravi, P.; Kumar, S.K.; Roeker, L.; Gonsalves, W.; Buadi, F.; Lacy, M.Q.; Go, R.S.; Dispenzieri, A.; Kapoor, P.; Lust, J.A.; et al. Revised diagnostic criteria for plasma cell leukemia: Results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Tuazon, S.A.; Holmberg, L.A.; Nadeem, O.; Richardson, P.G. A clinical perspective on plasma cell leukemia; current status and future directions. Blood Cancer J. 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Perrot, A.; Alegre, A.; Simpson, D.; Wang, M.C.; Spencer, A.; Delimpasi, S.; Hulin, C.; Sunami, K.; Facon, T.; et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br. J. Haematol. 2021, 194, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Spicka, I.; Moreau, P.; Martin, T.; Facon, T.; Martinez, G.; Oriol, A.; Koh, Y.; Lim, A.; Mikala, G.; Rosiñol, L.; et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high-risk cytogenetics: IKEMA subgroup analysis. Eur. J. Haematol. 2022, 39, 8042. [Google Scholar] [CrossRef]
- Martin, T.; Richardson, P.G.; Facon, T.; Moreau, P.; Perrot, A.; Spicka, I.; Bisht, K.; Inchauspe, M.; Casca, F.; Mace, S.; et al. Primary outcomes by 1q21+ status for isatuximab-treated patients with relapsed/refractory multiple myeloma: Subgroup analyses from ICARIA-MM and IKEMA. Haematologica 2022, 107, 2485–2491. [Google Scholar] [CrossRef]
- Knop, S.; Engelhardt, M.; Liebisch, P.; Meisner, C.; Holler, E.; Metzner, B.; Peest, D.; Kaufmann, M.; Bunjes, D.; Straka, C.; et al. Allogeneic transplantation in multiple myeloma: Long-term follow-up and cytogenetic subgroup analysis. Leukemia 2019, 33, 2710–2719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Arriba de la Fuente, F.; Montes Gaisán, C.; de la Rubia Comos, J. How to Manage Patients with Lenalidomide-Refractory Multiple Myeloma. Cancers 2023, 15, 155. https://doi.org/10.3390/cancers15010155
de Arriba de la Fuente F, Montes Gaisán C, de la Rubia Comos J. How to Manage Patients with Lenalidomide-Refractory Multiple Myeloma. Cancers. 2023; 15(1):155. https://doi.org/10.3390/cancers15010155
Chicago/Turabian Stylede Arriba de la Fuente, Felipe, Carmen Montes Gaisán, and Javier de la Rubia Comos. 2023. "How to Manage Patients with Lenalidomide-Refractory Multiple Myeloma" Cancers 15, no. 1: 155. https://doi.org/10.3390/cancers15010155
APA Stylede Arriba de la Fuente, F., Montes Gaisán, C., & de la Rubia Comos, J. (2023). How to Manage Patients with Lenalidomide-Refractory Multiple Myeloma. Cancers, 15(1), 155. https://doi.org/10.3390/cancers15010155






