RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Cell Line Culture and Treatment
2.3. Cell Proliferation Assay
2.4. Scratch/Wound Healing Assay
2.5. Cell Doubling Time (CDT)
2.6. RNA Extraction and Gene Expression by Real Time PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
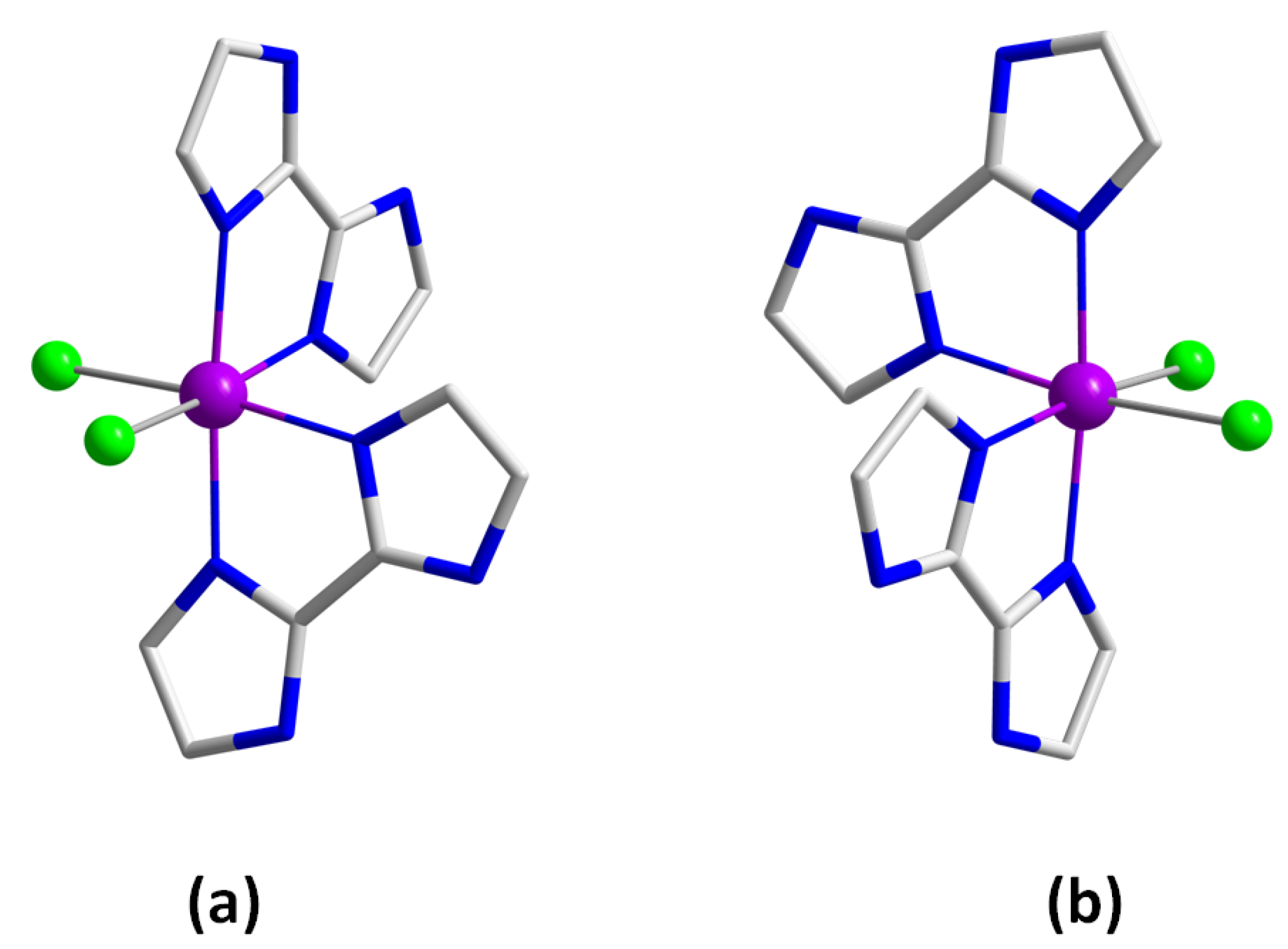
3.1. Preparation of Runat-BI
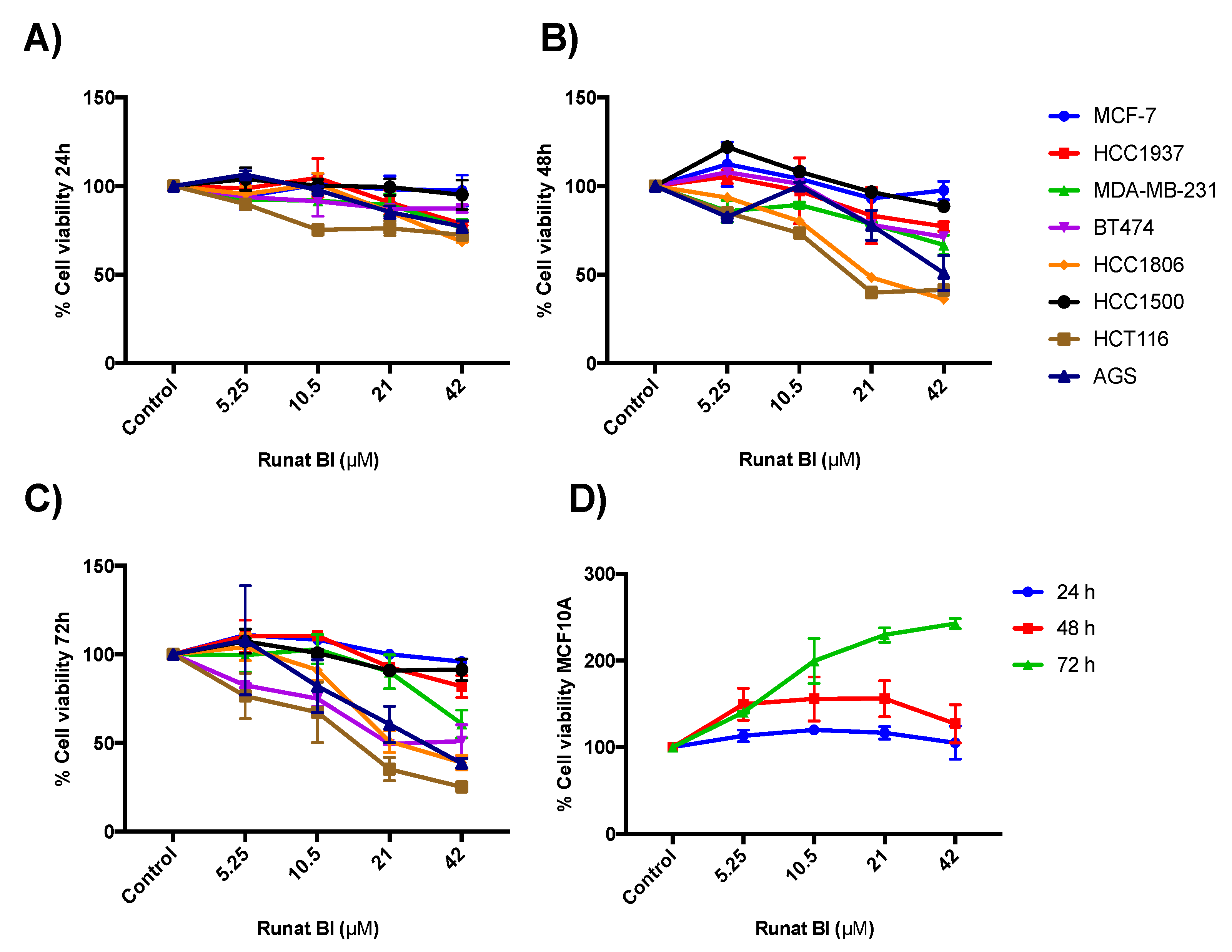
3.2. Runat-BI Reduces Proliferation in Cancer Cell Lines
3.3. Isomer 1 of Runat-BI Showed No Effect on Viability in the Cell Lines Studied
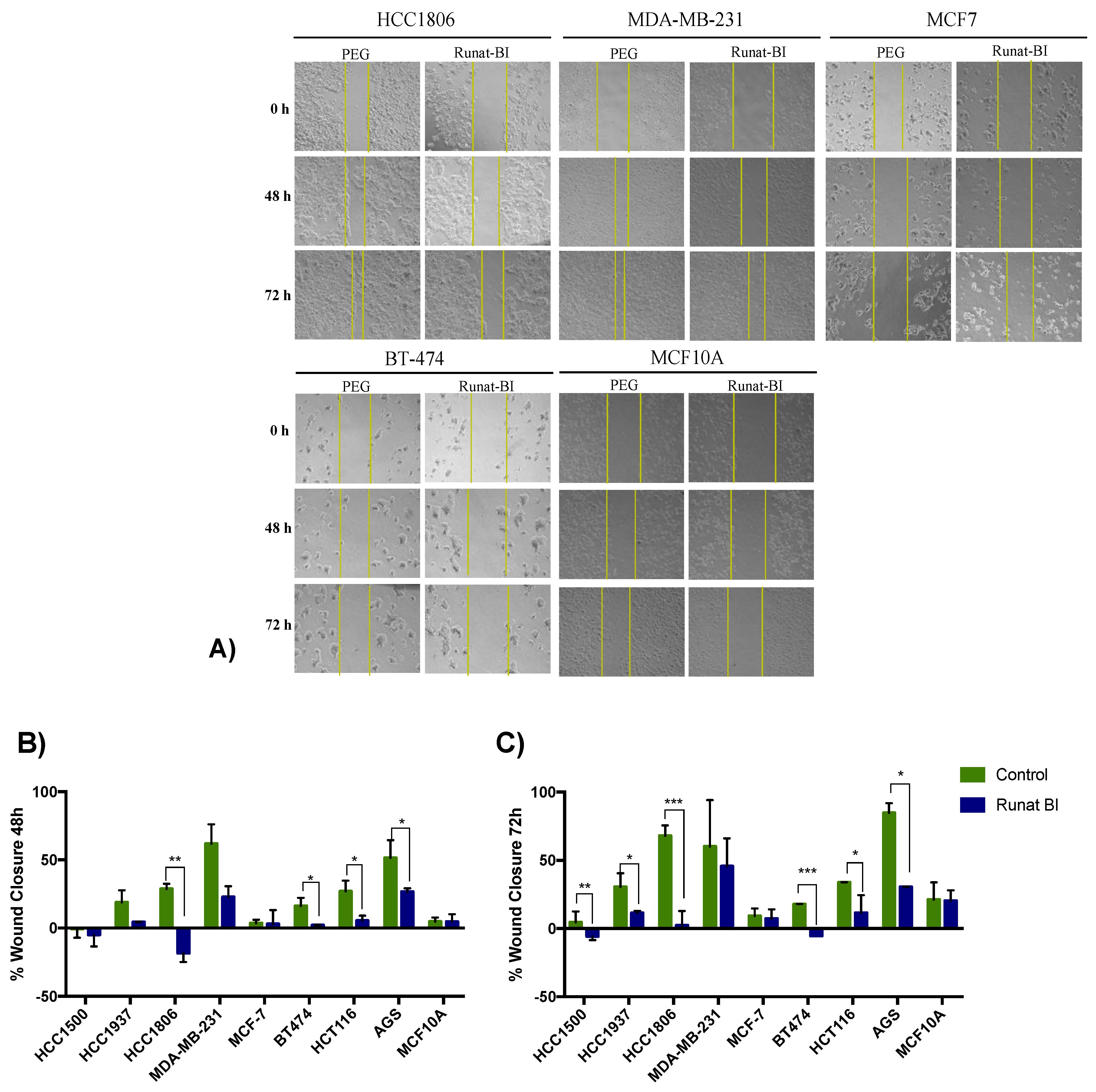
3.4. Migration Reduction in Cell Lines Treated with Runat-BI Measured by Wound Healing Assay
3.5. Relationship between Runat-BI Effect and Cell Growth Rate
3.6. Apoptosis Induced by Runat-BI Treatment
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Zielonke, N.; Kregting, L.M.; Heijnsdijk, E.A.M.; Veerus, P.; Heinävaara, S.; McKee, M.; de Kok, I.M.C.M.; de Koning, H.J.; van Ravesteyn, N.T.; EU-TOPIA Collaborators. The potential of breast cancer screening in Europe. Int. J. Cancer 2021, 148, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Montani, M.; Pazmay, G.V.B.; Hysi, A.; Lupidi, G.; Pettinari, R.; Gambini, V.; Tilio, M.; Marchetti, F.; Pettinari, C.; Ferraro, S.; et al. The water soluble ruthenium(II) organometallic compound [Ru(p-cymene)(bis(3,5 dimethylpyrazol-1-yl)methane)Cl]Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016, 107, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Hongthong, K.; Ratanaphan, A. BRCA1-Associated Triple-Negative Breast Cancer and Potential Treatment for Ruthenium-Based Compounds. Curr. Cancer Drug Targets 2016, 16, 606–617. