Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Galectin-3 in Colorectal Cancer Development and Progression
2.1. Role in Human Intestinal Inflammation and Colorectal Cancer
2.2. Direct Effects on Tumor Cells
2.3. Contribution to Colonic Inflammation and Immunosuppression
3. Galectin-3 Targeting by Bioactive Food Compounds in Colorectal Cancer Prevention and Therapy
3.1. Non-Digestible Carbohydrates
3.2. Polyphenols
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Zheng, Z.; Huang, X.; Yang, L.; Liu, J.; Wang, K.; Zhang, Y. Pectic Polysaccharide from Smilax China L. Ameliorated Ulcerative Colitis by Inhibiting the Galectin-3/NLRP3 Inflammasome Pathway. Carbohydr. Polym. 2022, 277, 118864. [Google Scholar] [CrossRef]
- Zhou, E.; Rifkin, S. Colorectal Cancer and Diet: Risk Versus Prevention, is Diet an Intervention? Gastroenterol. Clin. North Am. 2021, 50, 101–111. [Google Scholar] [CrossRef]
- Morrow, L.; Greenwald, B. Healthy Food Choices, Physical Activity, and Screening Reduce the Risk of Colorectal Cancer. Gastroenterol. Nurs. 2022, 45, 113–119. [Google Scholar] [CrossRef]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-Analyses of Prospective Observational Studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef]
- Carroll, K.L.; Fruge, A.D.; Heslin, M.J.; Lipke, E.A.; Greene, M.W. Diet as a Risk Factor for Early-Onset Colorectal Adenoma and Carcinoma: A Systematic Review. Front. Nutr. 2022, 9, 896330. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybylowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An Updated Systematic Review and Meta-Analysis on Adherence to Mediterranean Diet and Risk of Cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Pan, P.; Yu, J.; Wang, L.S. Colon Cancer: What we Eat. Surg. Oncol. Clin. N. Am. 2018, 27, 243–267. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Appleby, P.N.; Key, T.J. Fruit, Vegetable, and Fiber Intake in Relation to Cancer Risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 394S–398S. [Google Scholar] [CrossRef]
- Leenders, M.; Siersema, P.D.; Overvad, K.; Tjonneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Bastide, N.; Fagherazzi, G.; Katzke, V.; Kuhn, T.; et al. Subtypes of Fruit and Vegetables, Variety in Consumption and Risk of Colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 137, 2705–2714. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and Vegetables and Cancer Risk: A Review of Southern European Studies. Br. J. Nutr. 2015, 113 (Suppl. 2), S102–S110. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is Dietary Fibre Truly Protective Against Colon Cancer? A Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of Dietary Patterns and the Main Food Groups on Mortality and Recurrence in Cancer Survivors: A Systematic Review of Current Epidemiological Literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef]
- Bamia, C. Dietary Patterns in Association to Cancer Incidence and Survival: Concept, Current Evidence, and Suggestions for Future Research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J. Cell. Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The Family of Metazoan Metal-Independent Beta-Galactoside-Binding Lectins: Structure, Function and Molecular Evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Vasta, G.R. Galectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Eds.; by The Consortium of Glycobiology Editors, La Jolla, California: Cold Spring Harbor: New York, NY, USA, 2015; pp. 469–480. [Google Scholar]
- Di Lella, S.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of Acute and Chronic Inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-just how do they do those Things they do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An Open-Ended Story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Raimond, J.; Zimonjic, D.B.; Mignon, C.; Mattei, M.; Popescu, N.C.; Monsigny, M.; Legrand, A. Mapping of the Galectin-3 Gene (LGALS3) to Human Chromosome 14 at Region 14q21-22. Mamm. Genome 1997, 8, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-Ray Crystal Structure of the Human Galectin-3 Carbohydrate Recognition Domain at 2.1-A Resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of Galectin-3 Modulates T-Cell Growth and Apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and Function of a Large Family of Animal Lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, X.; Cheng, H.; Miller, M.C.; He, Z.; Gu, H.; Zhang, Z.; Raz, A.; Mayo, K.H.; Tai, G.; et al. Galectin-3 N-Terminal Tail Prolines Modulate Cell Activity and Glycan-Mediated Oligomerization/Phase Separation. Proc. Natl. Acad. Sci. USA 2021, 118, e2021074118. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Lazarevic, T.; Maksimovic, N.; Grk, M.; Volarevic, V.; GazdicJankovic, M.; Djukic, S.; Janicijevic, K.; MileticKovacevic, M.; Ljujic, B. Galectin 3 (LGALS3) Gene Polymorphisms are Associated with Biochemical Parameters and Primary Disease in Patients with End-Stage Renal Disease in Serbian Population. J. Clin. Med. 2022, 11, 3874. [Google Scholar] [CrossRef]
