Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics, Simulation, and Planning
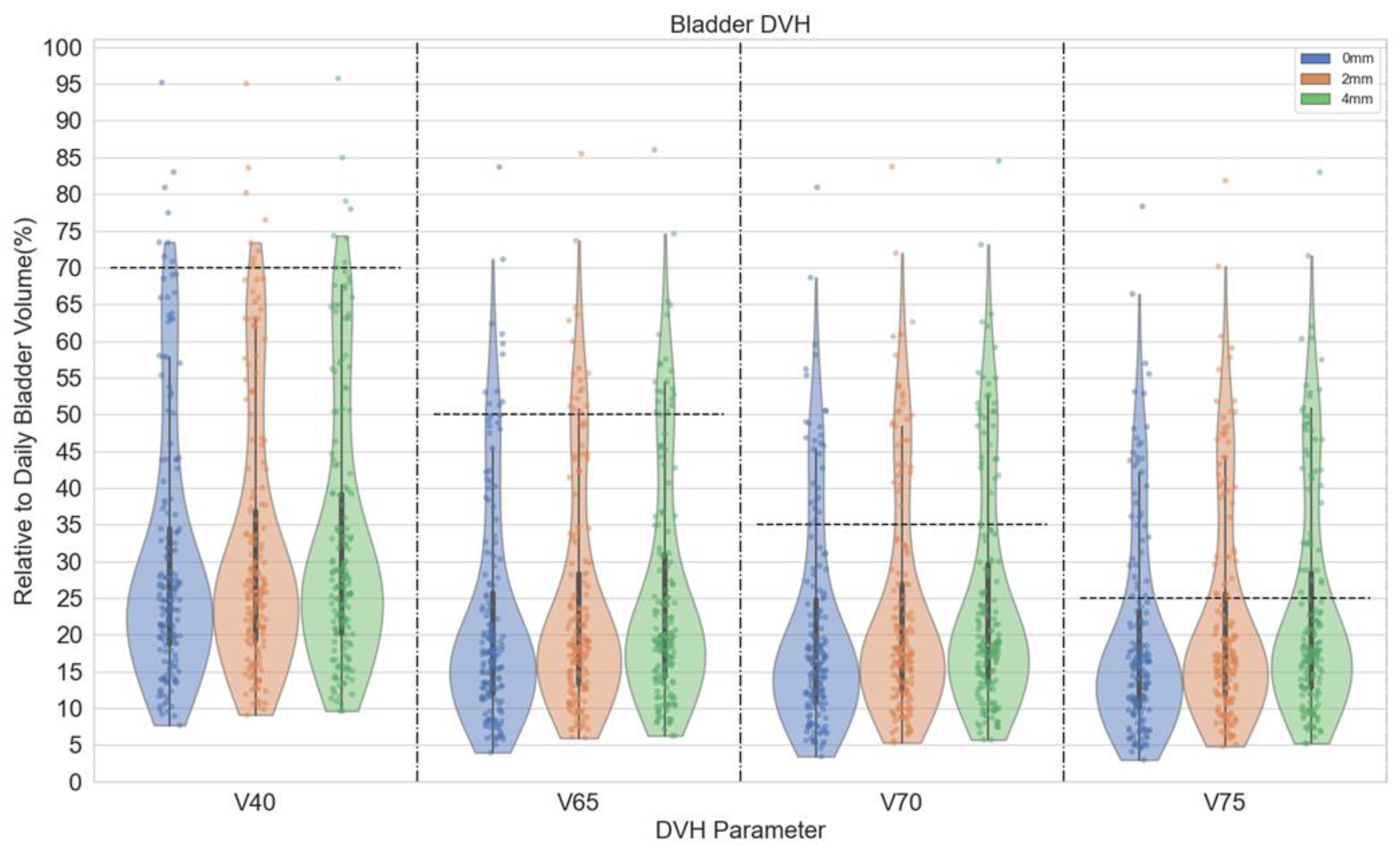
2.2. Data Acquisition and Analysis
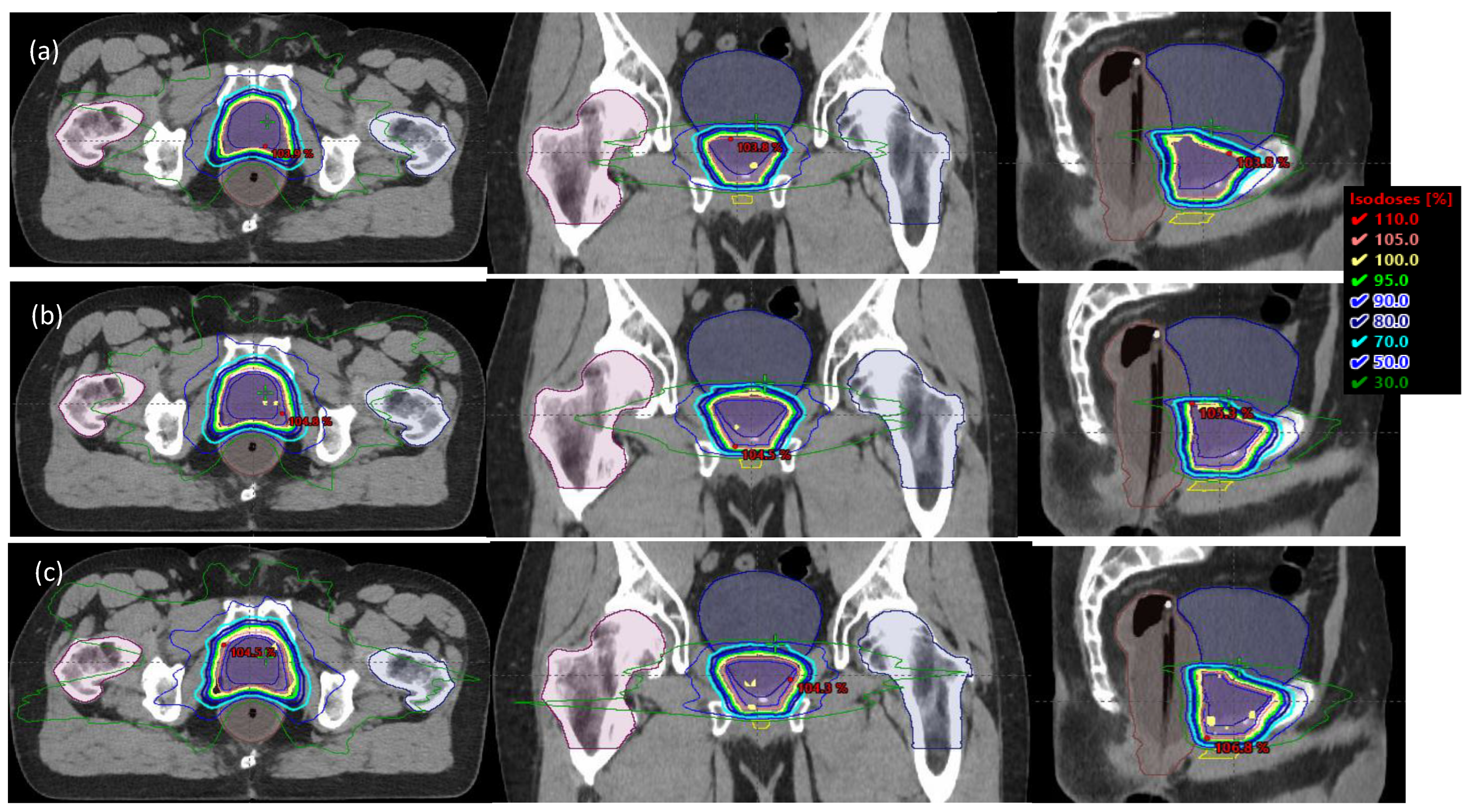
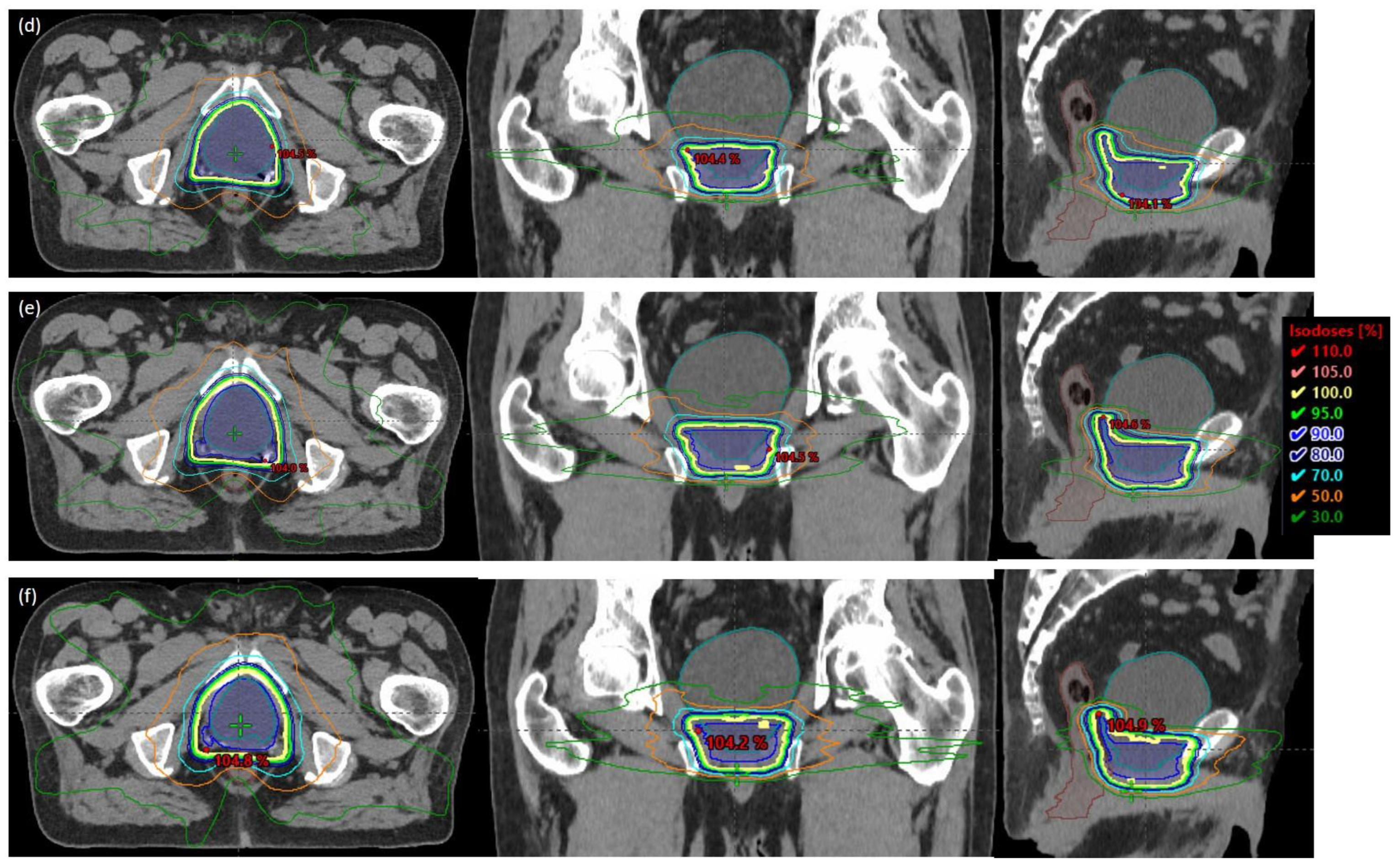
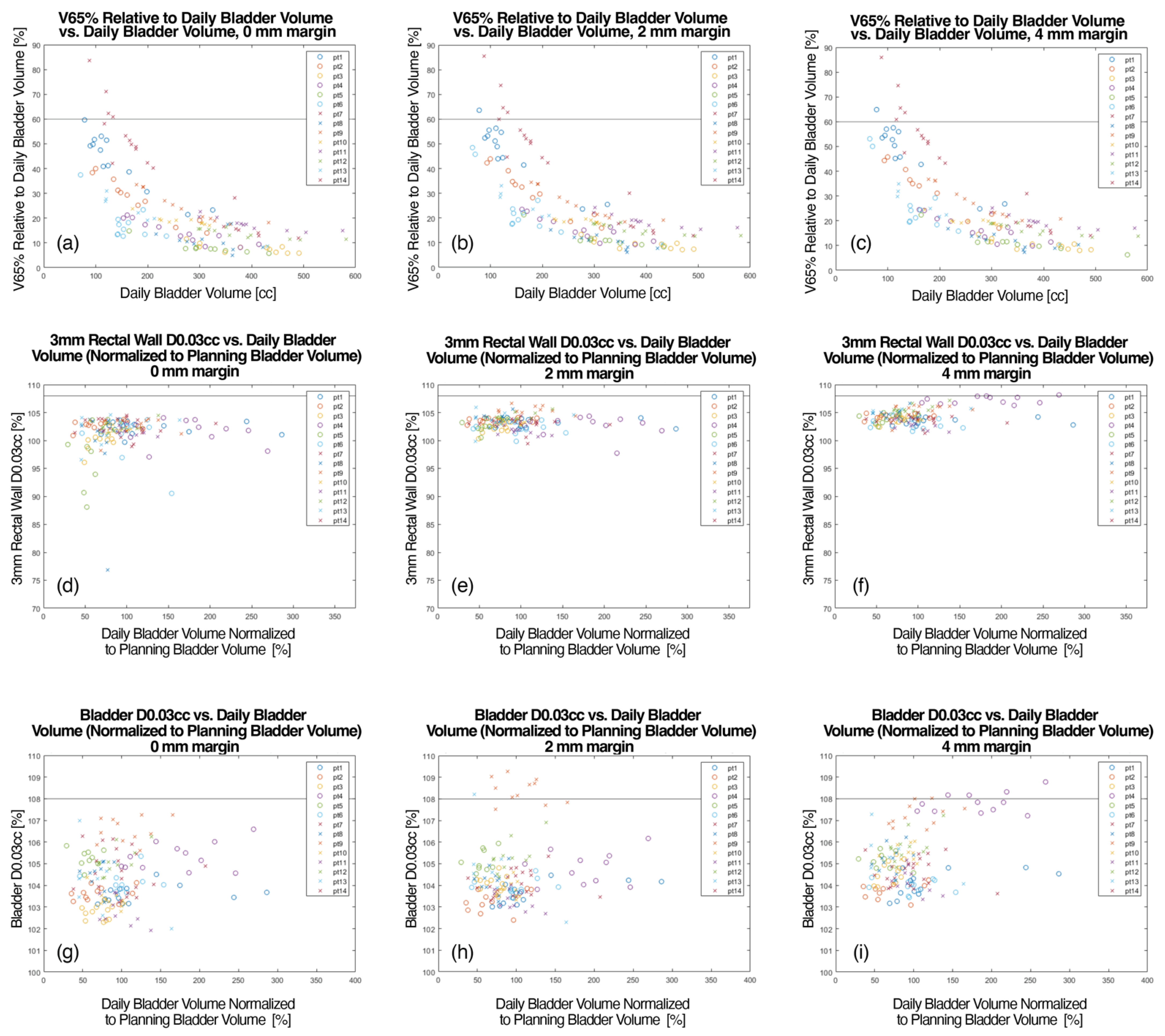
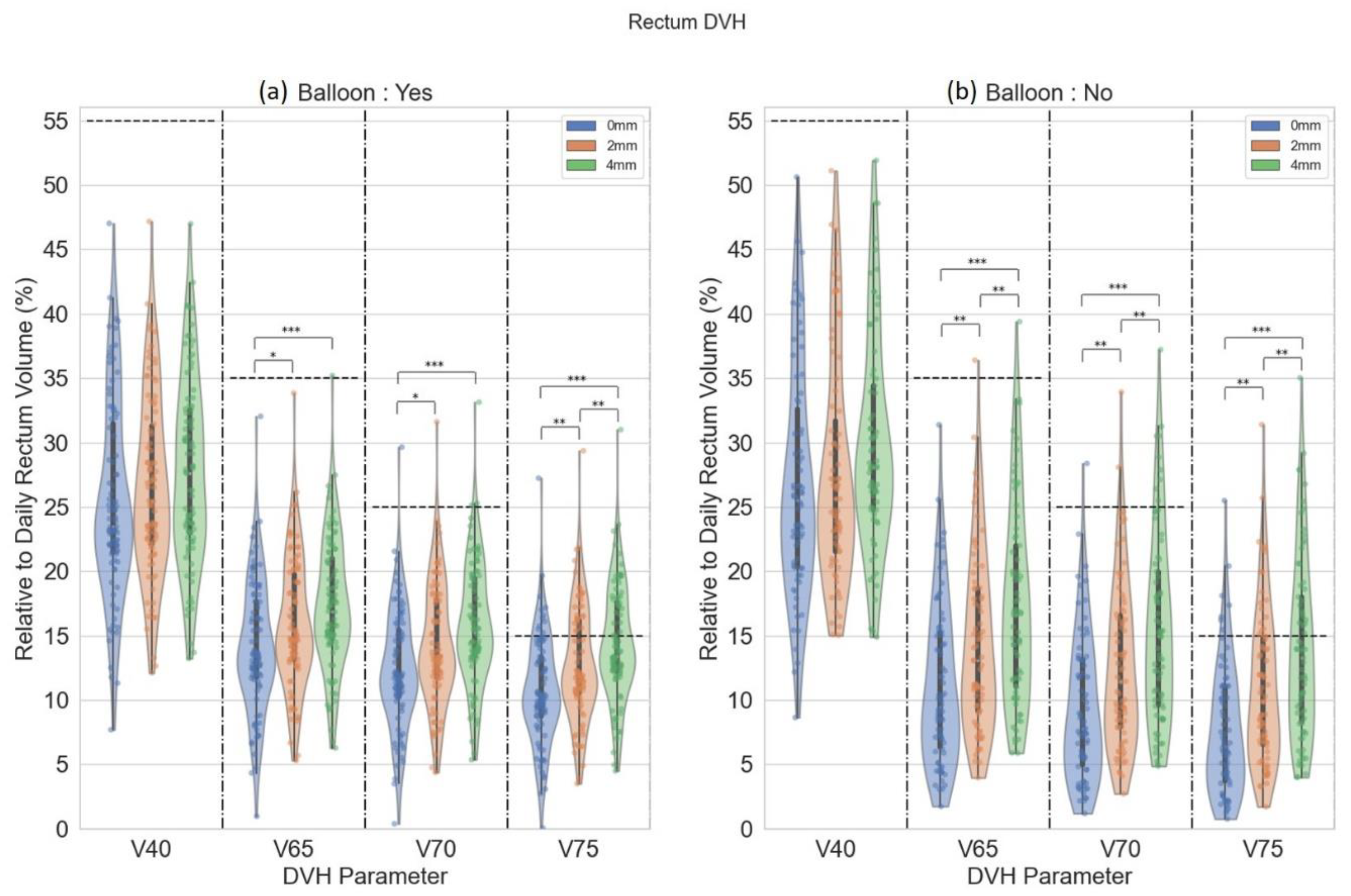
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym List | |
| ANOVA | analysis of variance |
| CBCT | cone beam computed tomography |
| CTV | Clinical target volume |
| DVH | dose volume histogram |
| ERB | endorectal balloon |
| FDK | Feldkamp-Davis-Kress |
| FROGG | Faculty of Radiation Oncology Genito-Urinary Group |
| GU | genitourinary |
| HU | hounsfield Unit |
| iCBCT | iterative cone beam computed tomography |
| IGRT | image-guided radiotherapy |
| MRI | magnetic resonance imaging |
| OAR | organ-at-risk |
| PTV | planning target volume |
| RT | radiotherapy |
| SBRT | stereotactic body radiotherapy |
| VMAT | volumetric modulated arc therapy |
References
- Pisansky, T.M.; Thampson, I.M.; Valicenti, R.K.; D’Amico, A.V.; Selvarajah, S. Adjuvant and alvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J. Urol. 2019, 202, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Michalski, J.M.; Lawton, C.; El Naqa, I.; Ritter, M.; O’Meara, E.; Seider, M.J.; Lee, W.R.; Rosenthal, S.A.; Pisansky, T.; Catton, C.; et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poortmans, P.; Bossi, A.; Vandeputte, K.; Bosset, M.; Miralbell, R.; Maingon, P.; Boehmer, D.; Budiharto, T.; Symon, Z.; van den Bergh, A.C. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother. Oncol. 2007, 84, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, M.A.; Kneebone, A.B.; Lehman, M.; Wiltshire, K.L.; Millar, J.L.; Mukherjee, R.K.; Shakespeare, T.P.; Tai, K.-H. Post-prostatectomy radiation therapy: Consensus guidelines of the Australian and New Zealand radiation oncology Genito-urinary group. Radiother. Oncol. 2008, 88, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, K.L.; Brock, K.K.; Haider, M.A.; Zwahlen, D.; Kong, V.; Chan, E.; Moseley, J.; Bayley, A.; Catton, C.; Chung, P.W. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1090–1099. [Google Scholar] [CrossRef]
- Haworth, A.; Paneghel, A.; Herschtal, A.; Duchesne, G.; Williams, S.; Tai, K.; Kron, T.; Roxby, P.; Soteriou, S.; Laferlita, M. Verification of target position in the post-prostatectomy cancer patient using cone beam CT. J. Med. Imaging Radiat. Oncol. 2009, 53, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Palma, D.A.; Scott, D.; McGregor, D.; Gaede, S.; Yartsev, S.; Bauman, G.; Louie, A.V.; Rodrigues, G. Inter- and intrafraction uncertainty in prostate bed image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, D.; Langen, K.M.; Mageras, G.; Dawson, L.; Di Yan, D.; Mundt, A.J.; Fraass, B.J.R.o. Assuring Safety and Quality in Image-guided Delivery of Radiation Therapy. Radiat. Oncol. 2013, 32, 33. [Google Scholar]