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [PubMed]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-activity relationships for ruthenium and osmium anticancer agents-towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Gałczyńska, K.; Drulis-Kawa, Z.; Arabski, M. Antitumor activity of Pt(II), Ru(III) and Cu(II) complexes. Molecules 2020, 25, 3492. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Naves, M.A.; Graminha, A.E.; Vegas, L.C.; Luna-Dulcey, L.; Honorato, J.; Menezes, A.C.S.; Batista, A.A.; Cominetti, M.R. Transport of the Ruthenium Complex [Ru(GA)(dppe)2]PF6 into Triple-Negative Breast Cancer Cells Is Facilitated by Transferrin Receptors. Mol. Pharm. 2019, 16, 1167–1183. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Van Beijnum, J.R.; Casini, A.; Nazarov, A.A.; Wagnieres, G.; van den Bergh, H.; Dyson, P.J.; Griffioen, A.W. Organometallic ruthenium(II) arene compounds with antiangiogenic activity. J. Med. Chem. 2011, 54, 3895–3902. [Google Scholar] [CrossRef]
- Kapitza, S.; Pongratz, M.; Jakupec, M.A.; Heffeter, P.; Berger, W.; Lackinger, L.; Keppler, B.K.; Marian, B. Heterocyclic complexes of ruthenium(III) induce apoptosis in colorectal carcinoma cells. J. Cancer Res. Clin. Oncol. 2005, 131, 101–110. [Google Scholar] [CrossRef]
- Lizardo, M.M.; Morrow, J.J.; Miller, T.E.; Hong, E.S.; Ren, L.; Mendoza, A.; Halsey, C.H.; Scacheri, P.C.; Helman, L.J.; Khanna, C. Upregulation of Glucose-Regulated Protein 78 in Metastatic Cancer Cells Is Necessary for Lung Metastasis Progression. Neoplasia 2016, 18, 699–710. [Google Scholar] [CrossRef]