- Hokama, A.; Mizoguchi, E.; Mizoguchi, A. Roles of Galectins in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 5133–5137. [Google Scholar] [CrossRef]
- Sundblad, V.; Quintar, A.A.; Morosi, L.G.; Niveloni, S.I.; Cabanne, A.; Smecuol, E.; Maurino, E.; Marino, K.V.; Bai, J.C.; Maldonado, C.A.; et al. Galectins in Intestinal Inflammation: Galectin-1 Expression Delineates Response to Treatment in Celiac Disease Patients. Front. Immunol. 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Mao, Y.S.; Chen, F.; Xia, D.X.; Zhao, T.Q. Palmitic Acid Up Regulates Gal-3 and Induces Insulin Resistance in Macrophages by Mediating the Balance between KLF4 and NF-kappaB. Exp. Ther. Med. 2021, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, F.; Zhong, W.; Jiang, X.; Zhang, F.; Ji, X.; Xue, M.; Qiu, Y.; Yu, J.; Hu, X.; et al. Secretory Galectin-3 Promotes Hepatic Steatosis Via Regulation of the PPARgamma/CD36 Signaling Pathway. Cell. Signal. 2021, 84, 110043. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Eisinger, K.; Hader, Y.; Buechler, C. Free Fatty Acids and IL-6 Induce Adipocyte Galectin-3 which is Increased in White and Brown Adipose Tissues of Obese Mice. Cytokine 2014, 69, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Li, X.T.; Yu, L.G.; Wang, L.; Shi, Z.Y.; Guo, X.L. Roles of Galectin-3 in Metabolic Disorders and Tumor Cell Metabolism. Int. J. Biol. Macromol. 2020, 142, 463–473. [Google Scholar] [CrossRef]
- Yue, F.; Xu, J.; Zhang, S.; Hu, X.; Wang, X.; Lu, X. Structural Features and Anticancer Mechanisms of Pectic Polysaccharides: A Review. Int. J. Biol. Macromol. 2022, 209, 825–839. [Google Scholar] [CrossRef]
- Tao, L.; Jin, L.; Dechun, L.; Hongqiang, Y.; Changhua, K.; Guijun, L. Galectin-3 Expression in Colorectal Cancer and its Correlation with Clinical Pathological Characteristics and Prognosis. Open Med. 2017, 12, 226–230. [Google Scholar] [CrossRef]
- Diaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef] [Green Version]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.R.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 Mediate Galectin-3-Induced Type II T-Cell Apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and Galectin-1 Bind Distinct Cell Surface Glycoprotein Receptors to Induce T Cell Death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical Localization of Six Galectin Subtypes in the Mouse Digestive Tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Papa Gobbi, R.; De Francesco, N.; Bondar, C.; Muglia, C.; Chirdo, F.; Rumbo, M.; Rocca, A.; Toscano, M.A.; Sambuelli, A.; Rabinovich, G.A.; et al. A Galectin-Specific Signature in the Gut Delineates Crohn’s Disease and Ulcerative Colitis from Other Human Inflammatory Intestinal Disorders. Biofactors 2016, 42, 93–105. [Google Scholar] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.G.; Subramanian, S. Serum Galectins as Potential Biomarkers of Inflammatory Bowel Diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef] [Green Version]
- Frol’ova, L.; Smetana, K., Jr.; Borovska, D.; Kitanovicova, A.; Klimesova, K.; Janatkova, I.; Malickova, K.; Lukas, M.; Drastich, P.; Benes, Z.; et al. Detection of Galectin-3 in Patients with Inflammatory Bowel Diseases: New Serum Marker of Active Forms of IBD? Inflamm. Res. 2009, 58, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef] [PubMed]
- SimovicMarkovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-Inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1beta in Macrophages. J. Crohns Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cibor, D.; Szczeklik, K.; Brzozowski, B.; Mach, T.; Owczarek, D. Serum Galectin 3, Galectin 9 and Galectin 3-Binding Proteins in Patients with Active and Inactive Inflammatory Bowel Disease. J. Physiol. Pharmacol 2019, 70. [Google Scholar] [CrossRef]
