Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management
Abstract
Simple Summary
Abstract
1. Background
2. Materials & Methods
3. Results
3.1. Demographical Characteristics
3.2. Tumor Characteristics
3.3. Treatment Characteristics:
3.4. Survival Characteristics by Age
3.5. Survival by Sex, Age, Tumor Size and Race
3.6. Survival by Tumor Stage, Grading and Metastasis at the Time of Diagnosis
3.7. Multivariate Analysis
4. Discussion
4.1. Genomic Alterations, Ongoing Investigations, and Insights in Future Therapeutic Approaches
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshida, E.J.; García, J.; Eisele, D.W.; Chen, A.M. Salivary gland malignancies in children. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Védrine, P.O.; Coffinet, L.; Temam, S.; Montagne, K.; Lapeyre, M.; Oberlin, O.; Orbach, D.; Simon, C.; Sommelet, D. Mucoepidermoid carcinoma of salivary glands in the pediatric age group: 18 clinical cases, including 11 second malignant neoplasms. Head Neck 2006, 28, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Boukheris, H.; Curtis, R.E.; Land, C.E.; Dores, G.M. Incidence of Carcinoma of the Major Salivary Glands According to the WHO Classification, 1992 to 2006: A Population-Based Study in the United States. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Jiang, H.; He, Z. Epithelial salivary gland tumors of children and adolescents in west China population: A clinicopathologic study of 79 cases. J. Oral Pathol. Med. 2008, 37, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Sharma, R.; Sinha, R.; Menon, P.S. Salivary gland neoplasms: An analysis of 74 cases. J. Maxillofac. Oral Surg. 2009, 8, 164–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kupferman, M.E.; De La Garza, G.O.; Santillan, A.A.; Williams, M.D.; Varghese, B.T.; Huh, W.; Roberts, D.; Weber, R.S. Outcomes of Pediatric Patients with Malignancies of the Major Salivary Glands. Ann. Surg. Oncol. 2010, 17, 3301–3307. [Google Scholar] [CrossRef]
- Byrd, S.A.; Spector, M.E.; Carey, T.; Bradford, C.R.; McHugh, J.B. Predictors of Recurrence and Survival for Head and Neck Mucoepidermoid Carcinoma. Otolaryngol. Neck Surg. 2013, 149, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, M.; Ferrari, A.; Marcon, I.; Collini, P.; Gandola, L.; Pizzi, N.R.; Casanova, M.; Mattavelli, F.; Scaramellini, G. Salivary gland neoplasms in children: The experience of the Istituto Nazionale Tumori of Milan. Pediatr. Blood Cancer 2006, 47, 806–810. [Google Scholar] [CrossRef]
- Karaa, M.; Goze, F.; Ezirganli, S.; Polat, S.; Muderris, S.; Elagoz, S. Neoplasms of the salivary glands in a Turkish adult population. Med. Oral Patol. Oral Y Cirugía Buccal 2010, 15, e880–e885. [Google Scholar] [CrossRef]
