Clinicopathological Features and Survival of Patients with Hepatocellular Carcinoma in Ethiopia: A Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
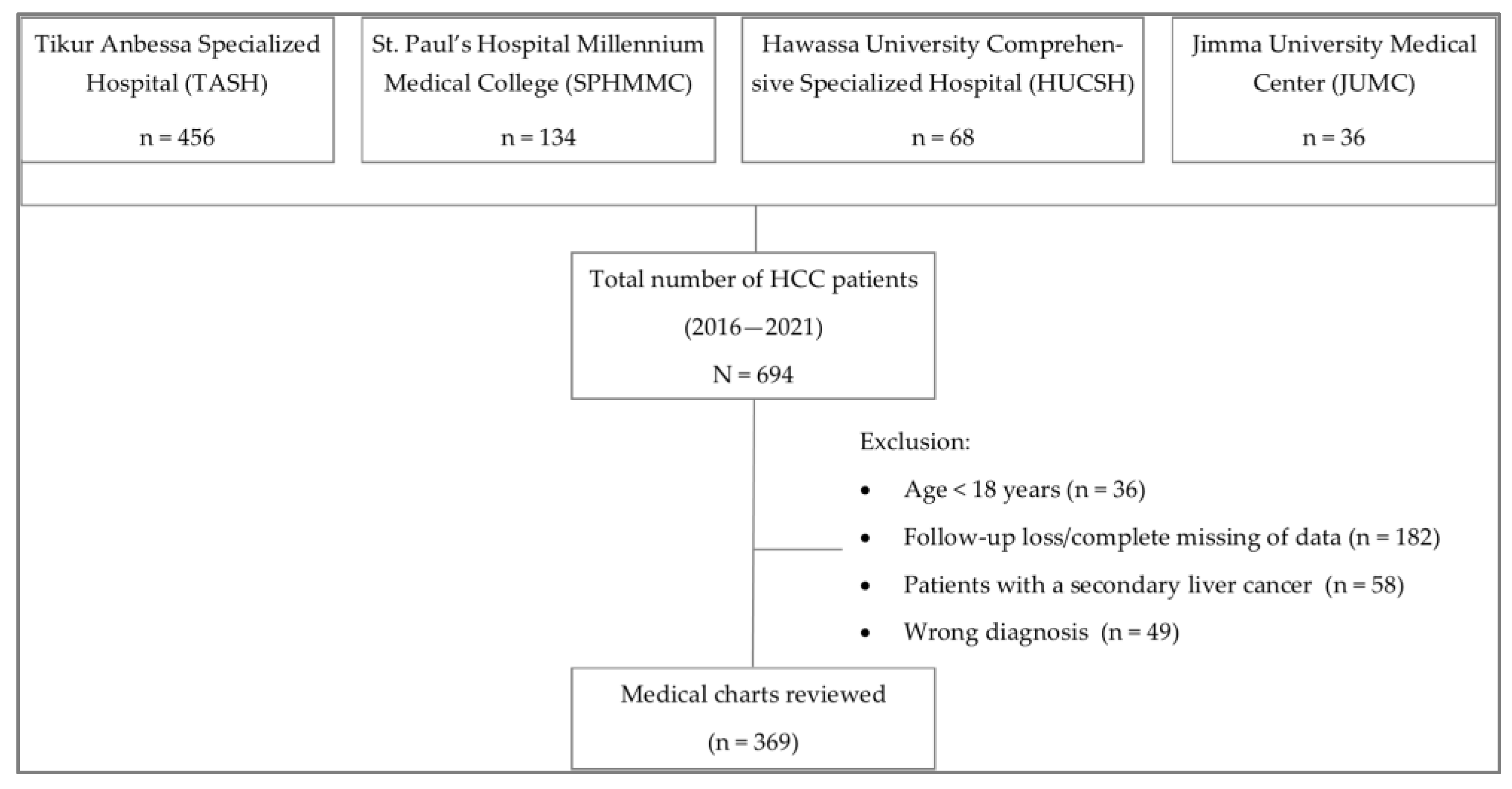
2.1. Study Design and Population
2.2. Methods
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics
3.2. Clinicopathological Features
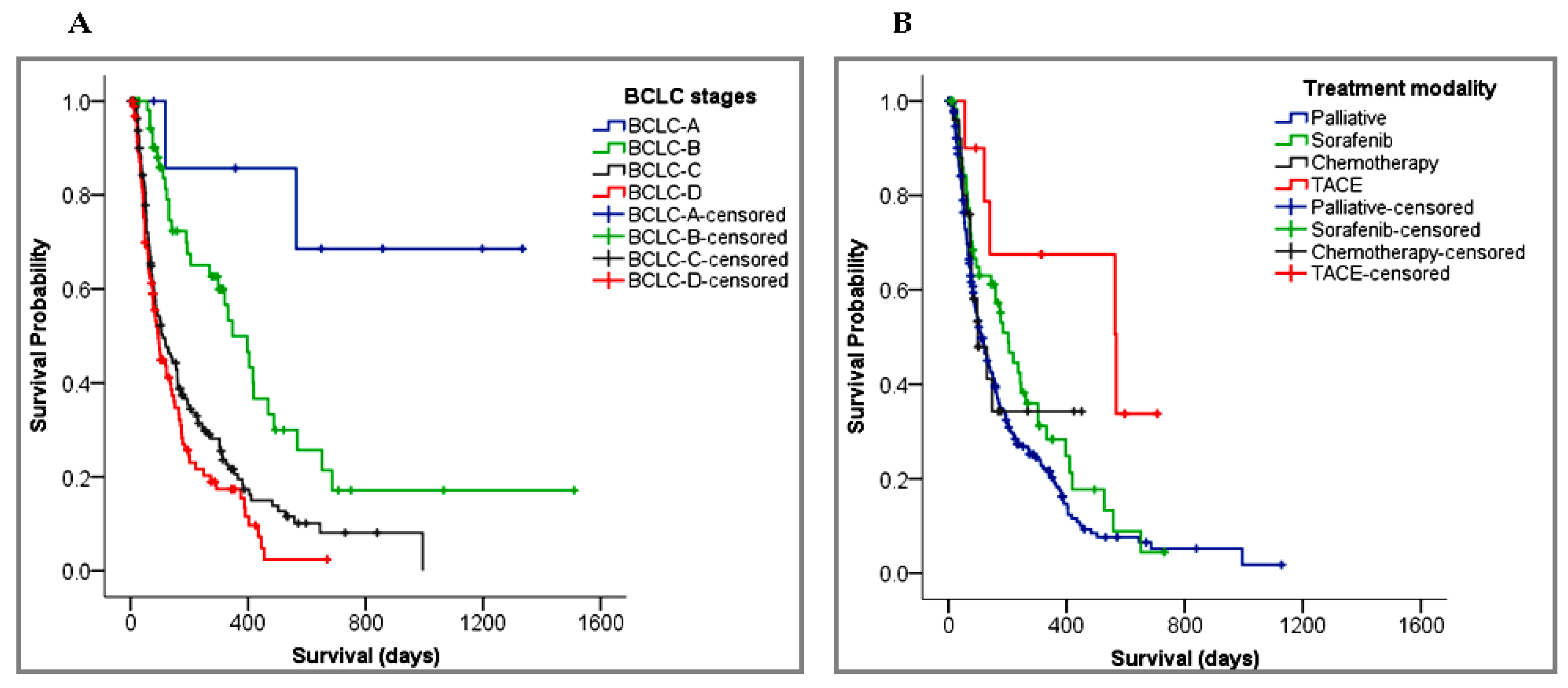
3.3. Treatment Modality
3.4. Survival Outcome
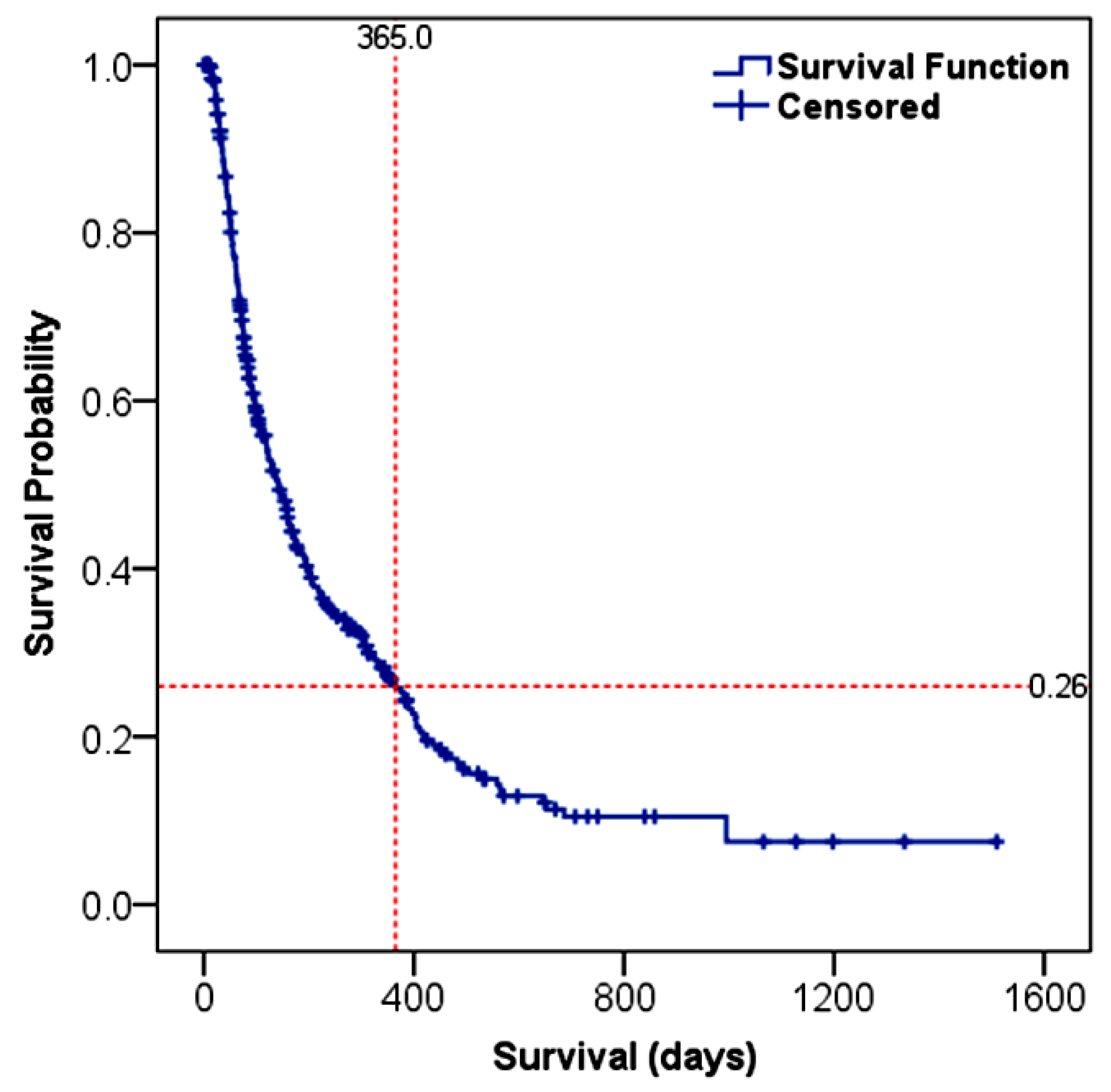
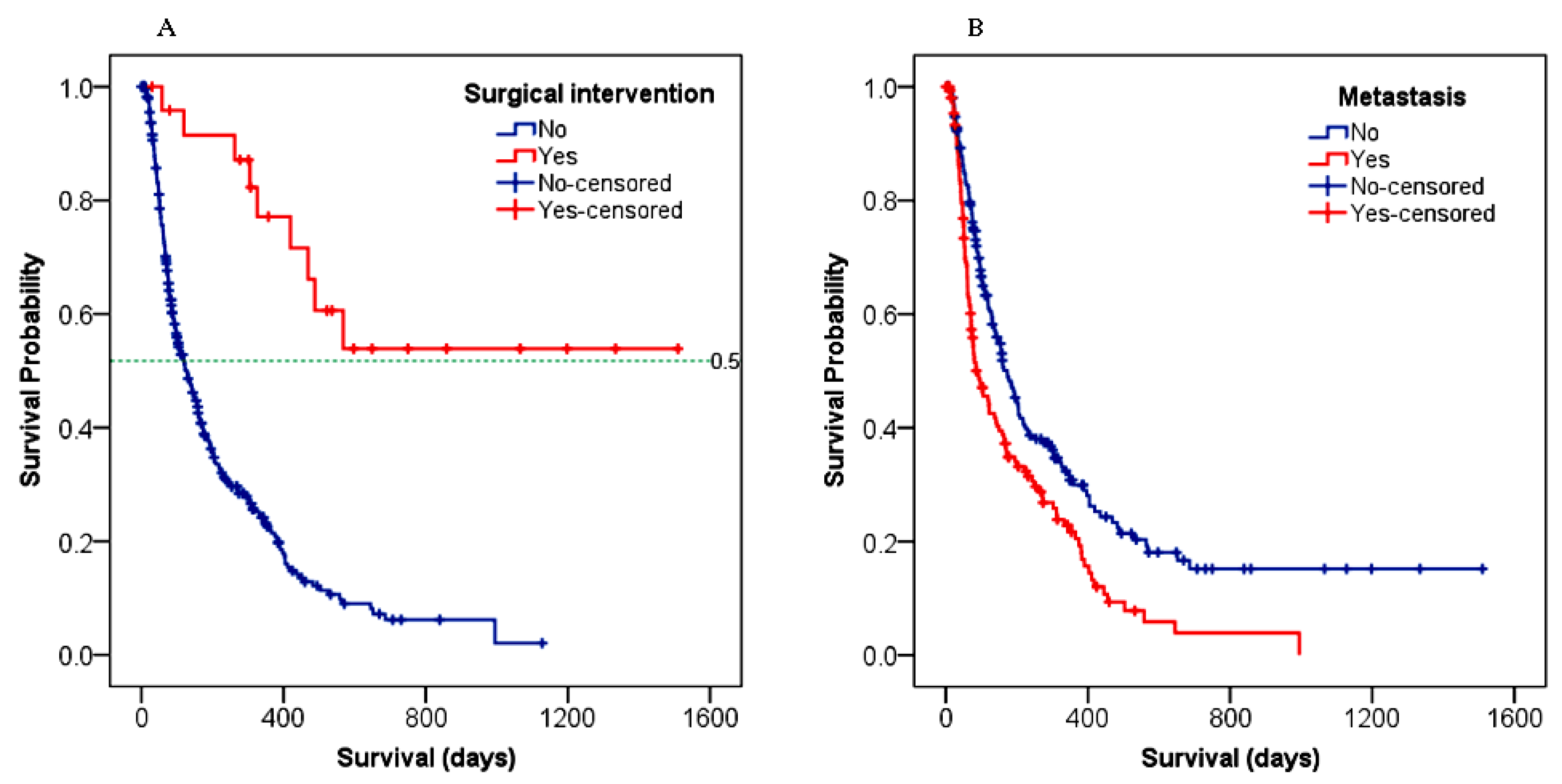
3.4.1. Overall Survival (OS)
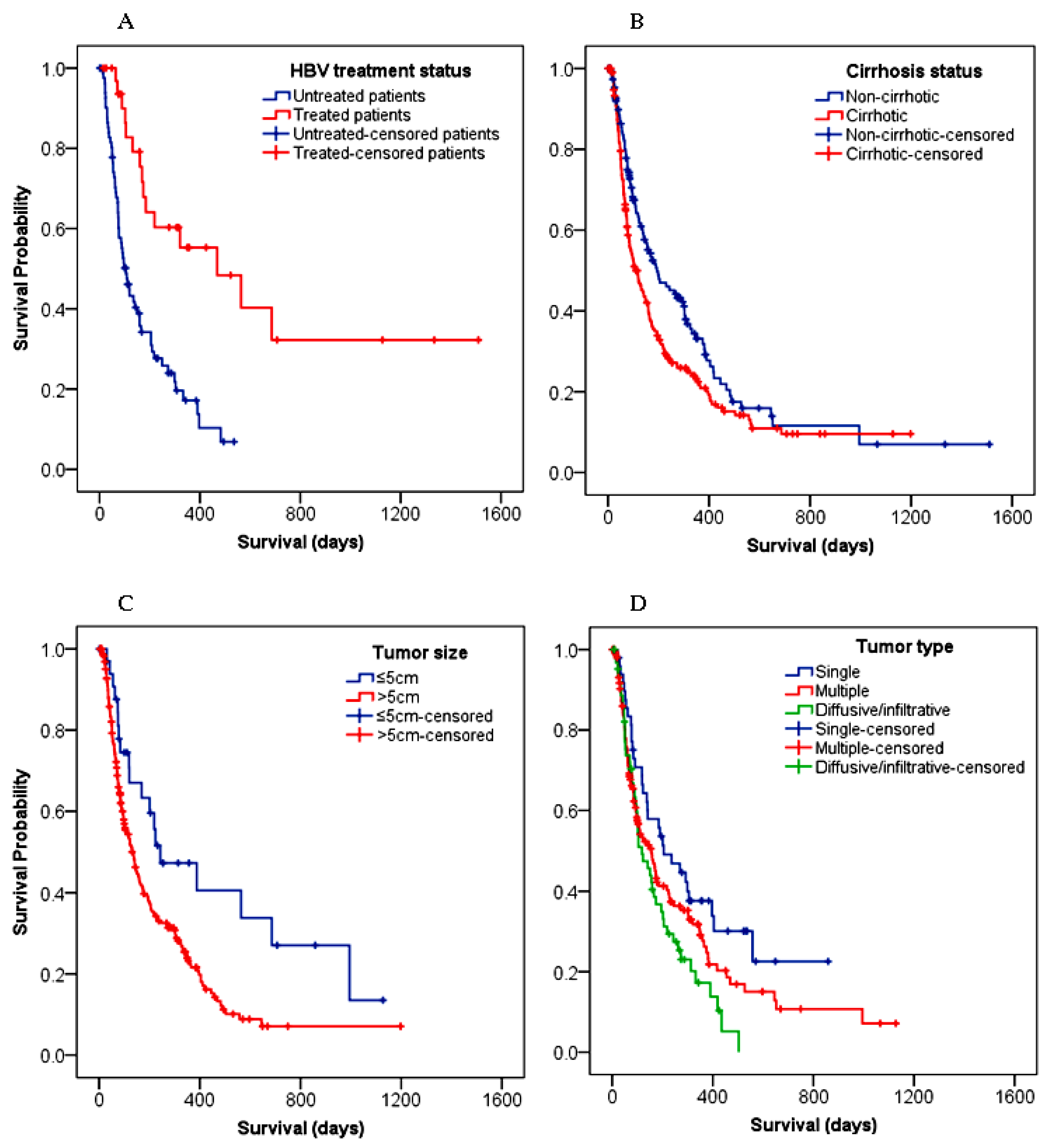