- Ramiandrisoa, F.; Duvergé, L.; Castelli, J.; Nguyen, T.D.; Servagi-Vernat, S.; de Crevoisier, R. Clinical to planning target volume margins in prostate cancer radiotherapy. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2016, 20, 629–639. [Google Scholar] [CrossRef]
- Gill, S.; Isiah, R.; Adams, R.; Dang, K.; Siva, S.; Tai, K.H.; Kron, T.; Foroudi, F. Conventional margins not sufficient for post-prostatectomy prostate bed coverage: An analysis of 477 cone-beam computed tomography scans. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 110, 235–239. [Google Scholar] [CrossRef]
- Yoon, S.; Cao, M.; Aghdam, N.; Shabsovich, D.; Kahlon, S.; Ballas, L.; Collins, S.; Steinberg, M.L.; Kishan, A.U. Prostate bed and organ-at-risk deformation: Prospective volumetric and dosimetric data from a phase II trial of stereotactic body radiotherapy after radical prostatectomy. Radiother. Oncol. 2020, 148, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Buyyounouski, M.K.; Pugh, S.; Chen, R.C.; Mann, M.; Kudchadker, R.; Konski, A.A.; Mian, O.Y.; Michalski, J.M.; Vigneault, E.; Valicenti, R.K.; et al. Primary Endpoint Analysis of a Randomized Phase III Trial of Hypofractionated vs. Conventional Post-Prostatectomy Radiotherapy: NRG Oncology GU003. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, S2–S3. [Google Scholar] [CrossRef]
