3T-MRI Artificial Intelligence in Patients with Invasive Breast Cancer to Predict Distant Metastasis Status: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Imaging and Data Analysis
- T2-weighted axial single-shot fast spin echo sequence with a modified Dixon technique (IDEAL) for intravoxel fat-water separation (TR/TE 3500–5200/120–135 ms, slice thickness 3.5 mm).
- Diffusion-weighted axial single-shot echo-planar with fat suppression sequence.
- (TR/TE 2700/58 ms, slice thickness 5 mm) with diffusion-sensitizing gradient with a b-value of 0, 500, and 1000 s/mm2.
- Dynamic 3D-T1w axial and sagittal gradient echo sequence with fat suppression after injection of 0.1 mmol/kg body weight of Gadoteric acid (Dotarem®, Guerbet S.p.A., Villepinte France, or Claricyclic®, GE Healthcare S.r.l, Chicago, IL, USA) at a rate of 2 mL/sec followed by a bolus of 15 mL saline flush (TR/TE 4/2 ms, slice thickness 2.4 mm), before, and five to ten times after intravenous contrast medium injection.
- Location on the breast quadrant;
- Margins: regular, irregular, lobulated, speculated, non-mass;
- Size (mm);
- Morphology: round, oval, or irregular.
2.3. Histologic Characteristics
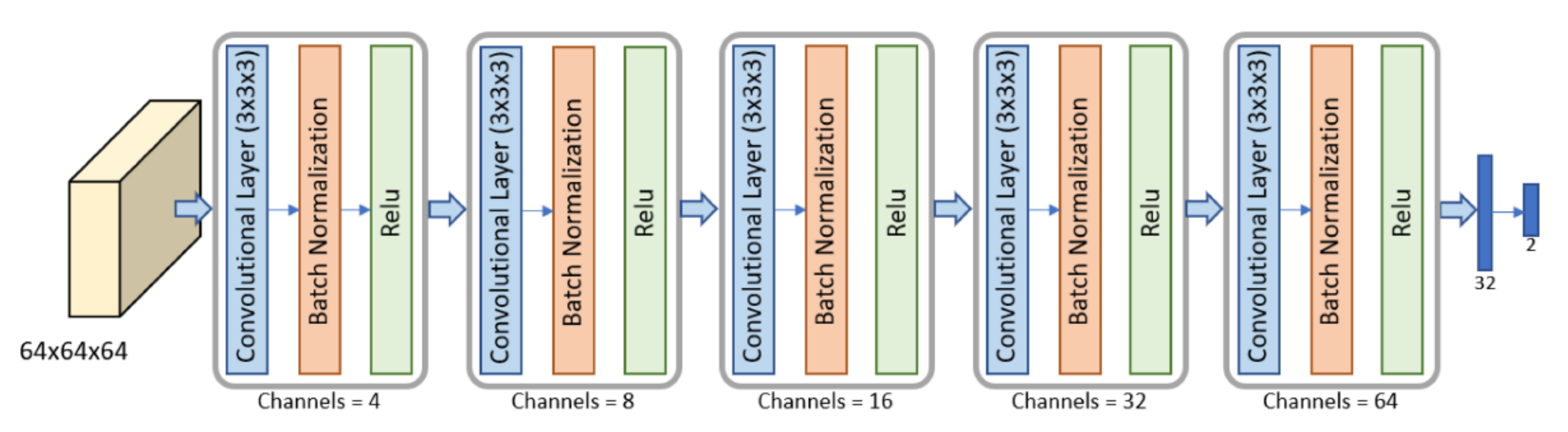
2.4. Segmentation and Pre-Processing
2.5. Volumes Extraction
2.6. Metastasis Prevision Assessment
2.7. Statistical Analysis
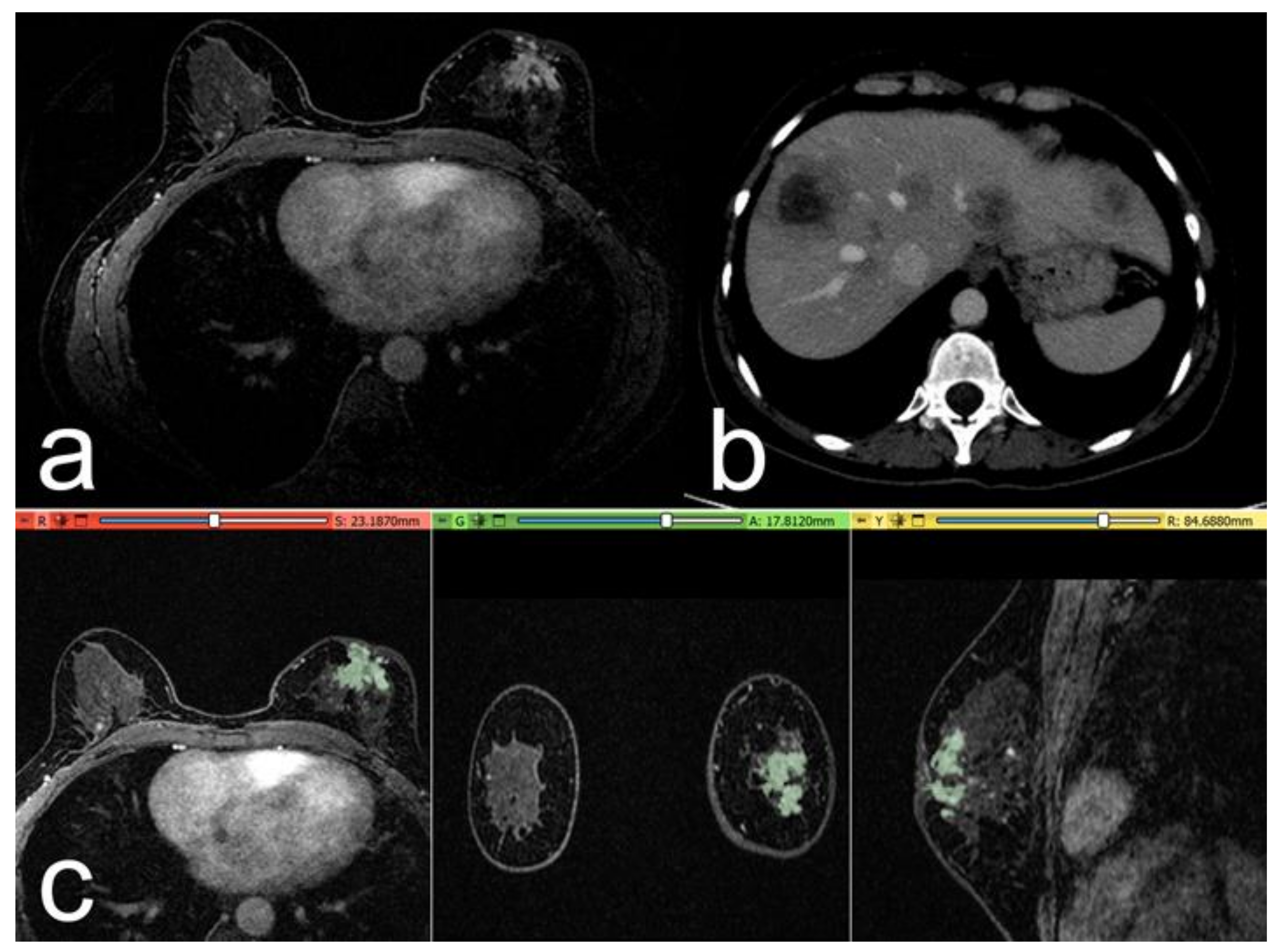
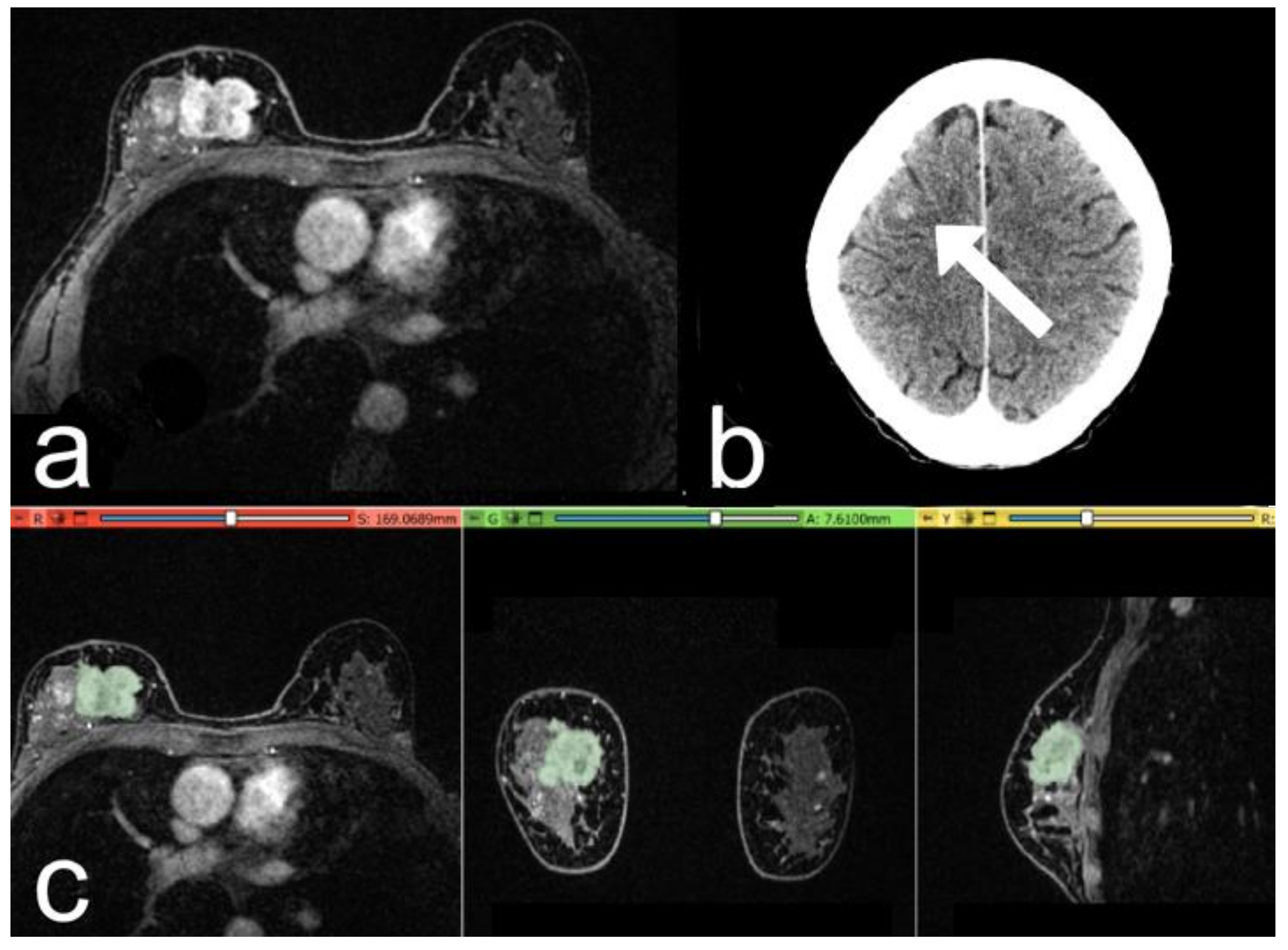
3. Results
- -
- Patients with distant metastases at follow-up (39/157 patients, 40 lesions);
- -
- Patients negative for distant metastasis (control group, 118/157 patients, 120 lesions).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Breast Cancer Facts and Figures 2013–2014; American Cancer Society, Inc.: Atlanta, GA, USA, 2013. [Google Scholar]