- Jovanovic, M.; Gajovic, N.; Zdravkovic, N.; Jovanovic, M.; Jurisevic, M.; Vojvodic, D.; Maric, V.; Arsenijevic, A.; Jovanovic, I. Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma. Mediators Inflamm. 2018, 2018, 8031328. [Google Scholar] [CrossRef] [Green Version]
- Nebbia, M.; Yassin, N.A.; Spinelli, A. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon Rectal Surg. 2020, 33, 305–317. [Google Scholar] [CrossRef]
- Endo, K.; Kohnoe, S.; Tsujita, E.; Watanabe, A.; Nakashima, H.; Baba, H.; Maehara, Y. Galectin-3 Expression is a Potent Prognostic Marker in Colorectal Cancer. Anticancer Res. 2005, 25, 3117–3121. [Google Scholar]
- Tsuboi, K.; Shimura, T.; Masuda, N.; Ide, M.; Tsutsumi, S.; Yamaguchi, S.; Asao, T.; Kuwano, H. Galectin-3 Expression in Colorectal Cancer: Relation to Invasion and Metastasis. Anticancer Res. 2007, 27, 2289–2296. [Google Scholar] [PubMed]
- Nagy, N.; Legendre, H.; Engels, O.; Andre, S.; Kaltner, H.; Wasano, K.; Zick, Y.; Pector, J.C.; Decaestecker, C.; Gabius, H.J.; et al. Refined Prognostic Evaluation in Colon Carcinoma using Immunohistochemical Galectin Fingerprinting. Cancer 2003, 97, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H. Galectins: Multipurpose Carbohydrate-Binding Proteins Implicated in Tumor Biology. Gastroenterology 1997, 113, 2003–2005. [Google Scholar] [PubMed]
- Dudas, S.P.; Yunker, C.K.; Sternberg, L.R.; Byrd, J.C.; Bresalier, R.S. Expression of Human Intestinal Mucin is Modulated by the Beta-Galactoside Binding Protein Galectin-3 in Colon Cancer. Gastroenterology 2002, 123, 817–826. [Google Scholar] [CrossRef]
- Nakamura, M.; Inufusa, H.; Adachi, T.; Aga, M.; Kurimoto, M.; Nakatani, Y.; Wakano, T.; Nakajima, A.; Hida, J.I.; Miyake, M.; et al. Involvement of Galectin-3 Expression in Colorectal Cancer Progression and Metastasis. Int. J. Oncol. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- Hegardt, P.; Widegren, B.; Li, L.; Sjogren, B.; Kjellman, C.; Sur, I.; Sjogren, H.O. Nitric Oxide Synthase Inhibitor and IL-18 Enhance the Anti-Tumor Immune Response of Rats Carrying an Intrahepatic Colon Carcinoma. Cancer Immunol. Immunother. 2001, 50, 491–501. [Google Scholar] [CrossRef]
- Kayser, K.; Zink, S.; Andre, S.; Schuring, M.P.; Hecker, E.; Klar, E.; Bovin, N.V.; Kaltner, H.; Gabius, H.J. Primary Colorectal Carcinomas and their Intrapulmonary Metastases: Clinical, Glyco-, Immuno- and Lectin Histochemical, Nuclear and Syntactic Structure Analysis with Emphasis on Correlation with Period of Occurrence of Metastases and Survival. APMIS 2002, 110, 435–446. [Google Scholar] [CrossRef]
- Mazurek, N.; Conklin, J.; Byrd, J.C.; Raz, A.; Bresalier, R.S. Phosphorylation of the Beta-Galactoside-Binding Protein Galectin-3 Modulates Binding to its Ligands. J. Biol. Chem. 2000, 275, 36311–36315. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.Q.; Rong, G.Q.; Wu, Y.; Pu, Y.W.; Ye, Z.Y.; Cao, C.; Yang, X.D.; Xing, C.G. Preliminary Proteomic Analysis of Radiation Response Markers in Rectal Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8841–8851. [Google Scholar]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of Galectin-3 in the Sera of Normal Controls and Cancer Patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Increased Circulation of Galectin-3 in Cancer Induces Secretion of Metastasis-Promoting Cytokines from Blood Vascular Endothelium. Clin. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers 2021, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Yamada, M.; Matsumoto, S.; Sakurazawa, N.; Kawano, Y.; Sekiguchi, K.; Yamada, T.; Matsutani, T.; Miyashita, M.; Yoshida, H. Blood Galectin-3 Levels Predict Postoperative Complications After Colorectal Cancer Surgery. J. Nippon Med. Sch. 2019, 86, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Yoon, G. Overexpression of Aquaporin-1 is a Prognostic Factor for Biochemical Recurrence in Prostate Adenocarcinoma. Pathol. Oncol. Res. 2017, 23, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, Z.; Li, N.; Zhang, M. Prognostic Value of Serum Aquaporin-1, Aquaporin-3 and Galectin-3 for Young Patients with Colon Cancer. Ann. Clin. Biochem. 2020, 57, 404–411. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, D.; Li, X.; Xie, X.; Ye, Y.; Wang, L. Galectin Family Members: Emerging Novel Targets for Lymphoma Therapy? Front. Oncol. 2022, 12, 889034. [Google Scholar] [CrossRef]