- Brabec, V.; Kasparkova, J. Ruthenium coordination compounds of biological and biomedical significance. DNA binding agents. Coord. Chem. Rev. 2018, 376, 75–94. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Jiang, H.; Zhang, Y.; Jiang, C.; Ma, X. Design, synthesis and pharmacological evaluation of three novel dehydroabietyl piperazine dithiocarbamate ruthenium (II) polypyridyl complexes as potential antitumor agents: DNA damage, cell cycle arrest and apoptosis induction. Molecules 2021, 26, 1453. [Google Scholar] [CrossRef]
- Popolin, C.P.; Cominetti, M.R. A Review of Ruthenium Complexes Activities on Breast Cancer Cells. Mini Rev. Med. Chem. 2017, 17, 1435–1441. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Misso, G.; Maione, F.; Montesarchio, D.; Caraglia, M.; Paduano, L.; Santamaria, R.; Irace, C. Breast Cancer Chemotherapeutic Options: A General Overview on the Preclinical Validation of a Multi-Target Ruthenium(III) Complex Lodged in Nucleolipid Nanosystems. Cells 2020, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent–Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. A Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.; Lupidi, G.; Pettinari, R.; Marchetti, F.; Di Nicola, C.; Falcioni, G.; Marchini, C.; et al. Evaluation of anticancer role of a novel ruthenium(II)-based compound compared with NAMI-A and cisplatin in impairing mitochondrial functionality and promoting oxidative stress in triple negative breast cancer models. Mitochondrion 2021, 56, 25–34. [Google Scholar] [CrossRef]
- Gaspari, A.P.S.; da Silva, R.S.; Carneiro, Z.A.; de Carvalho, M.R.; Carvalho, I.; Pernomian, L.; Ferreira, L.P.; Ramos, L.C.B.; de Souza, G.A.; Formiga, A. Improving Cytotoxicity against Breast Cancer Cells by Using Mixed-Ligand Ruthenium(II) Complexes of 2,2′-Bipyridine, Amino Acid, and Nitric Oxide Derivatives as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1602–1611. [Google Scholar] [CrossRef]
