Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Study Population
2.4. Study Size and Recruitment Procedure
2.5. Data Collection
2.6. Cohort Follow-Up
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Description of The Patients Included in Both Cohorts and Accuracy of the PSA Test
3.2. False-Positive Rate and Associated Sociodemographic and Clinical Characteristics
3.3. False-Positive Rate Associated with The Presence of Comorbidities
3.4. False-Positive Rate Associated with the Prescription of Medication
3.5. Description of the PCa Cases and their Main Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paschen, U.; Sturtz, S.; Fleer, D.; Lampert, U.; Skoetz, N.; Dahm, P. Assessment of prostate-specific antigen screening: An evidence-based report by the German Institute for Quality and Efficiency in Health Care. BJU Int. 2022, 129, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I. PSA screening—A matter of debate? Memo 2019, 12, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Ilic, D.; Neuberger, M.M.; Djulbegovic, M.; Dahm, P. Screening for prostate cancer. Cochrane Database Syst. Rev. 2013, 1, CD004720. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, A.R.; Ghaemi, E.O.; Ahmadi, A.R.; Marjani, A.; Moradi, A.; Saifi, A. Age related prostate specific antigen reference range among men in south-east caspian sea. Pakistan J. Biol. Sci. 2007, 10, 1496–1500. [Google Scholar] [CrossRef]
- Bernal-Soriano, M.C.; Lumbreras, B.; Hernández-Aguado, I.; Pastor-Valero, M.; López-Garrigos, M.; Parker, L.A. Untangling the association between prostate-specific antigen and diabetes: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2020, 59, 11–26. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Goldberg, K.C.; Platz, E.A.; Freedland, S.J. The influence of statin medications on prostate-specific antigen levels. J. Natl. Cancer Inst. 2008, 100, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Jayalath, V.H.; Ireland, C.; Fleshner, N.E.; Hamilton, R.J.; Jenkins, D.J. The relationship between metformin and serum prostate-specific antigen levels. Prostate 2016, 76, 1445–1453. [Google Scholar] [CrossRef]
- Marberger, M.; Freedland, S.J.; Andriole, G.L.; Emberton, M.; Pettaway, C.; Montorsi, F.; Teloken, C.; Rittmaster, R.S.; Somerville, M.C.; Castro, R. Usefulness of prostate specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: Lessons from the REDUCE study. BJU Int. 2012, 109, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Fenton, J.J.; Weyrich, M.S.; Durbin, S.; Liu, Y.; Bang, H.; Melnikow, J. Prostate-Specific Antigen-Based Screening for Prostate Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2018. [Google Scholar]
- Loeb, S.; van den Heuvel, S.; Zhu, X.; Bangma, C.H.; Schröder, F.H.; Roobol, M.J. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur. Urol. 2012, 61, 1110. [Google Scholar] [CrossRef]