- Nakahara, S.; Oka, N.; Wang, Y.; Hogan, V.; Inohara, H.; Raz, A. Characterization of the Nuclear Import Pathways of Galectin-3. Cancer Res. 2006, 66, 9995–10006. [Google Scholar] [CrossRef] [Green Version]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular Functions of Galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 Augments K-Ras Activation and Triggers a Ras Signal that Attenuates ERK but Not Phosphoinositide 3-Kinase Activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [Green Version]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A Novel Antiapoptotic Molecule with a Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Dagher, S.F.; Wang, J.L.; Patterson, R.J. Identification of Galectin-3 as a Factor in Pre-mRNA Splicing. Proc. Natl. Acad. Sci. USA 1995, 92, 1213–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavao, M.S. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the Interaction between Galectin-3 and Cell Adhesion Molecules in Cancer Metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of Immune Responses. Ann. New York Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- Du Toit, A. Endocytosis: Bend it Like Galectin 3. Nat. Rev. Mol. Cell Biol. 2014, 15, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hill, P.N.; Hsu, D.K.; Liu, F.T. Role of the Carboxyl-Terminal Lectin Domain in Self-Association of Galectin-3. Biochemistry 1998, 37, 4086–4092. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-Linked Complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.L.; Huang, E.Y.; Jhu, E.W.; Huang, Y.H.; Su, W.H.; Chuang, P.C.; Yang, K.D. Overexpression of Galectin-3 Enhances Migration of Colon Cancer Cells Related to Activation of the K-Ras-Raf-Erk1/2 Pathway. J. Gastroenterol. 2013, 48, 350–359. [Google Scholar] [CrossRef]
- Wu, K.L.; Huang, E.Y.; Yeh, W.L.; Hsiao, C.C.; Kuo, C.M. Synergistic Interaction between Galectin-3 and Carcinoembryonic Antigen Promotes Colorectal Cancer Metastasis. Oncotarget 2017, 8, 61935–61943. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.L.; Kuo, C.M.; Huang, E.Y.; Pan, H.M.; Huang, C.C.; Chen, Y.F.; Hsiao, C.C.; Yang, K.D. Extracellular Galectin-3 Facilitates Colon Cancer Cell Migration and is Related to the Epidermal Growth Factor Receptor. Am. J. Transl. Res. 2018, 10, 2402–2412. [Google Scholar]
- Barrow, H.; Rhodes, J.M.; Yu, L.G. The Role of Galectins in Colorectal Cancer Progression. Int. J. Cancer 2011, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Akita, K.; Yashiro, M.; Sawada, T.; Hirakawa, K.; Murata, T.; Nakada, H. Binding of Galectin-3, a Beta-Galactoside-Binding Lectin, to MUC1 Protein Enhances Phosphorylation of Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) and Akt, Promoting Tumor Cell Malignancy. J. Biol. Chem. 2015, 290, 26125–26140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Tang, J.W.; Owusu, L.; Sun, M.Z.; Wu, J.; Zhang, J. Galectin-3 in Cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef]
- Song, S.; Mazurek, N.; Liu, C.; Sun, Y.; Ding, Q.Q.; Liu, K.; Hung, M.C.; Bresalier, R.S. Galectin-3 Mediates Nuclear Beta-Catenin Accumulation and Wnt Signaling in Human Colon Cancer Cells by Regulation of Glycogen Synthase Kinase-3beta Activity. Cancer Res. 2009, 69, 1343–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Wang, J.; Yang, G.; Yu, N.; Huang, Z.; Xu, H.; Li, J.; Qiu, J.; Zeng, X.; Chen, S.; et al. Posttranscriptional Regulation of Galectin-3 by miR-128 Contributes to Colorectal Cancer Progression. Oncotarget 2017, 8, 15242–15251. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, N.; Byrd, J.C.; Sun, Y.; Hafley, M.; Ramirez, K.; Burks, J.; Bresalier, R.S. Cell-Surface Galectin-3 Confers Resistance to TRAIL by Impeding Trafficking of Death Receptors in Metastatic Colon Adenocarcinoma Cells. Cell Death Differ. 2012, 19, 523–533. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of Galectin3 in the Tumor Microenvironment and Tumor Metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809. [Google Scholar]
- Henderson, N.C.; Sethi, T. The Regulation of Inflammation by Galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The Role of Galectin-3 in Modulating Tumor Growth and Immunosuppression within the Tumor Microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef] [Green Version]
- SimovicMarkovic, B.; Nikolic, A.; Gazdic, M.; Nurkovic, J.; Djordjevic, I.; Arsenijevic, N.; Stojkovic, M.; Lukic, M.L.; Volarevic, V. Pharmacological Inhibition of Gal-3 in Mesenchymal Stem Cells Enhances their Capacity to Promote Alternative Activation of Macrophages in Dextran Sulphate Sodium-Induced Colitis. Stem Cells Int. 2016, 2016, 2640746. [Google Scholar]