- Tao, L.; Chu, L.; Wang , L.I.; Moy, L.; Brammer, M.; Song, C.; Green, M.; Kurian, A.W.; Gomez, S.L.; Clarke, C.A. Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control. 2016, 27, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, C.J.; Eckel, R.; van de Poll-Franse, L.V.; Coebergh, J.W.W.; Nortier, J.W.; Hölzel, D.; Breed, W.P.; Engel, J. Unfavourable pattern of metastases in M0 breast cancer patients during 1978–2008: A population-based analysis of the Munich Cancer Registry. Breast Cancer Res. Treat. 2011, 128, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.J.; Marinovich, M.L.; Patterson, J.A.; Wilcken, N.; Kiely, B.E.; Gebski, V.; Crossing, S.; Roder, D.M.; Gattellari, M.; Houssami, N. Incidence of metastatic breast cancer in an Australian population-based cohort of women with non-metastatic breast cancer at diagnosis. Med. J. Aust. 2012, 196, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology. 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, V.; Santucci, D.; Guerrieri, D.; Drudi, F.M.; Meggiorini, M.L.; de Felice, C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur. J. Radiol. 2014, 83, 2144–2150. [Google Scholar] [CrossRef]
- Santucci, D.; Faiella, E.; Calabrese, A.; Beomonte Zobel, B.; Ascione, A.; Cerbelli, B.; Iannello, G.; Soda, P.; de Felice, C. On the Additional Information Provided by 3T-MRI ADC in Predicting Tumor Cellularity and Microscopic Behavior. Cancers 2021, 13, 5167. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer. 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.M.; Wang, H.T.; Yu, T. The Application of Radiomics in Breast MRI: A Review. Technol. Cancer Res. Treat. 2020, 19, 1533033820916191. [Google Scholar] [CrossRef]
- Calabrese, A.; Santucci, D.; Landi, R.; Beomonte Zobel, B.; Faiella, E.; de Felice, C. Radiomics MRI for lymph node status prediction in breast cancer patients: The state of art. J. Cancer Res. Clin. Oncol. 2021, 147, 1587–1597. [Google Scholar] [CrossRef]
- Santucci, D.; Faiella, E.; Cordelli, E.; Calabrese, A.; Landi, R.; de Felice, C.; Beomonte Zobel, B.; Grasso, R.F.; Iannello, G.; Soda, P. The Impact of Tumor Edema on T2-Weighted 3T-MRI Invasive Breast Cancer Histological Characterization: A Pilot Radiomics Study. Cancers 2021, 13, 4635. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ha, W.; Vahedi, Z. Automatic Breast Tumor Diagnosis in MRI Based on a Hybrid CNN and Feature-Based Method Using Improved Deer Hunting Optimization Algorithm. Comput. Intell. Neurosci. 2021, 2021, 5396327. [Google Scholar] [CrossRef]