- Ma, T.M.; Ballas, L.K.; Wilhalme, H.; Sachdeva, A.; Chong, N.; Sharma, S.; Yang, T.; Basehart, V.; Reiter, R.E.; Saigal, C.; et al. Quality-of-Life Outcomes and Toxicity Profile among Patients with Localized Prostate Cancer after Radical Prostatectomy Treated with Stereotactic Body Radiation: The SCIMITAR Multicenter Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.S.; Voss, M.M.; Toesca, D.A.S.; Daniels, T.; Golafshar, M.A.; Keole, S.R.; Wong, W.W.; Rwigema, J.-C.; Davis, B.; Schild, S.E.; et al. Preliminary Analysis of a Phase II Trial of Stereotactic Body Radiotherapy for Prostate Cancer with High-Risk Features after Radical Prostatectomy. Adv. Radiat. Oncol. 2022, 101143. [Google Scholar] [CrossRef]
- Gardner, S.J.; Mao, W.H.; Liu, C.; Aref, I.; Elshaikh, M.; Lee, J.K.; Pradhan, D.; Movsas, B.; Chetty, I.J.; Siddiqui, F. Improvements in CBCT Image Quality Using a Novel Iterative Reconstruction Algorithm: A Clinical Evaluation. Adv. Radiat. Oncol. 2019, 4, 390–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.; Penoncello, G.P.; Hobbis, D.; Harrington, D.P.; Rong, Y. Technical Note: Characterization of novel iterative reconstructed cone beam CT images for dose tracking and adaptive radiotherapy on L-shape linacs. Med. Phys. 2022, 1–18. [Google Scholar] [CrossRef]