- Tsai, H.F.; Wu, C.S.; Chen, Y.L.; Liao, H.J.; Chyuan, I.T.; Hsu, P.N. Galectin-3 Suppresses Mucosal Inflammation and Reduces Disease Severity in Experimental Colitis. J. Mol. Med. 2016, 94, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Stieber-Gunckel, M.; Dunger, N.; Falk, W.; Obermeier, F.; Kunst, C. Galectin-3 Modulates Experimental Colitis. Digestion 2015, 92, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, H.Y.; Miyahara, Y.; Peng, G.; Wang, R.F. Tumor-Associated Galectin-3 Modulates the Function of Tumor-Reactive T Cells. Cancer Res. 2008, 68, 7228–7236. [Google Scholar] [CrossRef] [Green Version]
- Curciarello, R.; Steele, A.; Cooper, D.; MacDonald, T.T.; Kruidenier, L.; Kudo, T. The Role of Galectin-1 and Galectin-3 in the Mucosal Immune Response to CitrobacterRodentium Infection. PLoS ONE 2014, 9, e107933. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Shibata, M.; Gonda, K.; Nakajima, T.; Chida, S.; Noda, M.; Suzuki, S.; Nakamura, I.; Ohki, S.; Takenoshita, S. Association between Circulating Galectin-3 Levels and the Immunological, Inflammatory and Nutritional Parameters in Patients with Colorectal Cancer. Biomed. Rep. 2016, 5, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Sawa-Wejksza, K.; Dudek, A.; Lemieszek, M.; Kalawaj, K.; Kandefer-Szerszen, M. Colon Cancer-Derived Conditioned Medium Induces Differentiation of THP-1 Monocytes into a Mixed Population of M1/M2 Cells. Tumour Biol. 2018, 40, 1010428318797880. [Google Scholar] [CrossRef] [Green Version]
- Muller, S.; Schaffer, T.; Flogerzi, B.; Fleetwood, A.; Weimann, R.; Schoepfer, A.M.; Seibold, F. Galectin-3 Modulates T Cell Activity and is Reduced in the Inflamed Intestinal Epithelium in IBD. Inflamm. Bowel Dis. 2006, 12, 588–597. [Google Scholar] [CrossRef]
- Volarevic, V.; Zdravkovic, N.; Harrell, C.R.; Arsenijevic, N.; Fellabaum, C.; Djonov, V.; Lukic, M.L.; SimovicMarkovic, B. Galectin-3 Regulates Indoleamine-2,3-Dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and may Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 2019, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D. Unraveling how Tumor-Derived Galectins Contribute to Anti-Cancer Immunity Failure. Cancers 2021, 13, 4529. [Google Scholar] [CrossRef]
- Dumont, P.; Berton, A.; Nagy, N.; Sandras, F.; Tinton, S.; Demetter, P.; Mascart, F.; Allaoui, A.; Decaestecker, C.; Salmon, I. Expression of Galectin-3 in the Tumor Immune Response in Colon Cancer. Lab. Invest. 2008, 88, 896–906. [Google Scholar] [CrossRef] [Green Version]
- deKivit, S.; Kraneveld, A.D.; Garssen, J.; Willemsen, L.E. Glycan Recognition at the Interface of the Intestinal Immune System: Target for Immune Modulation Via Dietary Components. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S124–S132. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, D.; Kane, M.; Joshi, L.; Hickey, R.M. Detection of Galectin-3 Interaction with Commensal Bacteria. Appl. Environ. Microbiol. 2013, 79, 3507–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipova, M.; Bojarova, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunar, K.; Kren, V.; Etrych, T.; Janouskova, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochola, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [Green Version]
- Beukema, M.; Faas, M.M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact Via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Bishehsari, F.; Engen, P.A.; Preite, N.Z.; Tuncil, Y.E.; Naqib, A.; Shaikh, M.; Rossi, M.; Wilber, S.; Green, S.J.; Hamaker, B.R.; et al. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Prado, S.B.R.D.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-Induced Chemical Modifications of Papaya Pectin Inhibit Cancer Cell Proliferation. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Moslemi, M. Reviewing the Recent Advances in Application of Pectin for Technical and Health Promotion Purposes: From Laboratory to Market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- do Prado, S.B.R.; Castro-Alves, V.C.; Ferreira, G.F.; Fabi, J.P. Ingestion of Non-Digestible Carbohydrates from Plant-Source Foods and Decreased Risk of Colorectal Cancer: A Review on the Biological Effects and the Mechanisms of Action. Front. Nutr. 2019, 6, 72. [Google Scholar] [CrossRef]
