Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer
Abstract
Simple Summary
Abstract
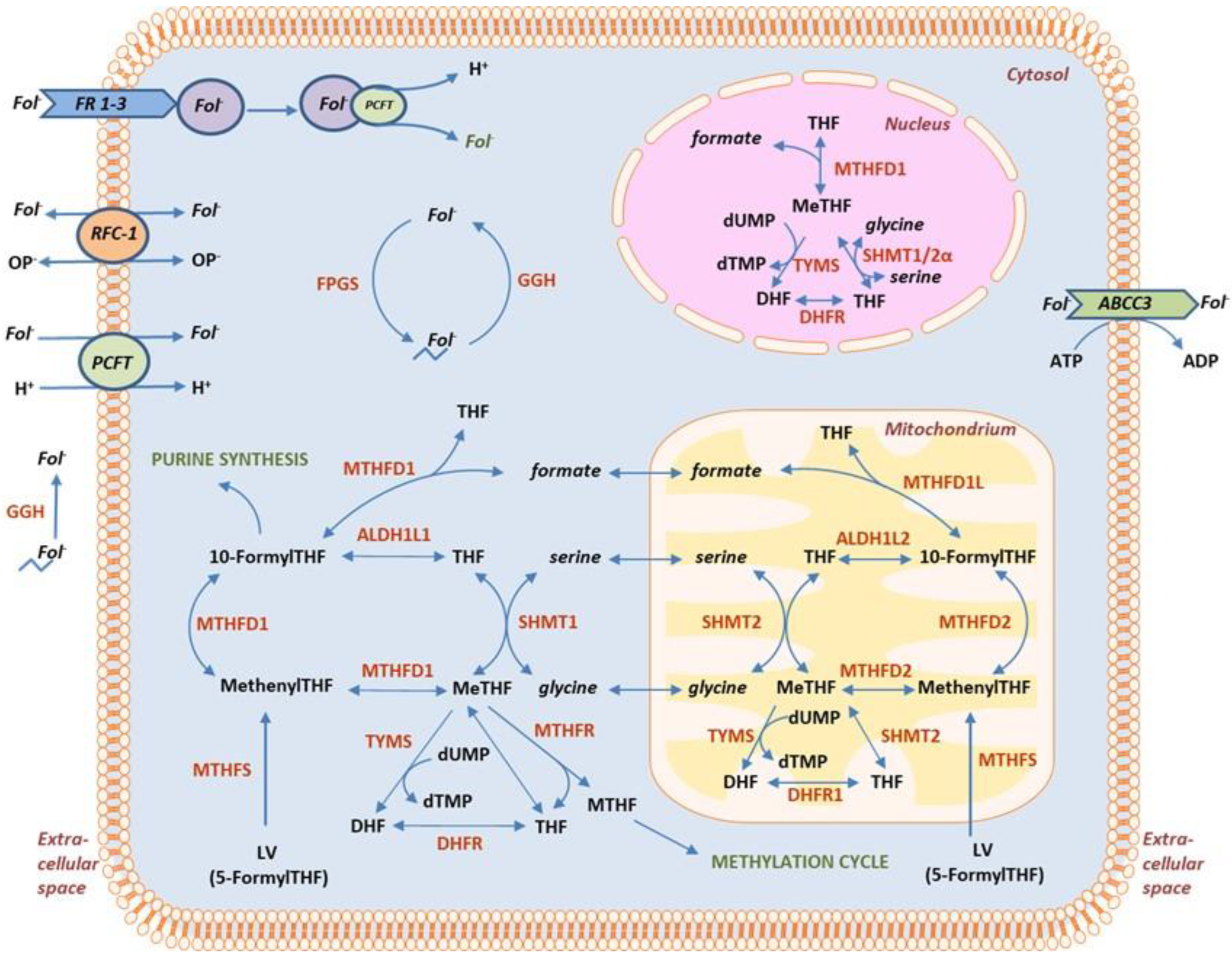
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Materials
2.3. Plasma Folate Analyses
2.4. Tissue Folate Analyses
2.5. LC-MS/MS Conditions
2.6. RNA Extraction, cDNA Synthesis and Real-Time Quantitative PCR
2.7. Statistics
3. Results
3.1. Demographic
3.2. Plasma Folate Levels after Two-Hour Infusion of LV
3.3. Folate Levels in Tissue Samples after Two-Hour Infusion of LV
3.4. Folates in Plasma versus Tissue after Two-Hour Infusion of LV
3.5. Tissue Folate Levels at Baseline and after Two-Hour Infusion versus Bolus Injection
3.6. Folate and Gene Expression Levels in Tissues at Baseline
3.7. Gene Expression Alterations in Tumor and Mucosa at Increasing Dosage of LV
3.8. Correlation between Gene Expression and Folate Levels
3.9. Impact of Ischemic Time on Gene Expression
3.10. Polyglutamated/Monoglutamated Folate Ratio after Two-Hour Infusion versus Bolus Injection of LV
4. Discussion
Strengths and Limitations of Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.J.; Tveit, K.M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Piedbois, P.; Buyse, M.; Rustum, Y.; Machover, D.; Erlichman, C.; Carlson, R.W.; Valone, F.; Labianca, R.; Doroshow, J.H.; Petrelli, N. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: Evidence in terms of response rate by the advanced colorectal cancer meta-analysis project. J. Clin. Oncol. 1992, 10, 896–903. [Google Scholar] [CrossRef]
- Cheeseman, S.L.; Joel, S.P.; Chester, J.D.; Wilson, G.; Dent, J.T.; Richards, F.J.; Seymour, M.T. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br. J. Cancer 2002, 87, 393–399. [Google Scholar] [CrossRef]
- Kohne, C.H.; Wils, J.; Lorenz, M.; Schoffski, P.; Voigtmann, R.; Bokemeyer, C.; Lutz, M.; Kleeberg, C.; Ridwelski, K.; Souchon, R.; et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J. Clin. Oncol. 2003, 21, 3721–3728. [Google Scholar] [CrossRef]
- Odin, E.; Wettergren, Y.; Carlsson, G.; Gustavsson, B. Determination of reduced folates in tumor and adjacent mucosa of colorectal cancer patients using LC-MS/MS. Biomed. Chromatogr. 2013, 27, 487–495. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Maeda, Y.; Tanaka, A.; Ogoshi, K.; Kamijo, A.; Murayama, C.; Tsukioka, S.; Sakamoto, E.; Fukui, Y.; et al. Molecular determinants of folate levels after leucovorin administration in colorectal cancer. Cancer Chemother. Pharmacol. 2010, 65, 735–742. [Google Scholar] [CrossRef]
- Taflin, H.; Wettergren, Y.; Odin, E.; Derwinger, K. Folate levels measured by LC-MS/MS in patients with colorectal cancer treated with different leucovorin dosages. Cancer Chemother. Pharmacol. 2014, 74, 1167–1174. [Google Scholar] [CrossRef]