- Pinkston, J.A.; Cole, P. Incidence Rates of Salivary Gland Tumors: Results from a Population-Based Study. Otolaryngol. Head Neck Surg. 1999, 120, 834–840. [Google Scholar] [CrossRef]
- Pires, F.R.; de Almeida, O.P.; de Araújo, V.C.; Kowalski, L.P. Prognostic Factors in Head and Neck Mucoepidermoid Carcinoma. Arch. Otolaryngol. Neck Surg. 2004, 130, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Ohta, H.; Shodo, R.; Matsuyama, H.; Takahashi, S. Clinicopathological features of mucoepidermoid carcinoma. J. Laryngol. Otol. 2014, 128, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Sun, M.Y.; Zhang, X.H.; Luo, X.L.; Sun, W.B. Analysis of postoperative survival rates of mucoepidermoid carcinoma in salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi 2006, 41, 709–712. [Google Scholar] [PubMed]
- Ali, S.; Sarhan, M.; Palmer, F.L.; Whitcher, M.; Shah, J.P.; Patel, S.G.; Ganly, I. Cause-Specific Mortality in Patients with Mucoepidermoid Carcinoma of the Major Salivary Glands. Ann. Surg. Oncol. 2013, 20, 2396–2404. [Google Scholar] [CrossRef]
- Chen, M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: A population-level analysis of 2400 patients. Head Neck 2013, 36, 158–163. [Google Scholar] [CrossRef]
- McHugh, C.H.; Roberts, D.B.; El-Naggar, A.K.; Hanna, E.Y.; Garden, A.S.; Kies, M.S.; Weber, R.S.; Kupferman, M.E. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2011, 118, 3928–3936. [Google Scholar] [CrossRef]
- Sultan, I.; Rodriguez-Galindo, C.; Al-Sharabati, S.; Guzzo, M.; Casanova, M.; Ferrari, A. Salivary gland carcinomas in children and adolescents: A population-based study, with comparison to adult cases. Head Neck 2010, 33, 1476–1481. [Google Scholar] [CrossRef]
- Liu, S.; Ow, A.; Ruan, M.; Yang, W.; Zhang, C.; Wang, L. Prognostic factors in primary salivary gland mucoepidermoid carcinoma: An analysis of 376 cases in an Eastern Chinese population. Int. J. Oral Maxillofac. Surg. 2014, 43, 667–673. [Google Scholar] [CrossRef]
- Chen, A.M.; Lau, V.H.; Farwell, D.G.; Luu, Q.; Donald, P.J. Mucoepidermoid carcinoma of the parotid gland treated by surgery and postoperative radiation therapy: Clinicopathologic correlates of outcome. Laryngoscope 2013, 123, 3049–3055. [Google Scholar] [CrossRef]
- Tonon, G.; Modi, S.; Wu, L.; Kubo, A.; Coxon, A.B.; Komiya, T.; O’Neil, K.; Stover, K.; Elnaggar, A.K.; Griffin, J.D.; et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003, 33, 208–213. [Google Scholar] [CrossRef]