- Gunning, A.P.; Bongaerts, R.J.; Morris, V.J. Recognition of Galactan Components of Pectin by Galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key Regulators of Galectin-Glycan Interactions. Proteomics 2016, 16, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of Human Cancer Cell Growth and Metastasis in Nude Mice by Oral Intake of Modified Citrus Pectin. J. Natl. Cancer Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.Y.; Huang, Z.L.; Yang, G.H.; Lu, W.Q.; Yu, N.R. Inhibitory Effect of Modified Citrus Pectin on Liver Metastases in a Mouse Colon Cancer Model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, P.; Lu, S.M.; Ling, Z.Q. Chemoprevention of Low-Molecular-Weight Citrus Pectin (LCP) in Gastrointestinal Cancer Cells. Int. J. Biol. Sci. 2016, 12, 746–756. [Google Scholar] [CrossRef]
- Odun-Ayo, F.; Mellem, J.; Naicker, T.; Reddy, L. Chemoprevention of Azoxymethane-Induced Colonic Carcinogenesis in Balb/C Mice using a Modified Pectin Alginate Probiotic. Anticancer Res. 2015, 35, 4765–4775. [Google Scholar]
- Li, Y.; Liu, L.; Niu, Y.; Feng, J.; Sun, Y.; Kong, X.; Chen, Y.; Chen, X.; Gan, H.; Cao, S.; et al. Modified Apple Polysaccharide Prevents Against Tumorigenesis in a Mouse Model of Colitis-Associated Colon Cancer: Role of Galectin-3 and Apoptosis in Cancer Prevention. Eur. J. Nutr. 2012, 51, 107–117. [Google Scholar] [CrossRef]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative Studies of the Antiproliferative Effects of Ginseng Polysaccharides on HT-29 Human Colon Cancer Cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The Inhibitory Effects of a Rhamnogalacturonan I (RG-I) Domain from Ginseng Pectin on Galectin-3 and its Structure-Activity Relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef]
- Maksymowicz, J.; Palko-Labuz, A.; Sobieszczanska, B.; Chmielarz, M.; Ferens-Sieczkowska, M.; Skonieczna, M.; Wikiera, A.; Wesolowska, O.; Sroda-Pomianek, K. The use of Endo-Cellulase and Endo-Xylanase for the Extraction of Apple Pectins as Factors Modifying their Anticancer Properties and Affecting their Synergy with the Active Form of Irinotecan. Pharmaceuticals 2022, 15, 732. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells Via an Interaction with the Neutral Sugar Side-Chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Oria, A.; Rodriguez-Gutierrez, G.; Alaiz, M.; Vioque, J.; Giron-Calle, J.; Fernandez-Bolanos, J. Pectin-Rich Extracts from Olives Inhibit Proliferation of Caco-2 and THP-1 Cells. Food Funct. 2019, 10, 4844–4853. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, R.S.; Pedrosa, L.F.; Diethelm, L.T.H.; Souza, T.; Shiga, T.M.; Fabi, J.P. The Purification of Pectin from Commercial Fruit Flours Results in a Jaboticaba Fraction that Inhibits Galectin-3 and Colon Cancer Cell Growth. Food Res. Int. 2020, 137, 109747. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.F.; Lopes, R.G.; Fabi, J.P. The Acid and Neutral Fractions of Pectins Isolated from Ripe and Overripe Papayas Differentially Affect Galectin-3 Inhibition and Colon Cancer Cell Growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Gunning, A.P.; Pin, C.; Morris, V.J. Galectin 3-Beta-Galactobiose Interactions. Carbohydr. Polym. 2013, 92, 529–533. [Google Scholar] [CrossRef]
- Hayashi, A.; Gillen, A.C.; Lott, J.R. Effects of Daily Oral Administration of Quercetin Chalcone and Modified Citrus Pectin on Implanted Colon-25 Tumor Growth in Balb-C Mice. Altern. Med. Rev. 2000, 5, 546–552. [Google Scholar]
- Bresalier, R.S.; Mazurek, N.; Sternberg, L.R.; Byrd, J.C.; Yunker, C.K.; Nangia-Makker, P.; Raz, A. Metastasis of Human Colon Cancer is Altered by Modifying Expression of the Beta-Galactoside-Binding Protein Galectin 3. Gastroenterology 1998, 115, 287–296. [Google Scholar] [CrossRef]
- Pedrosa, L.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; Prado, S.B.R.; Rogero, M.M.; Fabi, J.P. Effects of Pectins on Colorectal Cancer: Targeting Hallmarks as a Support for Future Clinical Trials. Food Funct. 2022, 13, 11438–11454. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study. Nutrients 2021, 13, 4295. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing Clinical and Immunological Effects of Anti-PD-1 with Belapectin, a Galectin-3 Inhibitor. J. Immunother. Cancer 2021, 9, e002371. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mileo, A.M.; Nistico, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Bracci, L.; Fabbri, A.; Del Corno, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Amintas, S.; Dupin, C.; Boutin, J.; Beaumont, P.; Moreau-Gaudry, F.; Bedel, A.; Krisa, S.; Vendrely, V.; Dabernat, S. Bioactive Food Components for Colorectal Cancer Prevention and Treatment: A Good Match. Crit. Rev. Food Sci. Nutr. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge Including Clinical Trials and Molecular Mechanism of Action. Biomed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [Green Version]