- Danenberg, P.V.; Gustavsson, B.; Johnston, P.; Lindberg, P.; Moser, R.; Odin, E.; Peters, G.J.; Petrelli, N. Folates as adjuvants to anticancer agents: Chemical rationale and mechanism of action. Crit. Rev. Oncol. Hematol. 2016, 106, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, R.L.; Ratain, M.J. Clinical pharmacokinetics of high-dose leucovorin calcium after intravenous and oral administration. J. Natl. Cancer Inst. 1990, 82, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.M.; Steger, G.G.; Rizovski, B.; Sieder, A.E.; Locker, G.; Gnant, M.F.; Jakesz, R.; Rainer, H. Pharmacokinetics of rac-leucovorin vs [S]-leucovorin in patients with advanced gastrointestinal cancer. Br. J. Clin. Pharmacol. 1994, 37, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Robinson, C.B.; Johnston, K.E. Bioactivity of [6R]-5-formyltetrahydrofolate, an unusual isomer, in humans and Enterococcus hirae, and cytochrome c oxidation of 10-formytetrahydrofolate to 10-formyldihydrofolate. Biochem. J. 2001, 354, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.W.; Reed, K.A.; Said, H.M. Transport of 5-methyltetrahydrofolate in basolateral membrane vesicles of rat liver. Am. J. Physiol. 1992, 262, G150–G158. [Google Scholar] [CrossRef] [PubMed]
- Voeller, D.M.; Allegra, C.J. Intracellular metabolism of 5-methyltetrahydrofolate and 5-formyltetrahydrofolate in a human breast-cancer cell line. Cancer Chemother. Pharmacol. 1994, 34, 491–496. [Google Scholar] [CrossRef]
- Carlsson, G.; Gustavsson, B.; Frosing, R.; Odin, E.; Hafstrom, L.O.; Spears, C.P.; Larsson, P.A. Antitumour effects of pure diastereoisomers of 5-formyltetrahydrofolate in hepatic transplants of a rodent colon carcinoma model. Biochem. Pharmacol. 1995, 50, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.D.; Quintero, C.M.; Stover, P.J. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 15163–15168. [Google Scholar] [CrossRef]
- Field, M.S.; Kamynina, E.; Chon, J.; Stover, P.J. Nuclear Folate Metabolism. Annu. Rev. Nutr. 2018, 38, 219–243. [Google Scholar] [CrossRef]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef]
- Ifergan, I.; Jansen, G.; Assaraf, Y.G. The reduced folate carrier (RFC) is cytotoxic to cells under conditions of severe folate deprivation. RFC as a double edged sword in folate homeostasis. J. Biol. Chem. 2008, 283, 20687–20695. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Rahat, B.; Hamid, A.; Najar, R.A.; Kaur, J. Identification of regulatory mechanisms of intestinal folate transport in condition of folate deficiency. J. Nutr. Biochem. 2015, 26, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, B.; Mohammed, Z.M.; Vaziri, N.D.; Said, H.M. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am. J. Clin. Nutr. 2007, 86, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Sirotnak, F.M.; Chello, P.L.; Moccio, D.M.; Kisliuk, R.L.; Combepine, G.; Gaumont, Y.; Montgomery, J.A. Stereospecificity at carbon 6 of fomyltetrahydrofolate as a competitive inhibitor of transport and cytotoxicity of methotrexate in vitro. Biochem. Pharmacol. 