3.4.2. Survival Predictors
4. Discussion
5. Conclusions
6. Current Challenges and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
Abbreviations
| AFP | Alpha-fetoprotein |
| BCLC | Barcelona Clinic Liver Cancer |
| ECOG | Eastern Cooperative Oncology Group |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| OS | Overall Survival |
| TNM | Tumor–Node–Metastasis |
References
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed]
- Kedar Mukthinuthalapati, V.V.P.; Sewram, V.; Ndlovu, N.; Kimani, S.; Abdelaziz, A.O.; Chiao, E.Y.; Abou-Alfa, G.K. Hepatocellular Carcinoma in Sub-Saharan Africa. JCO Glob. Oncol. 2021, 7, 756–766. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.; Kramvis, A. Epidemiology and Aetiology of Hepatocellular Carcinoma in Sub-Saharan Africa. Hepatoma Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Ministry of Health-Ethiopia National Strategic Plan for Prevention and Control of Viral Hepatitis in Ethiopia, 2021–2025. Available online: https://www.globalhep.org/sites/default/files/content/resource/files/2022-05/Final Hep NSP 2021-2025 Aug 27.pdf (accessed on 9 December 2022).
- Yang, J.D.; Mohamed, E.A.; Aziz, A.O.A.; Shousha, H.I.; Hashem, M.B.; Nabeel, M.M.; Abdelmaksoud, A.H.; Elbaz, T.M.; Afihene, M.Y.; Duduyemi, B.M.; et al. Characteristics, Management, and Outcomes of Patients with Hepatocellular Carcinoma in Africa: A Multicountry Observational Study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol. 2017, 2, 103–111. [Google Scholar] [CrossRef] [Green Version]
- GNI per Capita, PPP (Current International $)-Ethiopia|Data. Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD?end=2020&locations=ET&start=2000 (accessed on 9 June 2022).
- Greten, T.; Papendorf, F.; Kubicka, S.; Manns, M.P. Survival Rate in Patients with Hepatocellular Carcinoma: A Retrospective Analysis of 389 Patients. Br. J. Cancer 2005, 92, 1862–1868. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Benson, A.B.; Abbott, D.E.; Ahmed, A.; Anaya, D.A.; Anders, R.; Bachini, M.; Binder, D.; Burgoyne, A.; Cloyd, J.; Covey, A.M.; et al. NCCN Guidelines for Hepatobiliary Cancers Version 3.2022. Available online: https://www.nccn.org/home/member- (accessed on 9 December 2022).