- Moyer, V.A. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012, 157, 120–134. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.F.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kinoshita, H.; Nishizawa, K.; Mitsumori, K.; Ogawa, O.; Kamoto, T. Age-associated increase of prostate-specific antigen in a high level of men visiting urological clinics. Int. J. Urol. 2005, 12, 733–738. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar]
- Bernal-Soriano, M.C.; Parker, L.A.; López-Garrigos, M.; Hernández-Aguado, I.; Caballero-Romeu, J.P.; Gómez-Pérez, L.; Alfayate-Guerra, R.; Pastor-Valero, M.; García, N.; Lumbreras, B. Factors associated with false negative and false positive results of prostate-specific antigen (PSA) and the impact on patient health: Cohort study protocol. Medicine 2019, 98, e17451. [Google Scholar] [CrossRef]
- Bernal-Soriano, M.C.; Parker, L.A.; López-Garrigós, M.; Hernández-Aguado, I.; Gómez-Pérez, L.; Caballero-Romeu, J.P.; Pastor-Valero, M.; García, N.; Alfayate-Guerra, R.; Lumbreras, B. Do the Prostate-Specific Antigen (PSA) Tests That Are Ordered in Clinical Practice Adhere to the Pertinent Guidelines? J. Clin. Med. 2021, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wender, R.C.; Etzioni, R.B.; Thompson, I.M.; D’Amico, A.V.; Volk, R.J.; Brooks, D.D.; Dash, C.; Guessous, I.; Andrews, K.; et al. American Cancer Society guideline for the early detection of prostate cancer: Update 2010. CA Cancer J. Clin. 2010, 60, 70–98. [Google Scholar] [CrossRef] [Green Version]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef]
- Oesterling, J.E.; Jacobsen, S.J.; Chute, C.G.; Guess, H.A.; Girman, C.J.; Panser, L.A.; Lieber, M.M. Serum prostate-specific antigen in a community-based population of healthy men: Establishment of age-specific reference ranges. JAMA J. Am. Med. Assoc. 1993, 270, 860–864. [Google Scholar] [CrossRef]
- Luboldt, H.J.; Schindler, J.F.; RÜbben, H. Age-Specific Reference Ranges for Prostate-Specific Antigen as Marker for Prostate Cancer. EAU-EBU Update Ser. 2006, 5, 38–48. [Google Scholar] [CrossRef]
- Vickers, A.J.; Sjoberg, D.D.; Ulmert, D.; Vertosick, E.; Roobol, M.J.; Thompson, I.; Heijnsdijk, E.A.; De Koning, H.; Atoria-Swartz, C.; Scardino, P.T.; et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Cronin, A.M.; Aus, G.; Pihl, C.G.; Becker, C.; Pettersson, K.; Scardino, P.T.; Hugosson, J.; Lilja, H. Impact of recent screening on predicting the outcome of prostate cancer biopsy in men with elevated prostate-specific antigen: Data from the European Randomized Study of Prostate Cancer Screening in Gothenburg, Sweden. Cancer 2010, 116, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H.; Adolfsson, J.; Aly, M.; Nordström, T.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Clements, M.; et al. Prostate cancer screening in men aged 50-69 years (STHLM3): A prospective population-based diagnostic study. Lancet Oncol. 2015, 16, 1667–1676. [Google Scholar] [CrossRef]