1979, 28, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Horne, D.W. Transport of 5-formyltetrahydrofolate into primary cultured rat astrocytes. Arch. Biochem. Biophys. 2003, 410, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, E.; Tsukioka, S.; Oie, S.; Kobunai, T.; Tsujimoto, H.; Sakamoto, K.; Okayama, Y.; Sugimoto, Y.; Oka, T.; Fukushima, M.; et al. Folylpolyglutamate synthase and gamma-glutamyl hydrolase regulate leucovorin-enhanced 5-fluorouracil anticancer activity. Biochem. Biophys. Res. Commun. 2008, 365, 801–807. [Google Scholar] [CrossRef]
- Chen, L.; Qi, H.; Korenberg, J.; Garrow, T.A.; Choi, Y.J.; Shane, B. Purification and properties of human cytosolic folylpoly-gamma-glutamate synthetase and organization, localization, and differential splicing of its gene. J. Biol. Chem. 1996, 271, 13077–13087. [Google Scholar] [CrossRef]
- Danenberg, P.V.; Danenberg, K.D. Effect of 5, 10-methylenetetrahydrofolate on the dissociation of 5-fluoro-2’-deoxyuridylate from thymidylate synthetase: Evidence for an ordered mechanism. Biochemistry 1978, 17, 4018–4024. [Google Scholar] [CrossRef]
- Radparvar, S.; Houghton, P.J.; Houghton, J.A. Effect of polyglutamylation of 5,10-methylenetetrahydrofolate on the binding of 5-fluoro-2’-deoxyuridylate to thymidylate synthase purified from a human colon adenocarcinoma xenograft. Biochem. Pharmacol. 1989, 38, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.A.; Titus, S.A.; Ferguson, J.; Heineman, A.L.; Taylor, S.M.; Moran, R.G. Mammalian mitochondrial and cytosolic folylpolyglutamate synthetase maintain the subcellular compartmentalization of folates. J. Biol. Chem. 2014, 289, 29386–29396. [Google Scholar] [CrossRef] [PubMed]
- Galivan, J.; Ryan, T.J.; Chave, K.; Rhee, M.; Yao, R.; Yin, D. Glutamyl hydrolase. pharmacological role and enzymatic characterization. Pharmacol. Ther. 2000, 85, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Rustum, Y.M. Effects of diastereoisomers of 5-formyltetrahydrofolate on cellular growth, sensitivity to 5-fluoro-2’-deoxyuridine, and methylenetetrahydrofolate polyglutamate levels in HCT-8 cells. Cancer Res. 1991, 51, 3476–3481. [Google Scholar] [PubMed]
- Wright, J.E.; Pardo, M.; Sayeed-Shah, U.; Alperin, W.; Rosowsky, A. Leucovorin and folic acid regimens for selective expansion of murine 5,10-methylenetetrahydrofolate pools. Biochem. Pharmacol. 1995, 49, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Canet, M.J.; Merrell, M.D.; Harder, B.G.; Maher, J.M.; Wu, T.; Lickteig, A.J.; Jackson, J.P.; Zhang, D.D.; Yamamoto, M.; Cherrington, N.J. Identification of a functional antioxidant response element within the eighth intron of the human ABCC3 gene. Drug Metab. Dispos. 2015, 43, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Kusuhara, H.; Sugiyama, Y. Basolateral efflux mediated by multidrug resistance-associated protein 3 (Mrp3/Abcc3) facilitates intestinal absorption of folates in mouse. Pharm. Res. 2010, 27, 665–672. [Google Scholar] [CrossRef]