- El-Kott, A.F.; Shati, A.A.; Ali Al-Kahtani, M.; Alharbi, S.A. The Apoptotic Effect of Resveratrol in Ovarian Cancer Cells is Associated with Downregulation of Galectin-3 and Stimulating miR-424-3p Transcription. J. Food Biochem. 2019, 43, e13072. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Pei, C.; Zhang, Y.; Wang, P.; Zhang, B.; Fang, L.; Liu, B.; Meng, S. Berberine Alleviates Oxidized Low-Density Lipoprotein-Induced Macrophage Activation by Downregulating Galectin-3 Via the NF-kappaB and AMPK Signaling Pathways. Phytother. Res. 2019, 33, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.; Costa, R.; Rodrigues, I.; Castela, A.; Coelho, P.; Guerreiro, S.; Gomes, J.; Reis, C.; Soares, R. Xanthohumol and 8-Prenylnaringenin Reduce Type 2 Diabetes-Associated Oxidative Stress by Downregulating Galectin-3. Porto Biomed. J. 2018, 4, e23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, Z.; Jiang, M.; Sun, Y. Berberine as a Potential Agent for the Treatment of Colorectal Cancer. Front. Med. 2022, 9, 886996. [Google Scholar] [CrossRef]
- Chen, H.; Yin, S.; Xiong, Z.; Li, X.; Zhang, F.; Chen, X.; Guo, J.; Xie, M.; Mao, C.; Jin, L.; et al. Clinicopathologic Characteristics and Prognosis of Synchronous Colorectal Cancer: A Retrospective Study. BMC Gastroenterol. 2022, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Li, G.; Gao, Z. Xanthohumol Protects Against Azoxymethane-Induced Colorectal Cancer in Sprague-Dawley Rats. Environ. Toxicol. 2020, 35, 136–144. [Google Scholar] [CrossRef] [PubMed]

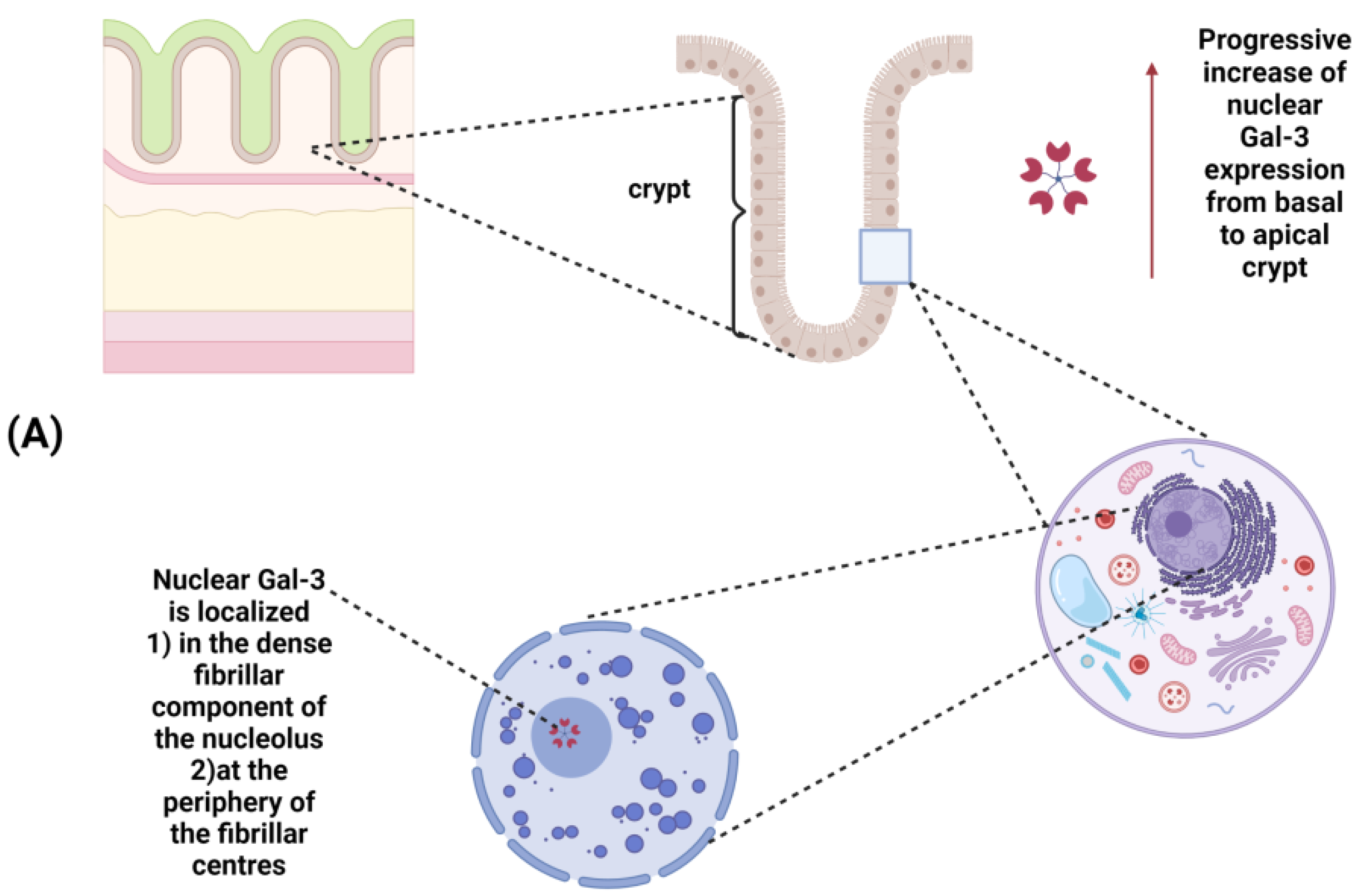


| Disease | Model | Immune Cell Type | Investigated Endpoints | Major Findings | Reference |
|---|---|---|---|---|---|
| UC | Human (10 patients) | Colonic macrophages | ↑Gal-3 expression | Attenuation of acute colitis | [47] |
| PBMCs | ↑Gal-3 secretion | ||||
| Mice (DSS- induced acute colitis) | ↓ Macrophages in colon | Gal-3 blocking (Gal-3−/− ko mice or pharmacological inhibition): ↓NLRP3 inflammasome ↓inflammatory cytokines (IL-1β, TNF-α) ↑IL-10 producing M2 phenotype | |||
| ↓DCs in colon | |||||
| ↓ Neutrophils in colon | |||||
| UC | Mice (DSS- induced acute colitis) | Colonic macrophages | Gal-3 blocking in MSC (pharmacological inhibition): ↑ immunosuppressive M2 phenotype ↑IL-10 | Attenuation of severity of colitis | [91] |
| UC | Mice (DSS- induced colitis) | T cells | Gal-3 blocking (Gal-3−/− ko mice): ↑ severe disease activity Recombinant Gal-3: ↑ Treg cell phenotype (FOXP3, ICOS, and PD-1 positive) Adoptive transfer of Gal-3 treated T cell: ↓Inhibition of colonic mucosa inflammation | Reduction of disease severity by inducing Treg | [92] |
| UC | Mice (DSS- induced colitis) | Mesenteric lymphnodes |
| [93] | |
| UC | Mice (DSS- induced colitis) | Macrophages | Gal-3 blocking (Gal-3−/− ko mice): ↓ IL-1-, TNF-α, IL-6 and IL-12-producing M1 phenotype ↑ IL-10-, IL-4, and TGF-β- producing M2 phenotype | Reduction of colon inflammation | [46] |
| DCs | Gal-3 blocking in DCs (pharmacological inhibition): ↑ DCs immunosuppressive function | ||||
| T cells | ↑ colon-infiltrated Tregs ↑pro-inflammatory Th1/Th17 phenotype | ||||
| CRC | In vitro, colon cancer cell lines from CRC patients | T cells | Recombinant Gal-3: ↓ tumor-reactive T cells ↑ cell apoptosis | Promotion of tumor immune tolerance | [94] |
| CRC | Mice (Gal-3−/− ko) | ↓ Macrophages in colon | ↑IL-6, KC |
| [95] |
| ↓ DCs in colon | |||||
| ↓ T cells in colon | |||||
| = Neutrophils in colon | |||||
| CRC | Human (50 patients) | PBMCs | ↑Gal-3 secretion ↑ IL-17 and IL-10 ↓ IL-12 ↑inflammatory parameters (NLR, WBC, CRP) ↓limphocytestimulation |
| [96] |
| CRC | In vitro, colon cancer cell lines (HT29, LS180, SW948, SW620) | Monocytes (THP1 cells) | ↑Gal-3 and IDO induction by tumor CM | Induction of immune suppressive macrophages | [97] |
| PectinSource and Modification | In Vivo Model | In Vitro Model | Galectin-3 Blocking | Main Results | Reference |
|---|---|---|---|---|---|