- Yang, W.J.; Cheon, S.H.; Kim, Y.S.; Kim, D.J.; Seong, H.D.; Song, Y.S.; Cho, S.Y.; Cho, I.R.; Kim, S.I.; Kim, S.J.; et al. Relationship between prostate-specific antigen and body mass index according to age: Lower prostate-specific antigen in middle-aged overweight and obese Korean men. Urol. Int. 2010, 85, 143–146. [Google Scholar] [CrossRef]
- Barlow, H.; Mao, S.; Khushi, M. Predicting High-Risk Prostate Cancer Using Machine Learning Methods. Data 2019, 4, 129. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.; Gelfond, J.; Goros, M.; Liss, M.A.; Liang, Y.; Ankerst, D.; Thompson, I.M., Jr.; Leach, R.J. The effect of 3-month finasteride challenge on biomarkers for predicting cancer outcome on biopsy: Results of a randomized trial. PLoS ONE 2018, 9, e0204823. [Google Scholar] [CrossRef]
- Choi, Y.H.; Cho, S.Y.; Cho, I.R. The different reduction rate of prostate-specific antigen in dutasteride and finasteride. Korean J. Urol. 2010, 51, 704–708. [Google Scholar] [CrossRef] [Green Version]
- Møller, M.H.; Kristiansen, I.S.; Beisland, C.; Rørvik, J.; Støvring, H. Trends in stage-specific incidence of prostate cancer in Norway, 1980–2010: A population-based study. BJU Int. 2016, 118, 547–555. [Google Scholar] [CrossRef]
- Cuocolo, R.; Stanzione, A.; Rusconi, G.; Petretta, M.; Ponsiglione, A.; Fusco, F.; Longo, N.; Persico, F.; Cocozza, S.; Brunetti, A.; et al. PSA-density does not improve bi-parametric prostate MR detection of prostate cancer in a biopsy naïve patient population. Eur. J. Radiol. 2018, 104, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Månsson, M.; Wallström, J.; Axcrona, U.; Carlsson, S.V.; Egevad, L.; Geterud, K.; Khatami, A.; Kohestani, K.; Pihl, C.G.; et al. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N. Engl. J. Med. 2022, 387, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.A.; Rabah, D.M.; Wahdan, I.H. Awareness of general public towards cancer prostate and screening practice in Arabic communities: A comparative multi-center study. Asian Pac. J. Cancer Prev. 2012, 13, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Cullati, S.; Charvet-Bérard, A.I.; Perneger, T.V. Cancer screening in a middle-aged general population: Factors associated with practices and attitudes. BMC Public Health. 2009, 29, 118. [Google Scholar] [CrossRef] [PubMed]
- Mirone, V.; Imbimbo, C.; Arcaniolo, D.; Franco, M.; La Rocca, R.; Venturino, L.; Spirito, L.; Creta, M.; Verze, P. Knowledge, attitudes, and practices towards prostate cancer screening amongst men living in the southern Italian peninsula: The Prevention and Research in Oncology (PRO) non-profit Foundation experience. World J. Urol. 2017, 35, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