- Liu, M.Z.; Mutasa, S.; Chang, P.; Siddique, M.; Jambawalikar, S.; Ha, R. A novel CNN algorithm for pathological complete response prediction using an I-SPY TRIAL breast MRI database. Magn. Reson. Imaging 2020, 73, 148–151. [Google Scholar] [CrossRef]
- Nguyen, S.; Polat, D.; Karbasi, P.; Moser, D.; Wang, L.; Hulsey, K.; Çobanoğlu, M.C.; Dogan, B.; Montillo, A. Preoperative Prediction of Lymph Node Metastasis from Clinical DCE MRI of the Primary Breast Tumor Using a 4D CNN. Med. Image Comput. Comput. Assist. Interv. 2020, 12262, 326–334. [Google Scholar] [CrossRef]
- Santucci, D.; Faiella, E.; Gravina, M.; Cordelli, E.; de Felice, C.; Beomonte Zobel, B.; Iannello, G.; Sansone, C.; Soda, P. CNN-Based Approaches with Different Tumor Bounding Options for Lymph Node Status Prediction in Breast DCE-MRI. Cancers 2022, 14, 4574. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.H.; Lin, Y.; Chan, S.; Zhou, J.; Chow, D.; Chang, P.; Kwong, T.; Yeh, D.C.; Wang, X.; et al. Prediction of breast cancer molecular subtypes on DCE-MRI using convolutional neural network with transfer learning between two centers. Eur. Radiol. 2021, 31, 2559–2567. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas: Breast Imaging Re-Porting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Gennari, A.; Conte, P.; Rosso, R.; Orlandini, C.; Bruzzi, P. Survival of metastatic breast carcinoma patients over a 20-year period: A retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005, 104, 1742–1750. [Google Scholar] [CrossRef]
- Dafni, U.; Grimani, I.; Xyrafas, A.; Eleftheraki, A.G.; Fountzilas, G. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res. Treat. 2010, 119, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Mauri, D.; Polyzos, N.P.; Salanti, G.; Pavlidis, N.; Ioannidis, J.P. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J. Natl. Cancer Inst. 2008, 100, 1780–1791. [Google Scholar] [CrossRef] [Green Version]