- Chiche, J.; Brahimi-Horn, M.C.; Pouyssegur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef]
- Field, M.S.; Szebenyi, D.M.; Perry, C.A.; Stover, P.J. Inhibition of 5,10-methenyltetrahydrofolate synthetase. Arch. Biochem. Biophys. 2007, 458, 194–201. [Google Scholar] [CrossRef]
- Anguera, M.C.; Suh, J.R.; Ghandour, H.; Nasrallah, I.M.; Selhub, J.; Stover, P.J. Methenyltetrahydrofolate synthetase regulates folate turnover and accumulation. J. Biol. Chem. 2003, 278, 29856–29862. [Google Scholar] [CrossRef]
- Bertrand, R.; Beauchemin, M.; Dayan, A.; Ouimet, M.; Jolivet, J. Identification and characterization of human mitochondrial methenyltetrahydrofolate synthetase activity. Biochim. Biophys. Acta 1995, 1266, 245–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hooijberg, J.H.; Peters, G.J.; Assaraf, Y.G.; Kathmann, I.; Priest, D.G.; Bunni, M.A.; Veerman, A.J.; Scheffer, G.L.; Kaspers, G.J.; Jansen, G. The role of multidrug resistance proteins MRP1, MRP2 and MRP3 in cellular folate homeostasis. Biochem. Pharmacol. 2003, 65, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Leung, G.K.K. More Than a Metabolic Enzyme: MTHFD2 as a Novel Target for Anticancer Therapy? Front. Oncol. 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Behring, M.; Hale, K.; Al Diffalha, S.; Wang, K.; Manne, U.; Varambally, S. MTHFD1L, A Folate Cycle Enzyme, Is Involved in Progression of Colorectal Cancer. Transl. Oncol. 2019, 12, 1461–1467. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Zhang, H.; Liang, B.; Zhang, J.; Zhang, Z.; Yang, Y. High expression of folate cycle enzyme MTHFD1L correlates with poor prognosis and increased proliferation and migration in colorectal cancer. J. Cancer 2020, 11, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Herbig, K.; Chiang, E.P.; Lee, L.R.; Hills, J.; Shane, B.; Stover, P.J. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J. Biol. Chem. 2002, 277, 38381–38389. [Google Scholar] [CrossRef]
- Spears, C.P.; Gustavsson, B.G.; Frosing, R. Folinic acid modulation of fluorouracil: Tissue kinetics of bolus administration. Investig. New Drugs 1989, 7, 27–36. [Google Scholar] [CrossRef]






| LV Dosage (mg/m2) | LV Level in Tumor (pmol/g), Median (Range) | LV Level in Mucosa (pmol/g), Median (Range) | p a |
|---|---|---|---|
| 60 | 3607 (1514–22310) | 4409 (3178–45928) | 0.0098 |
| 200 | 9126 (3725–49189) | 20326 (9783–73719) | 0.064 |
| 500 | 29723 (14978–83321) | 96437 (27652–207297) | 0.014 |
| LV Dosage (mg/m2) | Admini-stration | Folate Level in Tumor (pmol/g), Median (Range) | Folate Level in Mucosa (pmol/g), Median (Range) | p a,b | |
|---|---|---|---|---|---|
| MTHF | 60 | Infusion | 2946 (1557–16707) | 2371 (1447–14838) | 0.77 |
| Bolus | 1274 (935–1560) | 1000 (506–2194) | 0.43 | ||
| p c,d | 0.0002 | 0.0013 | |||