| Total | Symptomatic | Asymptomatic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total Cancer/Patients (109/1664; 6.5%) | False-Positive PSA Rate 1 (728/1555; 46.8%) | p-Value 2 | Total Cancer/Patients (17/303; 5.6) | False-Positive PSA Rate 1 (137/286; 47.9) | p-Value 2 | Total Cancer/Patients (92/1361; 6.8) | False-Positive PSA Rate 1 (591/1269; 46.6) | p-Value 2 |
| Age | <0.001 | 0.070 | <0.001 | ||||||
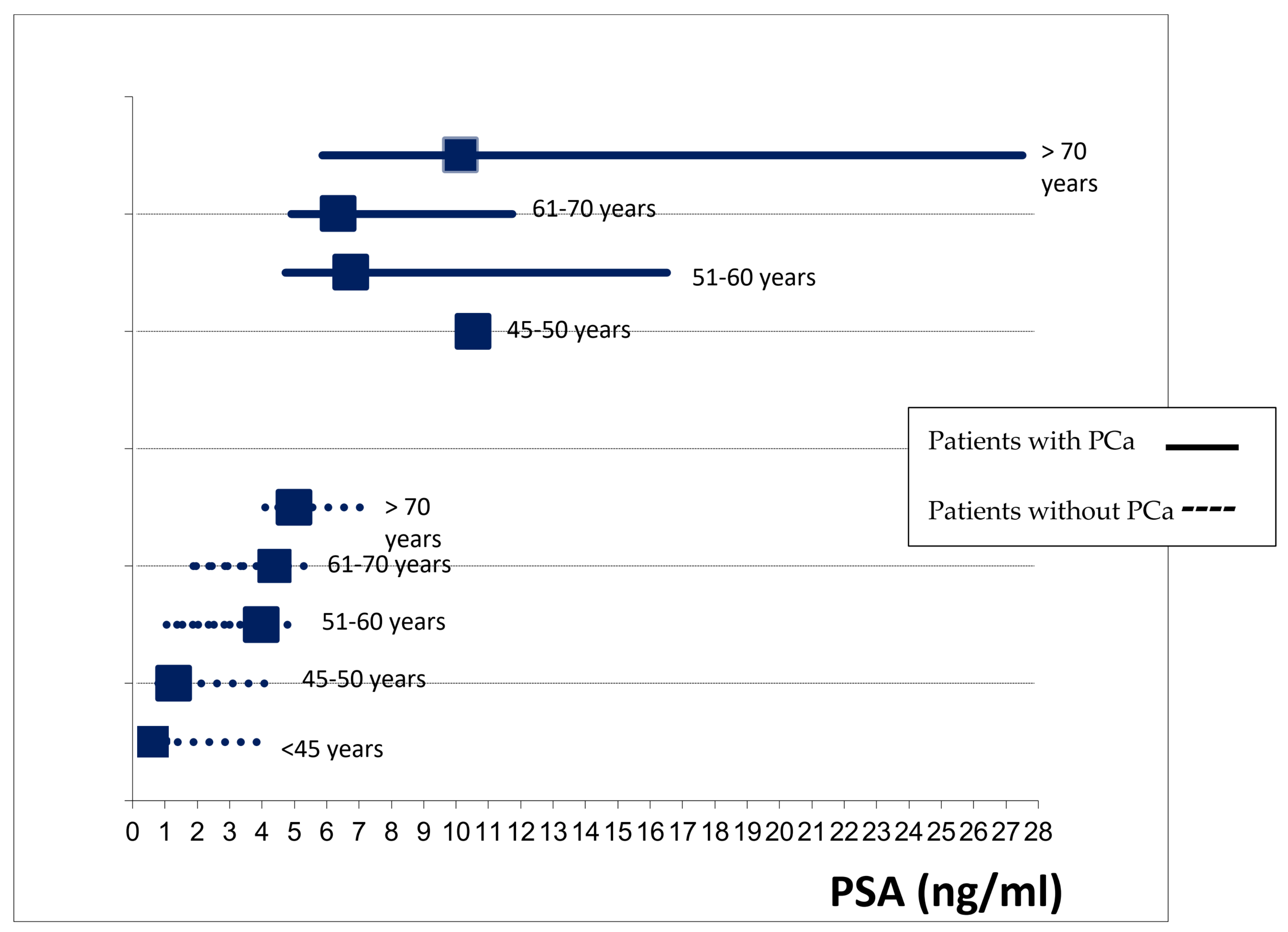
| <45 | 0/57; 0 | 15/57; 26.3 | 0/19; 0 | 5/19; 26.3 | 0/38; 0 | 10/38; 26.3 | |||
| 45–50 | 1/81; 1.2 | 22/80; 27.5 | 1/14; 7.1 | 6/13; 46.2 | 0/67; 0 | 16/67; 23.9 | |||
| 51–60 | 12/369; 3.3 | 139/357; 38.9 | 3/59; 5.1 | 21/56; 37.5 | 9/310; 2.9 | 118/301; 39.2 | |||
| 61–70 | 51/527; 9.7 | 229/476; 48.1 | 5/98; 5.1 | 52/93; 55.9 | 46/429; 10.7 | 177/383; 46.2 | |||
| >70 | 45/630; 7.1 | 323/585; 55.2 | 8/113; 7.1 | 53/105; 50.5 | 37/517; 7.2 | 270/480; 56.3 | |||
| PSA previous | 0.008 | 0.147 | 0.023 | ||||||
| No | 29/315; 9.2 | 154/286; 53.8 | 6/55; 10.9 | 28/49; 57.1 | 23/260; 8.8 | 126/237; 53.2 | |||
| Yes | 80/1347; 5.9 | 572/1267; 45.1 | 11/247; 4.5 | 108/236; 45.8 | 69/1100; 6.3 | 464/1031; 45 | |||
| Family history of PCa | 0.003 | 0.899 | 0.002 | ||||||
| No | 19/169; 11.2 | 89/150; 59.3 | 3/34; 8.8 | 16/31; 51.6 | 16/135; 11.9 | 73/119; 61.3 | |||
| Yes | 2/33; 6.1 | 17/31; 54.8 | 1/9; 11.1 | 4/8; 50 | 1/24; 4.2 | 13/23; 56.5 | |||
| Unknown | 88/1462; 6 | 622/1374; 45.3 | 13/260; 5 | 117/247; 47.4 | 75/1202; 6.2 | 505/1127; 44.8 | |||
| Tobacco | 0.274 | 0.056 | 0.677 | ||||||
| Never smoker | 21/377; 5.6 | 184/356; 51.7 | 4/69; 5.8 | 39/65; 60 | 17/308; 5.5 | 145/291; 49.8 | |||
| Current smoker | 27/362; 7.5 | 154/335; 46 | 4/66; 6.1 | 24/62; 38.7 | 23/296; 7.8 | 130/273; 47.6 | |||
| Ex-smoker | 40/546; 7.3 | 239/506; 47.2 | 6/103; 5.8 | 49/97; 50.5 | 34/44 | 190/409; 46.5 | |||
| Alcohol | 0.014 | 0.836 | 0.004 | ||||||
| Never drinker | 22/386; 5.7 | 168/364; 46.2 | 5/75; 6.7 | 38/70; 54.3 | 17/311; 5.5 | 130/294; 44.2 | |||
| Current drinker | 26/346; 7.5 | 181/320; 56.6 | 3/69; 4.3 | 36/66; 54.5 | 23/277; 8.3 | 145/254; 57.1 | |||
| Ex-drinker | 3/56; 5.4 | 31/53; 58.5 | 0/7; 0 | 3/7; 42.9 | 3/49/6.1 | 28/46; 60.9 | |||
| Variables | Total | With Symptoms | Without Symptoms | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Cancer/Patients (109/1664; 6.5%) | False-Positive PSA Rate 1 (728/1555; 46.8%) | p-Value 2 | Total Cancer/Patients (17/303; 5.6) | False-Positive PSA Rate 1 (137/286; 47.9) | p-Value 2 | Total Cancer/Patients (92/1361; 6.8) | False-Positive PSA Rate 1 (591/1269; 46.6) | p-Value 2 | |