- Daniels, B.; Kiely, B.E.; Lord, S.J.; Houssami, N.; Lu, C.Y.; Ward, R.L.; Pearson, S.A. Trastuzumab for metastatic breast cancer: Real world outcomes from an Australian whole-of-population cohort (2001–2016). Breast 2018, 38, 7–13. [Google Scholar] [CrossRef]
- Press, D.J.; Miller, M.E.; Liederbach, E.; Yao, K.; Huo, D. De novo metastasis in breast cancer: Occurrence and overall survival stratified by molecular subtype. Clin. Exp. Metastasis 2017, 34, 457–465. [Google Scholar] [CrossRef]
- Uhr, J.W.; Pantel, K. Controversies in clinical cancer dormancy. Proc. Natl. Acad. Sci. USA 2011, 108, 12396–12400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, S.; Tubiana, M.; Le, M.G.; Valleron, A.J.; Mouriesse, H.; Contesso, G.; Sarrazin, D. Breast cancer: Relationship between the size of the primary tumour and the probability of metastatic dissemination. Br. J. Cancer 1984, 49, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santucci, D.; Faiella, E.; Cordelli, E.; Sicilia, R.; de Felice, C.; Zobel, B.B.; Iannello, G.; Soda, P. 3T MRI-Radiomic Approach to Predict for Lymph Node Status in Breast Cancer Patients. Cancers 2021, 13, 2228. [Google Scholar] [CrossRef]
- Sopik, V.; Narod, S.A. The relationship between tumour size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Res. Treat. 2018, 170, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, J.J.; Hwangbo, L.; Kang, T.; Park, H. Diffusion-weighted Imaging of Invasive Breast Cancer: Relationship to Distant Metastasis-free Survival. Radiology 2019, 291, 300–307. [Google Scholar] [CrossRef]
- Xiao, W.; Zheng, S.; Yang, A.; Zhang, X.; Zou, Y.; Tang, H.; Xie, X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: A population-based study. Cancer Manag. Res. 2018, 10, 5329–5338. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Xia, L.; Sun, S. A pre-operative MRI-based brain metastasis risk-prediction model for triple-negative breast cancer. Gland Surg. 2021, 10, 2715–2723. [Google Scholar] [CrossRef]