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver Cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L. Changes in the Epidemiology of Hepatocellular Carcinoma in Asia. Cancers 2022, 14, 4473. [Google Scholar] [CrossRef]
- Jasirwan, C.O.M.; Fahira, A.; Siregar, L.; Loho, I. The Alpha-Fetoprotein Serum Is Still Reliable as a Biomarker for the Surveillance of Hepatocellular Carcinoma in Indonesia. BMC Gastroenterol. 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, B.T.; Feyissa, T.M.; Workneh, A.B.; Gudina, E.K.; Yizengaw, M.A. Chronic Liver Disease in Ethiopia with a Particular Focus on the Etiological Spectrums: A Systematic Review and Meta-Analysis of Observational Studies. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8740157. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, M.; Lei, Q.; Luo, Z.; Xue, Y.; Yao, D.; Lai, Y.; Ung, C.O.L.; Hu, H. Economic Burden and Quality of Life of Hepatocellular Carcinoma in Greater China: A Systematic Review. Front. Public Health 2022, 10, 801981. [Google Scholar] [CrossRef]
- Jinjuvadia, R.; Salami, A.; Lenhart, A.; Jinjuvadia, K.; Liangpunsakul, S.; Salgia, R. Hepatocellular Carcinoma: A Decade of Hospitalizations and Financial Burden in the United States. Am. J. Med. Sci. 2017, 354, 362. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Zhang, P.; Zhang, J.; Li, X.; Gan, D.; Cao, X.; Han, M.; Du, H.; Ye, Y. The Threshold of Alpha-Fetoprotein (AFP) for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0228857. [Google Scholar] [CrossRef]
- Hu, J.; Wang, N.; Yang, Y.; Ma, L.; Han, R.; Zhang, W.; Yan, C.; Zheng, Y.; Wang, X. Diagnostic Value of Alpha-Fetoprotein Combined with Neutrophil-to-Lymphocyte Ratio for Hepatocellular Carcinoma. BMC Gastroenterol. 2018, 18, 186. [Google Scholar] [CrossRef]
- Nimitrungtawee, N.; Inmutto, N.; Chattipakorn, S.C.; Chattipakorn, N. Extracellular Vesicles as a New Hope for Diagnosis and Therapeutic Intervention for Hepatocellular Carcinoma. Cancer Med. 2021, 10, 8253–8271. [Google Scholar] [CrossRef]
- Li, Q.; Xing, H.; Liu, D.; Li, H. Negative Impact of Low Body Mass Index on Liver Cirrhosis Patients with Hepatocellular Carcinoma. World J. Surg. Oncol. 2015, 13, 294. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim Gomaa, A.; Saad Hashim, M.; Waked, I. Comparing Staging Systems for Predicting Prognosis and Survival in Patients with Hepatocellular Carcinoma in Egypt. PLoS ONE 2014, 9, e90929. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Li, S. Clinical Characteristics and Prognosis of 2887 Patients with Hepatocellular Carcinoma A Single Center 14 Years Experience from China. Medicine 2019, 98, e14070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, K.; Wang, F.; Zhangyuan, G.; Yu, W.; Liu, Y.; Zhang, H.; Zhang, P.; Sun, B. Differences in the Prognostic Value of Tumor Size on Hepatocellular Cancer-Specific Survival Stratified by Gender in a SEER Population-Based Study. United Eur. Gastroenterol. J. 2019, 7, 933–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Wu, J.; Wang, B.; Zhu, X.; Shi, X.; Ding, Y. Importance of Tumor Size at Diagnosis as a Prognostic Factor for Hepatocellular Carcinoma Survival: A Population-Based Study. Cancer Manag. Res. 2018, 10, 4401–4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Peng, X.L.; Lihua, W.; Fengna, Y.; Huiwen, Y.; Zhou, Y.D.; Yang, Z. Antiviral Therapy Reduces Mortality in Hepatocellular Carcinoma Patients with Low-Level Hepatitis B Viremia. J. Hepatocell. Carcinoma 2021, 27, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.J.; Rosinski, A.A.; Huynh, C.T.; Raman, S.S.; Lu, D.S.K. Long-Term Survival after Surveillance and Treatment in Patients with Chronic Viral Hepatitis and Hepatocellular Carcinoma. Hepatol. Commun. 2017, 1, 595–608. [Google Scholar] [CrossRef]