| Cardiovascular Disease | 0.702 | 0.846 | 0.637 | ||||||
| No | 103/1508; 6.8 | 660/1405; 47 | 15/281; 5.3 | 127/266; 47.7 | 88/1227; 7.2 | 533/1139; 46.8 | |||
| Yes | 6/156; 3.8 | 68/150; 45.3 | 2/22; 9.1 | 10/20; 50 | 4/134; 3 | 58/130; 44.6 | |||
| Hyperlipidemia | 0.016 | 0.814 | 0.012 | ||||||
| No | 96/1391; 6.9 | 624/1295; 48.2 | 16/263; 6.1 | 119/247; 48.2 | 80/1128; 7.1 | 505/1048; 48.2 | |||
| Yes | 13/273; 4.8 | 104/260; 40 | 1/40; 2.5 | 18/39; 46.2 | 12/233; 5.2 | 86/221;38.9 | |||
| Type II Diabetes Mellitus | 0.002 | 0.008 | 0.019 | ||||||
| No | 98/1450; 6.8 | 654/1352; 48.4 | 15/275; 5.5 | 131/260; 50.4 | 83/1175; 7.1 | 523/1092; 47.9 | |||
| Yes | 11/214; 5.1 | 74/203; 36.5 | 2/28; 7.1 | 6/26; 23.1 | 9/186; 4.8 | 68/177: 38.4 | |||
| Urinary tract infection | <0.001 | 0.007 | <0.001 | ||||||
| No | 108/1617; 6.7 | 691/1509; 45.8 | 17/293; 5.8 | 128/46.4; 46.4 | 91/1324; 6.9 | 563/1233; 45.7 | |||
| Yes | 1/47; 2.1 | 37/46; 80.4 | 0 | 9/10; 90 | 1/37; 2.7 | 28/36; 77.8 | |||
| IMC > 30 | 0.310 | 0.927 | 0.348 | ||||||
| No | 48/667; 7.2 | 291/619; 47 | 7/127; 5.5 | 52/120; 43.3 | 41/540; 7.6 | 239/499; 47.9 | |||
| Yes | 20/333; 6 | 137/313; 43.8 | 2/56; 3.6 | 23/54; 42.6 | 18/277; 6.5 | 114/259; 44% | |||
| HTA | 0.175 | 0.590 | 0.212 | ||||||
| No | 18/352; 5.1 | 164/334; 49.1 | 2/70; 2.9 | 33/68; 48.5 | 16/282; 5.7 | 131/266; 49.2 | |||
| Yes | 82/1154; 7.1 | 481/1072; 44.9 | 14/204; 6.9 | 85/190; 44.7 | 68/950; 7.2 | 396/882; 44.9 | |||
| Total | Symptomatic | Asymptomatic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total Cancer/Patients (109/1664; 6.5%) | False-Positive PSA Rate 1 (728/1555; 46.8%) | p-value 2 | Total Cancer/Patients (17/303; 5.6) | False-Positive PSA Rate 1 (137/286; 47.9) | p-value 2 | Total Cancer/Patients (92/1361; 6.8) | False-Positive PSA Rate 1 (591/1269; 46.6) | p-Value 2 |
| Treatment | 0.003 | 0.611 | 0.003 | ||||||
| No | 1/334; 0.3 | 180/333; 54.1 | 0/59; 0 | 30/59; 50.8 | 1/275; 0.4 | 150/274; 54.7 | |||
| Yes | 108/1330; 8.1 | 548/1222; 44.8 | 17/244; 7 | 107/227; 47.1 | 91/1086; 8.4 | 441/995; 44.3 | |||
| Statins | 0.138 | 0.122 | 0.363 | ||||||
| No | 82/1201; 6.8 | 537/1119; 48 | 11/218; 5 | 105/207; 50.7 | 71/983; 7.2 | 432/912; 47.4 | |||
| Yes | 27/463; 5.8 | 191/436; 43.8 | 6/85; 7.1 | 32/79; 40.5 | 21/378; 5.6 | 159/357; 44.5 | |||
| Metformin | 0.415 | 0.059 | 0.979 | ||||||
| No | 101/1456; 6.1 | 629/1355; 46.4 | 16/267; 6 | 115/251; 45.8 | 85/1189; 7.1 | 514/1104; 46.6 | |||
| Yes | 8/208; 3.8 | 99/200; 49.5 | 1/36; 2.8 | 22/35; 62.9 | 7/172; 4.1 | 77/165; 46.7 | |||
| Treatment for BPH | 0.082 | 0.497 | 0.103 | ||||||
| No | 70/1305; 5.4 | 592/1235; 47.9 | 12/226; 5.3 | 105/214; 49.1 | 58/1079; 5.4 | 487/1021; 47.7 | |||