| (MeTHF + THF) | 60 | Infusion | 2213 (1087–10720) | 1393 (599–8736) | 0.064 |
| Bolus | 2651 (1001–3493) | 1726 (1065–2856) | 0.037 | ||
| p c,d | 0.79 | 0.47 | |||
| MTHF | 200 | Infusion | 6422 (2526–22,753) | 4971 (3711–30,230) | 0.49 |
| Bolus | 2310 (849–3457) | 2246 (1404–2822) | 0.46 | ||
| p c,d | 0.0016 | 0.0004 | |||
| (MeTHF + THF) | 200 | Infusion | 2597 (1073–11,277) | 2262 (1302–5138) | 0.56 |
| Bolus | 3620 (2639–11,184 | 2306 (1515–2928) | 0.0078 | ||
| p c,d | 0.35 | 0.56 | |||
| MTHF | 500 | Infusion | 10988 (8342–37,823) | 19884 (8136–46,085) | 0.16 |
| Bolus | 3392 (2551–7645) | 2956 (2195–6026) | 0.57 | ||
| p c,d | 0.0002 | 0.0003 | |||
| (MeTHF + THF) | 500 | Infusion | 5047 (2052–9492) | 4995 (1503–14,360) | 0.56 |
| Bolus | 4909 (2765–7713) | 3032 (2241–4670) | 0.019 | ||
| p c,d | 0.73 | 0.096 |
| Dose LV (mg/m2) | Time (Minutes), Median (Range) | p a | ||||
|---|---|---|---|---|---|---|
| Two-Hour Infusion | n | Bolus Injection | n | |||
| 60 | 120 (60–181) | 10 | 42 (22–120) | 10 | 0.0015 | |
| 200 | 84 (27–205) | 10 | 50 (20–175) | 7 | 0.41 | |
| 500 | 96 (66–197) | 10 | 45 (17–105) | 7 | 0.0054 | |
| Gene | Gene expression (ΔCt), Median (Range) | p a | |
|---|---|---|---|
| Mucosaadj | Mucosares | ||
| ABCC3 | 6.83 (4.84–11.01) | 6.80 (4.73–8.80) | 0.82 |
| RFC-1 | 10.87 (9.12–12.96) | 10.88 (8.09–12.98) | 0.89 |
| PCFT | 10.92 (7.17–16.32) | 11.52 (4.36–16.86) | 0.27 |
| FPGS | 10.12 (7.22–11.26) | 10.13 (7.37–11.42) | 0.75 |
| GGH | 9.68 (6.39–11.58) | 9.74 (5.96–12.96) | 0.45 |
| TYMS | 10.40 (6.70–11.91) | 10.35 (7.68–12.82) | 0.63 |
| MTHFS | 11.75 (9.61–13.26) | 11.65 (8.10–13.19) | 0.63 |
| MTHFD1L | 13.56 (10.22–15.23) | 13.87 (10.80–15.09) | 0.74 |
| MTHFD2 | 10.66 (7.11–13.08) | 10.05 (6.16–13.30) | 0.47 |
| SHMT1 | 9.26 (6.10–11.01) | 9.07 (4.34–10.58) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taflin, H.; Odin, E.; Carlsson, G.; Gustavsson, B.; Wettergren, Y.; Bexe Lindskog, E. Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer. Cancers 2023, 15, 258. https://doi.org/10.3390/cancers15010258
Taflin H, Odin E, Carlsson G, Gustavsson B, Wettergren Y, Bexe Lindskog E. Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer. Cancers. 2023; 15(1):258. https://doi.org/10.3390/cancers15010258
Chicago/Turabian StyleTaflin, Helena, Elisabeth Odin, Göran Carlsson, Bengt Gustavsson, Yvonne Wettergren, and Elinor Bexe Lindskog. 2023. "Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer" Cancers 15, no. 1: 258. https://doi.org/10.3390/cancers15010258
APA StyleTaflin, H., Odin, E., Carlsson, G., Gustavsson, B., Wettergren, Y., & Bexe Lindskog, E. (2023). Increasing Dosage of Leucovorin Results in Pharmacokinetic and Gene Expression Differences When Administered as Two-Hour Infusion or Bolus Injection to Patients with Colon Cancer. Cancers, 15(1), 258. https://doi.org/10.3390/cancers15010258