- Saade, R.E.; Bell, D.; Garcia, J.; Roberts, D.; Weber, R. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol. Neck Surg. 2016, 142, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Tan, M.; Bishop, J.A.; Jones, S.; Sausen, M.; Ha, P.K.; Agrawal, N. Whole-Exome Sequencing of Salivary Gland Mucoepidermoid Carcinoma. Clin. Cancer Res. 2017, 23, 283–288. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, I.D. t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol. 2009, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Miyabe, S.; Nakayama, T.; Fujiyoshi, Y.; Hattori, H.; Shimozato, K.; Inagaki, H. Impact of CRTC1/3-MAML2 fusions on histological classification and prognosis of mucoepidermoid carcinoma. Histopathology 2011, 59, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; McDermott, J.D.; Schrock, A.B.; Elvin, J.A.; Gay, L.; Karam, S.D.; Raben, D.; Somerset, H.; Ali, S.M.; Ross, J.S.; et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequentBAP1,PIK3CA, and other actionable genomic alterations. Ann. Oncol. 2017, 28, 748–753. [Google Scholar] [CrossRef]
- Nakano, T.; Yamamoto, H.; Hashimoto, K.; Tamiya, S.; Shiratsuchi, H.; Nakashima, T.; Nishiyama, K.-I.; Higaki, Y.; Komune, S.; Oda, Y. HER2 and EGFR gene copy number alterations are predominant in high-grade salivary mucoepidermoid carcinoma irrespective of MAML2 fusion status. Histopathology 2013, 63, 378–392. [Google Scholar] [CrossRef]
- Press, M.F.; Pike, M.C.; Hung, G.; Zhou, J.Y.; Ma, Y.; George, J.; El-Naggar, A.K.; Dietz-Band, J.; James, W.; Slamon, D.J.; et al. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: Correlation with poor prognosis. Cancer Res. 1994, 54, 5675–5682. [Google Scholar]
- Cros, J.; Sbidian, E.; Hans, S.; Roussel, H.; Scotte, F.; Tartour, E.; Brasnu, D.; Laurent-Puig, P.; Bruneval, P.; Blons, H.; et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann. Oncol. 2013, 24, 2624–2629. [Google Scholar] [CrossRef]
- Yoshimura, T.; Higashi, S.; Yamada, S.; Noguchi, H.; Nomoto, M.; Suzuki, H.; Ishida, T.; Takayama, H.; Hirano, Y.; Yamashita, M.; et al. PCP4/PEP19 and HER2 Are Novel Prognostic Markers in Mucoepidermoid Carcinoma of the Salivary Gland. Cancers 2021, 14, 54. [Google Scholar] [CrossRef]
- Lujan, B.; Hakim, S.; Moyano, S.; Nadal, A.; Caballero, M.; Diaz, A.; Valera, A.; Carrera, M.; Cardesa, A.; Alos, L. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br. J. Cancer 2010, 103, 510–516. [Google Scholar] [CrossRef]
- Zhou, X.; Edmonson, M.N.; Wilkinson, M.R.; Patel, A.; Wu, G.; Liu, Y.; Li, Y.; Zhang, Z.; Rusch, M.C.; Parker, M.; et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat. Genet. 2015, 48, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Casalone, E.; Ferrante, D.; Romanelli, A.; Grosso, F.; Guarrera, S.; Righi, L.; Vatrano, S.; Pelosi, G.; Libener, R.; et al. Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosom. Cancer 2014, 54, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.K.; Teknos, T.N.; Toland, A.E.; Senter, L.; Nagy, R. Salivary Gland Cancer in BRCA-Positive Families. JAMA Otolaryngol. Neck Surg. 2014, 140, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Arolt, C.; Meyer, M.; Hoffmann, F.; Wagener-Ryczek, S.; Schwarz, D.; Nachtsheim, L.; Beutner, D.; Odenthal, M.; Guntinas-Lichius, O.; Buettner, R.; et al. Expression Profiling of Extracellular Matrix Genes Reveals Global and Entity-Specific Characteristics in Adenoid Cystic, Mucoepidermoid and Salivary Duct Carcinomas. Cancers 2020, 12, 2466. [Google Scholar] [CrossRef]
- Boahene, D.K.O.; Olsen, K.D.; Lewis, J.E.; Pinheiro, A.D.; Pankratz, V.S.; Bagniewski, S.M. Mucoepidermoid Carcinoma of the Parotid Gland. Arch. Otolaryngol. Neck Surg. 2004, 130, 849–856. [Google Scholar] [CrossRef]
- Xu, B.; Dogan, S.; Al Rasheed, M.R.H.; Ghossein, R.; Katabi, N. Androgen receptor immunohistochemistry in salivary duct carcinoma: A retrospective study of 188 cases focusing on tumoral heterogeneity and temporal concordance. Hum. Pathol. 2019, 93, 30–36. [Google Scholar] [CrossRef]
- Fushimi, C.; Tada, Y.; Takahashi, H.; Nagao, T.; Ojiri, H.; Masubuchi, T.; Matsuki, T.; Miura, K.; Kawakita, D.; Hirai, H.; et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann. Oncol. 2017, 29, 979–984. [Google Scholar] [CrossRef]
- Ito, F.A.; Ito, K.; Della Coletta, R.; Vargas, P.A.; Lopes, M.A. Immunohistochemical study of androgen, estrogen and progesterone receptors in salivary gland tumors. Braz. Oral Res. 2009, 23, 393–398. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ito, Y.; Fujii, K.; Masaki, A.; Beppu, S.; Kawakita, D.; Ijichi, K.; Shimozato, K.; Inagaki, H. Androgen Receptor–Positive Mucoepidermoid Carcinoma: Case Report and Literature Review. Int. J. Surg. Pathol. 2015, 23, 243–247. [Google Scholar] [CrossRef]
- Le, X.; Baik, C.; Bauman, J.; Gilbert, J.; Brose, M.; Grilley-Olsen, J.; Patil, T.; McDermott, R.; Raez, L.; Johnson, J.; et al. Larotrectinib Treatment for Patients With TRK Fusion-Positive Salivary Gland Cancers. Oncologist 2022, oyac080. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Ueyama, Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer 2018, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Rack, S.; Feeney, L.; Hapuarachi, B.; Adderley, H.; Woodhouse, L.; Betts, G.; Burghel, G.; Harrington, K.; Metcalf, R. Evaluation of the Clinical Utility of Genomic Profiling to Inform Selection of Clinical Trial Therapy in Salivary Gland Cancer. Cancers 2022, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Rack, S.; Li, Y.; McKay, C.; Wallace, A. Metcalf, R. PROSPERO: A study to determine the utility of focused genomic profiling to guide selection of drug therapy in salivary gland cancer. J. Clin. Oncol. 2019, 37, 6086. [Google Scholar] [CrossRef]