- Scolaro, C.; Geldbach, T.J.; Rochat, S.; Dorcier, A.; Gossens, C.; Bergamo, A.; Cocchietto, M.; Tavernelli, I.; Sava, G.; Rothlisberger, U.; et al. Influence of hydrogen-bonding substituents on the cytotoxicity of RAPTA compounds. Organometallics 2006, 25, 756–765. [Google Scholar] [CrossRef]
- Popolin, C.P.; Reis, J.P.B.; Becceneri, A.B.; Graminha, A.E.; Almeida, M.A.P.; Corrêa, R.S.; Colina-Vegas, L.A.; Ellena, J.; Batista, A.A.; Cominetti, M.R. Cytotoxicity and anti-tumor effects of new ruthenium complexes on triple negative breast cancer cells. PLoS ONE 2017, 12, e0183275. [Google Scholar] [CrossRef]
- Wu, Q.; He, J.; Mei, W.; Zhang, Z.; Wu, X.; Sun, F. Arene ruthenium(II) complex, a potent inhibitor against proliferation, migration and invasion of breast cancer cells, reduces stress fibers, focal adhesions and invadopodia. Metallomics 2014, 6, 2204–2212. [Google Scholar] [CrossRef]
- Bergamo, A.; Masi, A.; Dyson, P.; Sava, G. Modulation of the metastatic progression of breast cancer with an organometallic ruthenium compound. Int. J. Oncol. 2008, 33, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Hartinger, C.G.; Nazarov, A.A.; Dyson, P.J. Organometallic antitumour agents with alternative modes of action. Med. Organomet. Chem. 2010, 32, 57–80. [Google Scholar] [CrossRef]
- Kladnik, J.; Coverdale, J.P.C.; Kljun, J.; Burmeister, H.; Lippman, P.; Ellis, F.G.; Jones, A.M.; Ott, I.; Romero-Canelón, I.; Turel, I. Organoruthenium complexes with benzo-fused pyrithiones overcome platinum resistance in ovarian cancer cells. Cancers 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Orts-Arroyo, M.; Gutiérrez, F.; Gil-Tebar, A.; Ibarrola-Villava, M.; Jiménez-Martí, E.; Silvestre-Llora, A.; Castro, I.; Ribas, G.; Martínez-Lillo, J. A novel adenine-based diruthenium(III) complex: Synthesis, crystal structure, electrochemical properties and evaluation of the anticancer activity. J. Inorg. Biochem. 2022, 232, 111812–111819. [Google Scholar] [CrossRef] [PubMed]