- Roberts, L.R. Untreated Chronic Hepatitis B Is Associated With a Higher Risk of Extrahepatic Malignancies. J. Clin. Oncol. 2022, 40, 3357–3360. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Xu, H.; Xiao, P.; Gao, Y. Hepatocellular Carcinoma in the Noncirrhotic Liver: A Literature Review. Eur. J. Gastroenterol. Hepatol. 2019, 31, 743–748. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, Y.H.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lin, H.C.; Lee, R.C.; Chiou, Y.Y.; Lee, F.Y.; Huo, T.I. Performance Status in Patients with Hepatocellular Carcinoma: Determinants, Prognostic Impact, and Ability to Improve the Barcelona Clinic Liver Cancer System. Hepatology 2013, 57, 112–119. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hsieh, P.-M.; Lin, H.-Y.; Hung, C.-M.; Lo, G.-H.; Hsu, Y.-C.; Lu, I.-C.; Lee, C.-Y.; Wu, T.-C.; Yeh, J.-H.; et al. Surgical Resection Significantly Promotes the Overall Survival of Patients with Hepatocellular Carcinoma: A Propensity Score Matching Analysis. BMC Gastroenterol. 2020, 21, 220. [Google Scholar] [CrossRef]
- Guo, H.; Wu, T.; Lu, Q.; Li, M.; Guo, J.Y.; Shen, Y.; Wu, Z.; Nan, K.J.; Lv, Y.; Zhang, X.F. Surgical Resection Improves Long-Term Survival of Patients with Hepatocellular Carcinoma across Different Barcelona Clinic Liver Cancer Stages. Cancer Manag. Res. 2018, 10, 361. [Google Scholar] [CrossRef]
- Sultan, A.; Anugwom, C.M.; Wondifraw, Z.; Braimoh, G.A.; Bane, A.; Debes, J.D. Single Center Analysis of Therapy and Outcomes of Hepatocellular Carcinoma in Sub-Saharan Africa: An Ethiopian Experience. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1007. [Google Scholar] [CrossRef] [PubMed]
- Khetsuriani, N.; Mosina, L.; Van Damme, P.; Mozalevskis, A.; Datta, S.; Tohme, R.A. Progress Toward Hepatitis B Control—World Health Organization European Region, 2016–2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Auta, A.; Adewuyi, E.O.; Kureh, G.T.; Onoviran, N.; Adeloye, D. Hepatitis B Vaccination Coverage among Health-Care Workers in Africa: A Systematic Review and Meta-Analysis. Vaccine 2018, 36, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Graber-stiehl, I. Africa’s Silent Epidemic: Hepatitis Now Kills More People Worldwide than HIV, Tuberculosis or Malaria. Tackling the Hepatitis B Virus in Africa Is Key to Fighting Back. Nature 2018, 564, 24–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
| Characteristics | HCC Patients, N (%) 1 |
|---|---|
| Age, years (mean ± SD) | 52.0 ± 15.56 |
| Sex Male Female | 246 (67) 123 (33) |
| Educational status (n = 327) None 2 Primary Secondary College/University | 133 (41) 74 (22) 68 (21) 52 (16) |
| Economic status (monthly income, ETB 3) (n = 288) <500 501–1000 1001–5000 5001–10,000 >10,000 | 127 (44) 8 (3) 79 (27) 63 (22) 11 (4) |
| Alcohol use (n = 366), yes | 123 (37) |
| Smoking status (n = 366), yes | 38 (10) |
| Physical exercise 4 (n = 325), no | 314 (97) |
| Variables | Frequency, N (%) |
|---|---|
| BMI 1 (n = 96) Underweight (<18.5 kg/m2) Normal weight (18.5–24.9 kg/m2) Overweight (≥25 kg/m2) | 47 (49) 45 (47) 4 (4) |
| Comorbidities (n = 368) Yes No | 100 (27) 268 (73) |
| Performance score 2 (n = 350) ECOG 0 ECOG 1 ECOG 2 ECOG 3 ECOG 4 | 17 (5) 115 (33) 135 (39) 71 (20) 12 (3) |
| BCLC stage 3 (n = 326) BCLC-A BCLC-B BCLC-C BCLC-D | 8 (2) 55 (17) 166 (51) 97 (30) |