- van Herk, M.; Remeijer, P.; Rasch, C.; Lebesque, J.V. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. 2000, 47, 1121–1135. [Google Scholar] [CrossRef]
- van Herk, M.; Remeijer, P.; Lebesque, J.V. Inclusion of geometric uncertainties in treatment plan evaluation. Int. J. Radiat. Oncol. 2002, 52, 1407–1422. [Google Scholar] [CrossRef]
- Laughlin, B.S.; Lo, S.; Vargas, C.E.; DeWees, T.A.; Van der Walt, C.; Tinnon, K.; Beckett, M.; Hobbis, D.; Schild, S.E.; Wong, W.W.; et al. Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 1: Consistent Organs at Risk Management with Advanced Image Guidance. Cancers 2023, 15, 16. [Google Scholar] [CrossRef]
- Bell, L.J.; Cox, J.; Eade, T.; Rinks, M.; Herschtal, A.; Kneebone, A. Determining optimal planning target volume and image guidance policy for post-prostatectomy intensity modulated radiotherapy. Radiat. Oncol. 2015, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.J.; Eade, T.; Hruby, G.; Bromley, R.; Kneebone, A. Implementing daily soft tissue image guidance with reduced margins for post-prostatectomy radiotherapy: Research-based changes to clinical practice. J. Med. Radiat. Sci. 2019, 66, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Both, S.; Wang, K.K.-H.; Plastaras, J.P.; Deville, C.; Bar Ad, V.; Tochner, Z.; Vapiwala, N. Real-Time Study of Prostate Intrafraction Motion During External Beam Radiotherapy with Daily Endorectal Balloon. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Lamb, J.; Casado, M.; Wang, X.; Ma, T.M.; Low, D.; Sheng, K.; Yang, Y.; Gao, Y.; Basehart, V.; et al. Magnetic resonance imaging-guided versus computed tomography-guided stereotactic body radiotherapy for prostate cancer (MIRAGE): Interim analysis of a phase III randomized trial. J. Clin. Oncol. 2022, 40, 255. [Google Scholar] [CrossRef]
- Cao, M.; Gao, Y.; Yoon, S.M.; Yang, Y.; Sheng, K.; Ballas, L.K.; Basehart, V.; Sachdeva, A.; Felix, C.; Low, D.A.; et al. Interfractional Geometric Variations and Dosimetric Benefits of Stereotactic MRI Guided Online Adaptive Radiotherapy (SMART) of Prostate Bed after Radical Prostatectomy: Post-Hoc Analysis of a Phase II Trial. Cancers 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Bharat, S.; Michalski, J.M.; Gay, H.A.; Hou, W.-H.; Parikh, P.J. Adaptive Radiation Therapy for Postprostatectomy Patients Using Real-Time Electromagnetic Target Motion Tracking During External Beam Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.J.; Eade, T.; Hruby, G.; Bromley, R.; Kneebone, A. Intra-fraction displacement of the prostate bed during post-prostatectomy radiotherapy. Radiat. Oncol. 2021, 16, 20. [Google Scholar] [CrossRef]