| Variation | Study Population | Patients with Metastasis | Control Group | p-Value | ||
|---|---|---|---|---|---|---|
| Kinetic Curve | I | n | 21 | 7 | 14 | |
| % | 13.1% | 17.5% | 11.7% | |||
| II | n | 71 | 15 | 56 | ||
| 0.962 | ||||||
| % | 44.4% | 37.5% | 46.7% | |||
| III | n | 68 | 18 | 50 | ||
| % | 42.5% | 45.0% | 41.7% | |||
| Margins | Regular | n | 4 | 0 | 4 | |
| % | 2.5% | 0.0% | 3.3% | |||
| Irregular | n | 86 | 21 | 65 | ||
| % | 53.8% | 52.5% | 54.2% | |||
| Lobulated | n | 25 | 7 | 18 | 0.349 | |
| % | 15.6% | 17.5% | 15.0% | |||
| Spiculated | n | 33 | 6 | 27 | ||
| % | 20.6% | 15.0% | 22.5% | |||
| Non-mass | n | 12 | 6 | 6 | ||
| % | 7.5% | 15.0% | 5.0% | |||
| Variation | Study Population | Patients with Metastasis | Control Group | p-Value | ||
|---|---|---|---|---|---|---|
| Histology | IDC | n | 127 | 30 | 97 | |
| % | 79.4% | 75.0% | 80.8% | |||
| ILC | n | 33 | 10 | 23 | 0.433 | |
| % | 20.6% | 25.0% | 19.2% | |||
| Molecular subtype | Luminal A | n | 59 | 11 | 48 | |
| % | 36.9% | 27.5% | 40.0% | |||
| Luminal B | n | 69 | 18 | 51 | ||
| % | 43.1% | 45.0% | 42.5% | |||
| HER2+ | n | 13 | 3 | 10 | 0.079 | |
| % | 8.1% | 7.5% | 8.3% | |||
| Triple negative | n | 19 | 8 | 11 | ||
| 11.9% | 20.0% | 9.2% | ||||
| ER Status | Negative | n | 31 | 12 | 19 | |
| % | 19.4% | 30.0% | 15.8% | |||
| Positive | n | 129 | 28 | 101 | 0.195 | |
| % | 80.6% | 70.0% | 84.2% | |||
| PgR Status | Negative | n | 58 | 23 | 35 | |
| % | 36.3% | 57.5% | 29.2% | |||
| Positive | n | 102 | 17 | 85 | 0.001 * | |
| % | 63.7% | 42.5% | 70.8% | |||
| HER2 Status | Negative | n | 137 | 32 | 105 | |
| % | 85.6% | 80.0% | 87.5% | |||
| Positive | n | 23 | 8 | 15 | 0.044 * | |
| 14.4% | 20.0% | 12.5% | ||||
| Grade | 1 | n | 19 | 4 | 15 | |
| % | 11.9% | 10.0% | 12.5% | |||
| 2 | n | 71 | 15 | 56 | 0.225 | |
| % | 44.4% | 37.5% | 46.7% | |||
| 3 | n | 70 | 21 | 49 | ||
| % | 43.8% | 52.5% | 40.8% |