| Sign and symptoms 4 Abdominal pain Ascites Anorexia Weight loss Jaundice | 344 (94) 195 (53) 171 (47) 141 (38) 56 (15) |
| Degree of pain (n = 340) Mild Moderate Severe | 139 (41) 178 (52) 23 (7) |
| History of cirrhosis (n = 369) Present Absent | 213 (58) 156 (42) |
| Cirrhosis (n = 213) Compensated Decompensated | 7 (3) 206 (97) |
| Viral hepatitis (n = 355) Positive Negative | 165 (47) 190 (53) |
| Type of viral hepatitis 5 (n = 165) HBV HCV HBV + HCV | 117 (71) 46 (28) 2 (1) |
| Type of tumor (n = 266) Single Multiple Diffuse/infiltrative | 50 (19) 152 (57) 64 (24) |
| Tumor size (n = 262) ≤5 cm >5 cm | 34 (13) 228 (87) |
| Metastasis (n = 366) Yes No | 157 (43) 209 (57) |
| Liver function estimates 6 ALT, U/L, median [IQR] AST, U/L, median [IQR] ALP, U/L, median [IQR] Total bilirubin, mg/dL, median [IQR] Albumin, g/dL, mean ± SD | 43.0 [26–80] 82.0 [44–146.8] 203.5 [129–326.3] 1.0 [0.6–1.7] 3.4 ± 0.74 |
| AFP 7 (n = 295) ≤400 ng/mL >400 ng/mL | 185 (63) 110 (37) |
| BCLC Stage | Treatment Given (n = 324) | ||||
|---|---|---|---|---|---|
| Surgery | TACE | Sorafenib | Chemotherapy 1 | Palliative | |
| BCLC-A (Early) | 7 | 1 | 0 | 0 | 0 |
| BCLC-B (Intermediate) | 13 | 5 | 9 | 1 | 26 |
| BCLC-C (Advanced) | 5 | 2 | 36 | 12 | 110 |
| BCLC-D (Terminal) | 0 | 0 | 6 | 2 | 89 |
| Covariates | Univariate Analysis | Multivariate Analysis (with HCC Treatment) | Multivariate Analysis (without HCC Treatment) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years) | 1.00 (0.99–1.01) | 0.744 | - | - | - | - |
| Gender | 1.00 (0.77–1.29) | 0.986 | - | - | - | - |
| Tumor size (cm) | 1.06 (1.03–1.10) | <0.001 ** | - | - | - | - |
| ECOG Score | 1.39 (1.22–1.57) | <0.001 ** | - | - | - | - |
| BCLC stage | 1.72 (1.44–2.04) | <0.001 ** | 1.23 (0.89–1,69) | 0.206 | 1.46 (1.08–1.96) | 0.013 * |
| Viral hepatitis 1 | 0.98 (0.77–1.26) | 0.897 | - | - | - | - |
| HBV treatment | 0.29 (0.16–0.53) | <0.001 ** | 0.42 (0.22–0.79) | 0.007 * | 0.33 (0.18–0.61) | <0.001 ** |
| HCV treatment | 0.26 (0.03–1.89) | 0.181 | - | - | - | - |
| Metastasis | 1.58 (1.24–2.02) | <0.001 ** | 0.94 (0.59–1.50) | 0.793 | 1.05 (0.66–1.65) | 0.851 |
| Cirrhosis | 1.38 (1.07–1.77) | 0.012 * | 1.45 (0.93–2.27) | 0.103 | 1.51 (0.97–2.35) | 0.069 |
| AFP (ng/mL) | 1.00 (1.00–1.00) | 0.934 | - | - | - | - |
| HCC treatment 2 | 0.51 (0.38–0.67) | <0.001 ** | 0.67 (0.53–0.86) | 0.001 * | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abza, G.B.; Ahmed, J.H.; Yesufe, A.A.; Seife, E.; Erkie, M.; Spriet, I.; Chelkeba, L.; Annaert, P. Clinicopathological Features and Survival of Patients with Hepatocellular Carcinoma in Ethiopia: A Multicenter Study. Cancers 2023, 15, 193. https://doi.org/10.3390/cancers15010193
Abza GB, Ahmed JH, Yesufe AA, Seife E, Erkie M, Spriet I, Chelkeba L, Annaert P. Clinicopathological Features and Survival of Patients with Hepatocellular Carcinoma in Ethiopia: A Multicenter Study. Cancers. 2023; 15(1):193. https://doi.org/10.3390/cancers15010193
Chicago/Turabian StyleAbza, Getahun Befirdu, Jemal Hussien Ahmed, Abdu Adem Yesufe, Edom Seife, Mengistu Erkie, Isabel Spriet, Legese Chelkeba, and Pieter Annaert. 2023. "Clinicopathological Features and Survival of Patients with Hepatocellular Carcinoma in Ethiopia: A Multicenter Study" Cancers 15, no. 1: 193. https://doi.org/10.3390/cancers15010193
APA StyleAbza, G. B., Ahmed, J. H., Yesufe, A. A., Seife, E., Erkie, M., Spriet, I., Chelkeba, L., & Annaert, P. (2023). Clinicopathological Features and Survival of Patients with Hepatocellular Carcinoma in Ethiopia: A Multicenter Study. Cancers, 15(1), 193. https://doi.org/10.3390/cancers15010193