| Citrus (pH and temperature-modified pectin) 1% in drinking water | NCR nu/nu mice injected with LSLiM6 cells | - | ↓ binding to HUVEC ↓ HUVEC chemotaxis/ capillary tube formation | Therapeutic effect: ↓ tumor growth ↓tumor- associated blood vessels ↓ NM and LM | [114] |
| Citrus (modified pectin) 1–5% in drinking water | Balb/c mice spleen injected with CT26 cells | - | = expression in blood and LM | Therapeutic effect: ↓ tumor growth ↓ LM | [115] |
| Citrus(LMW pectin PYKTIN, Centrax Int.) 1–5% in drinking water | Nude mice engrafted with SW-480 cells | - | ↓ expression | Therapeutic effect: ↓ tumor growth ↑ 5-FU effect ↑ apoptosis in tumor tissue | [116] |
| Citrus(modified pectin with alginate and L. acidophilus) | AOM-treated Balb/c mice | - | ↓ expression in colonic crypts and blood vessels | Chemopreventive effect: ↓ precancerous lesions | [117] |
| Apple(modified pectin from red Fuji apples) 2.5–10% in pellet diet | DMH/DSS- treated ICS mice | - | ↓ expression in serum ↓ galactose binding to SW-1116 | Chemopreventive effect: ↓ colon inflammation ↓ tumorigenesis ↑ colonic EC apoptosis ↑ caspase-3 activation | [118] |
| Smilax china L. (pectin) | DSS-treated Balb/c mice | - | ↓ Gal- 3/NLRP3 inflammasome interaction | Therapeutic/preventive effects: ↓ UC histo- pathological damage ↓ inflammatory mediators | [3] |
| Citrus(modified pectin, EcoNugenics) 0.1–25 mM | - | DLD-1 | ↓ extracellular expression | ↓ cell migration | [81] |
| Citrus (LMW pectin PYKTIN, Centrax Int.) 0.625–10 mg/mL | - | SW-480 | ↓ expression | ↓ cell proliferation ↑ cell cycle arrest ↓ EMT | [116] |
| Ginseng (HG-rich pectin) Ginseng(temperature-modified pectin) | - | HT-29 HT-29 | ND | ↓ cell proliferation ↑ cell cycle arrest ↓↓ cell proliferation ↑ apoptosis ↑ caspase-3 activation | [119] |
| ND | |||||
| Ginseng (RG-I-4 pectic fragment) | - | HT-29 | ↓ rGal3- induced RBC agglutination ↓ binding to Jurkat cells | ↓ cell adhesion ↓ ASF-induced cell aggregation | [120] |
| Apple(enzyme- modified, enriched in RG-I regions) | - | HCT 116 Caco-2 HT-29 | ↓ expression | ↑ irinotecan effect ↓ cell viability ↑ apoptosis ↑ ROS ↓ LPS-induced inflammatory mediators | [121] |
| Sugar beet(enzyme- or alkali- modified pectin) 0.2–1 mg/mL | - | HT-29 DLD-1 | Galactose/ arabinose- mediated | ↓ cell proliferation ↑ apoptosis | [122] |
| Olive (heat- and acid-modified pectin) 1–10 mg/mL | - | Caco-2 | ↓ rGal3-induced RBC agglutination | ↓ cell proliferation | [123] |
| Jaboticaba (pectin from fruit flour) 0.25–2 mg/mL | - | HCT116 | ↓ rGal3- induced RBC agglutination | ↓ cell viability | [124] |
| Papaya (pectin from fruit pulp) 0.025–0.2% | - | HCT116 HT-29 | ↓ rGal3- induced RBC agglutination Gal-3 gene knockdown | ↓ cell viability ↓ cell proliferation | [108,125] |
| Polyphenol | In Vivo Model | In Vitro Model | Galectin-3 Blocking | Main Results | Reference |
|---|---|---|---|---|---|
| Resveratrol 25–100 µM | - | Human ovarian cancer cell lines SKOV3 OVCAR-3 | ↓ expression (↑ mir- 424-3p/↓ NF- kB activation) | ↓ cell proliferation ↑ apoptosis ↓ cell migration and invasion ↑ sensitivity to cisplatin | [140] |
| Quercetin oral intake 100 mg/kg | HFD fed ApoE−/− C57BL/6J mice | RAW264.7 macrophage cell line | ↓ expression | ↓ atherosclerotic lesions ↓ NLRP3 inflammasome activation | [141] |
| Berberine 5–25 µM | - | Ox-LDL- activated THP-1 | ↓ expression (↓ NF- kB/AMPK signaling) | ↓ cell activation | [142] |
| Xanthohumol in drinking water 0.1% | HFD fed C57BL/6J mice | - | ↓ expression in kidney and liver | ↓ AGE in kidney ↓ 3-NT in liver | [143] |
| 8- prenylnaringenin in drinking water 0.1% | HFD fed C57BL/6J mice | - | ↓ expression in kidney and liver | ↓ AGE ↓ 3-NT in liver and kidney | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aureli, A.; Del Cornò, M.; Marziani, B.; Gessani, S.; Conti, L. Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors. Cancers 2023, 15, 52. https://doi.org/10.3390/cancers15010052
Aureli A, Del Cornò M, Marziani B, Gessani S, Conti L. Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors. Cancers. 2023; 15(1):52. https://doi.org/10.3390/cancers15010052
Chicago/Turabian StyleAureli, Anna, Manuela Del Cornò, Beatrice Marziani, Sandra Gessani, and Lucia Conti. 2023. "Highlights on the Role of Galectin-3 in Colorectal Cancer and the Preventive/Therapeutic Potential of Food-Derived Inhibitors" Cancers 15, no. 1: 52. https://doi.org/10.3390/cancers15010052