| Yes | 39/359; 10.9 | 136/320; 42.5 | 5/77; 6.5 | 32/72; 44.4 | 34/282; 12.1 | 104/248; 41.9 | |||
| Diuretic | 0.245 | 0.801 | 0.157 | ||||||
| No | 96/1482; 6.5 | 656/47.3 | 16/270; 5.9 | 121/254; 47.6 | 80/1212; 6.6 | 535/1132; 47.3 | |||
| Yes | 13/182; 7.1 | 72/169; 42.6 | 1/33/3 | 16/32; 50 | 12/149; 8.1 | 56/137; 40.9 | |||
| ASA | 0.234 | 0.481 | 0.329 | ||||||
| No | 95/1412; 6.7 | 625/1317; 47.5 | 11/266; 4.1 | 124/255; 48.6 | 84/1146; 7.3 | 501/1062; 47.2 | |||
| Yes | 14/252; 5.6 | 103/238; 43.3 | 6/37; 16.2 | 13/31; 41.9 | 8/215; 3.7 | 90/207; 43.5 | |||
| Gleason | p-Value | |||
|---|---|---|---|---|
| PSA Levels | 6 | 7 | 8–10 | |
| PSA (ng/mL) (median, IQR) | 6.66 (4.91–10.13) | 6.32 (5.15–8.89) | 13.37 (6.44–31.24) | 0.004 |
| Total | Without PCa | With PCa | |||
|---|---|---|---|---|---|
| Variables | PSA Levels (ng/mL) (Median, IQR) | PSA Levels (ng/mL) (Median, IQR) | p-Value | PSA Levels (ng/mL) (Median, IQR) | p-Value |
| Hyperlipidemia | 0.009 | 0.082 | |||
| • No | 4.53 (1.89–6.36) | 4.44 (1.67–6.01) | 8.20 (5.18–16.84) | ||
| • Yes | 4.27 (1.24–5.15) | 4.24 (1.18–5.13) | 5.87 (4.44–6.61) | ||
| Diabetes | 0.001 | 0.974 | |||
| • No | 4.53 (1.90–6.26) | 4.45 (1.73–5.91) | 6.82 (5.08–16.80) | ||
| • Yes | 4.19 (1–5.39) | 4.04 (0.92–5.08) | 7.98 (5.93–14.45) | ||
| UTI | <0.001 | 0.993 | |||
| • No | 4.45 (1.69–6.07) | 4.36 (1.15–5.77) | 6.86 (5.09–16.54) | ||
| • Yes | 5.71 (4.64–10.33) | 5.70 (4.64–9.51) | 10.33 | ||
| Pharmacological treatment | <0.001 | 0.484 | |||
| • No | 4.70 (2.29–6.35) | 4.70 (2.29–6.34) | 5.68 (5.08–6.28) | ||
| • Yes | 4.39 (1.64–6.07) | 4.32 (1.41–5.74) | 7.17 (5.11–16.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumbreras, B.; Parker, L.A.; Caballero-Romeu, J.P.; Gómez-Pérez, L.; Puig-García, M.; López-Garrigós, M.; García, N.; Hernández-Aguado, I. Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data. Cancers 2023, 15, 261. https://doi.org/10.3390/cancers15010261
Lumbreras B, Parker LA, Caballero-Romeu JP, Gómez-Pérez L, Puig-García M, López-Garrigós M, García N, Hernández-Aguado I. Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data. Cancers. 2023; 15(1):261. https://doi.org/10.3390/cancers15010261
Chicago/Turabian StyleLumbreras, Blanca, Lucy Anne Parker, Juan Pablo Caballero-Romeu, Luis Gómez-Pérez, Marta Puig-García, Maite López-Garrigós, Nuria García, and Ildefonso Hernández-Aguado. 2023. "Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data" Cancers 15, no. 1: 261. https://doi.org/10.3390/cancers15010261
APA StyleLumbreras, B., Parker, L. A., Caballero-Romeu, J. P., Gómez-Pérez, L., Puig-García, M., López-Garrigós, M., García, N., & Hernández-Aguado, I. (2023). Variables Associated with False-Positive PSA Results: A Cohort Study with Real-World Data. Cancers, 15(1), 261. https://doi.org/10.3390/cancers15010261