| Variation | Study Population | Patients with Metastasis | Control Group | p-Value | ||
|---|---|---|---|---|---|---|
| Menopause | Pre- | n | 71 | 17 | 54 | |
| % | 44.4% | 42.5% | 45.0% | |||
| Post- | n | 89 | 23 | 66 | 0.784 | |
| % | 55.6% | 57.5% | 55.0% | |||
| Hormone Therapy | None | n | 109 | 39 | 35 | |
| % | 90.8% | 97.5% | 29.2% | |||
| Positive | n | 11 | 1 | 85 | 0.168 | |
| % | 9.2% | 2.5% | 70.8% | |||
| Family History | No relatives | n | 118 | 32 | 86 | |
| % | 73.8% | 80.0% | 71.7% | |||
| ≥1 relative with BC | n | 42 | 8 | 34 | 0.303 | |
| 26.3% | 20.0% | 28.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, A.; Santucci, D.; Gravina, M.; Faiella, E.; Cordelli, E.; Soda, P.; Iannello, G.; Sansone, C.; Zobel, B.B.; Catalano, C.; et al. 3T-MRI Artificial Intelligence in Patients with Invasive Breast Cancer to Predict Distant Metastasis Status: A Pilot Study. Cancers 2023, 15, 36. https://doi.org/10.3390/cancers15010036
Calabrese A, Santucci D, Gravina M, Faiella E, Cordelli E, Soda P, Iannello G, Sansone C, Zobel BB, Catalano C, et al. 3T-MRI Artificial Intelligence in Patients with Invasive Breast Cancer to Predict Distant Metastasis Status: A Pilot Study. Cancers. 2023; 15(1):36. https://doi.org/10.3390/cancers15010036
Chicago/Turabian StyleCalabrese, Alessandro, Domiziana Santucci, Michela Gravina, Eliodoro Faiella, Ermanno Cordelli, Paolo Soda, Giulio Iannello, Carlo Sansone, Bruno Beomonte Zobel, Carlo Catalano, and et al. 2023. "3T-MRI Artificial Intelligence in Patients with Invasive Breast Cancer to Predict Distant Metastasis Status: A Pilot Study" Cancers 15, no. 1: 36. https://doi.org/10.3390/cancers15010036
APA StyleCalabrese, A., Santucci, D., Gravina, M., Faiella, E., Cordelli, E., Soda, P., Iannello, G., Sansone, C., Zobel, B. B., Catalano, C., & de Felice, C. (2023). 3T-MRI Artificial Intelligence in Patients with Invasive Breast Cancer to Predict Distant Metastasis Status: A Pilot Study. Cancers, 15(1), 36. https://doi.org/10.3390/cancers15010036