- Vargas, C.; Saito, A.I.; Hsi, W.C.; Indelicato, D.; Falchook, A.; Zengm, Q.; Oliver, K.; Keole, S.; Dempsey, J. Cine-magnetic resonance imaging assessment of intrafraction motion for prostate cancer patients supine or prone with and without a rectal balloon. Am. J. Clin. Oncol. 2010, 33, 11–16. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laughlin, B.S.; Yu, N.Y.; Lo, S.; Duan, J.; Welchel, Z.; Tinnon, K.; Beckett, M.; Schild, S.E.; Wong, W.W.; Keole, S.R.; et al. Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance. Cancers 2023, 15, 40. https://doi.org/10.3390/cancers15010040
Laughlin BS, Yu NY, Lo S, Duan J, Welchel Z, Tinnon K, Beckett M, Schild SE, Wong WW, Keole SR, et al. Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance. Cancers. 2023; 15(1):40. https://doi.org/10.3390/cancers15010040
Chicago/Turabian StyleLaughlin, Brady S., Nathan Y. Yu, Stephanie Lo, Jingwei Duan, Zachary Welchel, Katie Tinnon, Mason Beckett, Steven E. Schild, William W. Wong, Sameer R. Keole, and et al. 2023. "Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance" Cancers 15, no. 1: 40. https://doi.org/10.3390/cancers15010040
APA StyleLaughlin, B. S., Yu, N. Y., Lo, S., Duan, J., Welchel, Z., Tinnon, K., Beckett, M., Schild, S. E., Wong, W. W., Keole, S. R., Rwigema, J.-C. M., Vargas, C. E., & Rong, Y. (2023). Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy—Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance. Cancers, 15(1), 40. https://doi.org/10.3390/cancers15010040