- Haddad, R.; Colevas, A.D.; Krane, J.F.; Cooper, D.; Glisson, B.; Amrein, P.C.; Weeks, L.; Costello, R.; Posner, M. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003, 39, 724–727. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Offin, M.; Buonocore, D.J.; Myers, M.L.; Venkatesh, A.; Razavi, P.; Ginsberg, M.S.; Ulaner, G.A.; Solit, D.B.; et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): Results from a phase II basket trial. J. Clin. Oncol. 2019, 37 (Suppl. S15), 6001. [Google Scholar] [CrossRef]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T.A. Molecular and Cellular Modelling of Salivary Gland Tumors Open New Landscapes in Diagnosis and Treatment. Cancers 2020, 12, 3107. [Google Scholar] [CrossRef]
- Grisanti, S.; Amoroso, V.; Buglione, M.; Rosati, A.; Gatta, R.; Pizzocaro, C.; Ferrari, V.D.; Marini, G. Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: A case report and review of literature. J. Med. Case Rep. 2008, 2, 320. [Google Scholar] [CrossRef]
- Locati, L.D.; Bossi, P.; Perrone, F.; Potepan, P.; Crippa, F.; Mariani, L.; Casieri, P.; Orsenigo, M.; Losa, M.; Bergamini, C.; et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol. 2009, 45, 574–578. [Google Scholar] [CrossRef]
- Hanna, G.J.; Guenette, J.P.; Chau, N.G.; Sayehli, C.M.; Wilhelm, C.; Metcalf, R.; Ho, A.L.; Metcalf, R.; Wong, D.J.; Brose, M.; et al. Tipifarnib in recurrent, metastatic HRAS-mutant salivary gland cancer. Cancer 2020, 126, 3972–3981. [Google Scholar] [CrossRef]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Keam, B.; Algazi, A.P.; Damian, S.; Hong, R.-L.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef]
- Dreyfuss, A.I.; Clark, J.R.; Fallon, B.G.; Posner, M.R.; Norris, C.M., Jr.; Miller, D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer 1987, 60, 2869–2872. [Google Scholar] [CrossRef] [PubMed]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef] [PubMed]





| Overall | Age ≤ 18 | Age > 18 | |
|---|---|---|---|
| n (%) | 4507 (100.0) | 165 (3.7) | 4342 (96.3) |
| Age (Mean ± SD) | 55.1 ± 18.9 | 13.5 ± 3.6 | 56.6 ± 17.4 |
| Gender (%) | |||
| Male | 2156 (47.9) | 80 (48.5) | 2076 (47.8) |
| Female | 2351 (52.1) | 85 (51.5) | 2266 (52.2) |
| Race n (%) | |||
| White | 3394 (75.3) | 119 (72.1) | 3275 (75.4) |
| Black | 579 (12.8) | 29 (17.6) | 550 (12.7) |
| Asian or Pacific Islander | 444 (9.9) | 14 (8.5) | 430 (9.9) |
| American Indian/Alaska Native | 31 (0.7) | 1 (0.6) | 30 (6.9) |
| Unknown | 59 (1.3) | 2 (1.2) | 57 (1.3) |
| Overall | Age ≤ 18 | Age > 18 | |
|---|---|---|---|
| n (%) | 4507 (100.0) | 165 (3.7) | 4342 (96.3) |
| Grade n (%) | |||
| Well-differentiated | 1023 (22.6) | 52 (31.2) * | 971 (22.4) * |
| Moderately differentiated | 1767 (39.2) | 79 (47.9) | 1688 (38.9) |
| Poorly differentiated | 496 (11.0) | 5 (3.0) * | 491 (11.3) * |
| Undifferentiated | 503 (11.2) | 10 (6.1) * | 493 (11.4) * |