- Bakewell, S.; Conde, I.; Fallah, Y.; McCoy, M.; Jin, L.; Shajahan-Haq, A.N. Inhibition of DNA repair pathways and induction of ROS are potential mechanisms of action of the small molecule inhibitor bold-100 in breast cancer. Cancers 2020, 12, 2647. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanell-Fernández, M.; Oltra, S.S.; Orts-Arroyo, M.; Ibarrola-Villava, M.; Carrasco, F.; Jiménez-Martí, E.; Cervantes, A.; Castro, I.; Martínez-Lillo, J.; Ribas, G. RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines. Cancers 2023, 15, 69. https://doi.org/10.3390/cancers15010069
Albanell-Fernández M, Oltra SS, Orts-Arroyo M, Ibarrola-Villava M, Carrasco F, Jiménez-Martí E, Cervantes A, Castro I, Martínez-Lillo J, Ribas G. RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines. Cancers. 2023; 15(1):69. https://doi.org/10.3390/cancers15010069
Chicago/Turabian StyleAlbanell-Fernández, Marta, Sara S. Oltra, Marta Orts-Arroyo, Maider Ibarrola-Villava, Fany Carrasco, Elena Jiménez-Martí, Andrés Cervantes, Isabel Castro, José Martínez-Lillo, and Gloria Ribas. 2023. "RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines" Cancers 15, no. 1: 69. https://doi.org/10.3390/cancers15010069
APA StyleAlbanell-Fernández, M., Oltra, S. S., Orts-Arroyo, M., Ibarrola-Villava, M., Carrasco, F., Jiménez-Martí, E., Cervantes, A., Castro, I., Martínez-Lillo, J., & Ribas, G. (2023). RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines. Cancers, 15(1), 69. https://doi.org/10.3390/cancers15010069