| Unknown | 429 (9.5) | 14 (8.5) | 415 (9.6) |
| Stage n (%) | |||
| Localized | 2650 (58.8) | 99 (60.0) | 2551 (58.8) |
| Regional | 1007 (22.3) | 49 (29.7) * | 958 (22.1) * |
| Distant | 412 (9.1) | 8 (4.8) * | 404 (9.3) * |
| Unstaged | 149 (3.3) | 4 (2.4) | 145 (3.3) |
| Tumor Size | |||
| Microscopic | 15 (0.3) | 0 (0.0) | 15 (0.3) |
| <2cm | 1363 (30.2) | 41 (24.8) * | 1322 (30.4) * |
| 2–4 cm | 924 (20.5) | 47 (28.5) * | 877 (20.2) * |
| >4cm | 282 (6.3) | 15 (0.9) | 267 (6.1) |
| Lymph Node Involvement n (%) | |||
| Yes | 739 (16.4) | 25 (15.1) | 714 (16.4) |
| No | 2153 (47.8) | 101 (61.2) | 2052 (47.3) |
| Overall | Age ≤ 18 | Age > 18 | |
|---|---|---|---|
| n (%) | 4507 (100.0) | 165 (3.7) | 4342 (96.3) |
| Treatment n (%) | |||
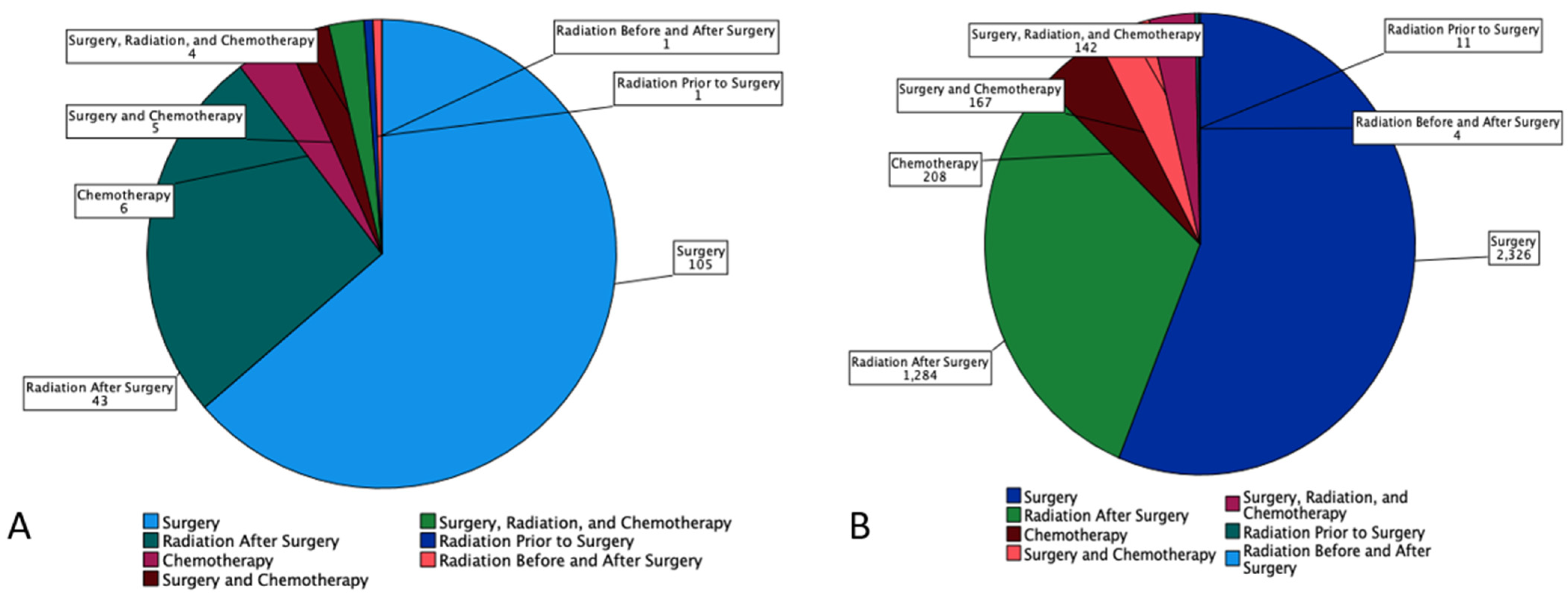
| Surgery Only | 2431 (53.9) | 105 (63.6) * | 2326 (53.5) * |
| Radiation Prior to Surgery | 12 (0.3) | 1 (0.6) | 11 (0.3) |
| Radiation After Surgery | 1327 (29.4) | 43 (26.1) | 1284 (29.6) |
| Radiation Before and After Surgery | 5 (0.1) | 1 (0.6) | 4 (0.1) |
| Chemotherapy | 214 (4.7) | 6 (3.6) | 208 (4.8) |
| Combined Surgery and Chemotherapy | 172 (3.8) | 5 (3.0) | 167 (3.8) |
| Combination of Surgery, Radiation, Chemotherapy | 146 (3.2) | 4 (2.4) | 142 (3.3) |
| Survival by treatment (years ± SD) | |||
| Surgery Only | 7.3 ± 5.4 | 8 ± 5.8 | 7.2 ± 5.3 |
| Radiation Prior to Surgery | 5.8 ± 5.7 | 15.9 | 4.2 ± 3.8 |
| Radiation After Surgery | 6.8 ± 5.1 | 8.1 ± 4.6 | 6.8 ± 5.1 |
| Radiation Before and After Surgery | 5.5 ± 4.4 | 7.6 | 5.0 ± 4.8 |
| Chemotherapy | 2.1 ± 2.4 | 0.9 | 2.1 ± 2.4 |
| Combined Surgery and Chemotherapy | 4.4 ± 4.1 | 7.8 ± 5.6 | 4.0 ± 3.9 |
| Combination of Surgery, Radiation, Chemotherapy | 4.4 ± 4.1 | 9.2 ± 4.8 | 4.2 ± 4.0 |
| Unknown | 3.1 ± 4.3 | 0.6 ± 1.0 | 3.1 ± 4.3 |
| Overall Mortality n (%) | 1252 (27.8) | 9 (5.5) *** | 1243 (28.6) |
| Cancer-Specific Mortality n (%) | 569 (12.6) | 5 (3.0) *** | 564 (13.0) |
| Variables | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|
| ANOVA F Value (p-Value) | Hazard Ratio (p-Value) | Confidence Interval (CI) | |
| Age > 18 | 6.1 (0.014) | 7.4 (0.005) | 1.8–29.7 |
| Male Gender | 19.5 (<0.001) | 0.8 (0.04) | 0.7–1.0 |
| Asian or Pacific Islander Race | 2.4 (0.063) | 0.5 (0.001) | 0.3–0.8 |
| Poorly differentiated Grade | 124.6 (<0.001) | 3.8 (<0.001) | 2.6–5.6 |
| Undifferentiated Grade | 4.5 (<0.001) | 3.1–6.4 | |
| Regional Extent of Disease | 129.4 (<0.001) | 2.1 (<0.001) | 1.7–2.7 |
| Distant Extent of Disease | 3.2 (<0.001) | 2.4–4.2 | |
| Trial Number (Name) | Study Title | Study Type | Study Arms | Primary Outcome | Status (on 09/14/2022) |
|---|---|---|---|---|---|
| NCT01473784 | Transoral robotic surgery in treating patients with benign or malignant tumors of the head and neck | Single arm, open label | Transoral robotic surgery (TORS) using the Da Vinci robotic surgical system | Feasibility of TORS | Recruiting |
| NCT01586767 | Intensity-modulated or proton radiation therapy for sinonasal malignancy | Phase II, non-randomized | Proton beam therapy vs. IMRT | Local control rate at 2 years | Recruiting |
| NCT04249947 | P-PSMA-101 CAR-T Cells in the treatment of subjects with metastatic castration-resistant prostate cancer (mCRPC) and advanced salivary gland cancers (SGC) | Phase II, non-randomized | P-PSMA-101 CAR-T cells single and multiple doses following conditioning regimen | Safety, maximum tolerated dose and efficacy | Recruiting |
| NCT03602079 | Study of A166 in patients with relapsed/refractory cancers expressing HER2 antigen or having amplified HER2 gene | Phase I–II, non-randomized | Phase I: Six dose levels Phase II: Treatment with A166 with recommended phase II dose | Maximum tolerated dose, number of patients with dose limiting toxicities | Active, not recruiting |
| NCT00003251 | Amifostine plus chemotherapy and radiation therapy in treating patients with advanced, unresectable head and neck cancer | Phase I/II | N/A | N/A | Unknown * |
| NCT01220583 | Radiation therapy with or without chemotherapy in treating patients with high-risk malignant salivary gland tumors that have been removed by surgery | Phase II/III, randomized | 3D-CRT or IMRT vs. 3D-CRT or IMRT with cisplatin | PFS | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Khan, J.; Waheed, A.; Karki, N.R.; Goodbee, M.; Yasinzai, A.Q.K.; Tareen, B.; Wali, A.; Khan, K.A.; Zarak, M.S.; et al. Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management. Cancers 2023, 15, 250. https://doi.org/10.3390/cancers15010250
Ullah A, Khan J, Waheed A, Karki NR, Goodbee M, Yasinzai AQK, Tareen B, Wali A, Khan KA, Zarak MS, et al. Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management. Cancers. 2023; 15(1):250. https://doi.org/10.3390/cancers15010250
Chicago/Turabian StyleUllah, Asad, Jaffar Khan, Abdul Waheed, Nabin Raj Karki, Mya Goodbee, Abdul Qahar Khan Yasinzai, Bisma Tareen, Agha Wali, Khaleel Ahmad Khan, Muhammad Samsoor Zarak, and et al. 2023. "Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management" Cancers 15, no. 1: 250. https://doi.org/10.3390/cancers15010250
APA StyleUllah, A., Khan, J., Waheed, A., Karki, N. R., Goodbee, M., Yasinzai, A. Q. K., Tareen, B., Wali, A., Khan, K. A., Zarak, M. S., Khan, I., Garcia, A. A., Khan, A., Khan, M., Jogezai, S., Ahmad, J., Zarate, L. V., Patel, N., Karim, N. A., & Heneidi, S. (2023). Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management. Cancers, 15(1), 250. https://doi.org/10.3390/cancers15010250










