RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Cell Line Culture and Treatment
2.3. Cell Proliferation Assay
2.4. Scratch/Wound Healing Assay
2.5. Cell Doubling Time (CDT)
2.6. RNA Extraction and Gene Expression by Real Time PCR (RT-qPCR)
2.7. Statistical Analysis
3. Results
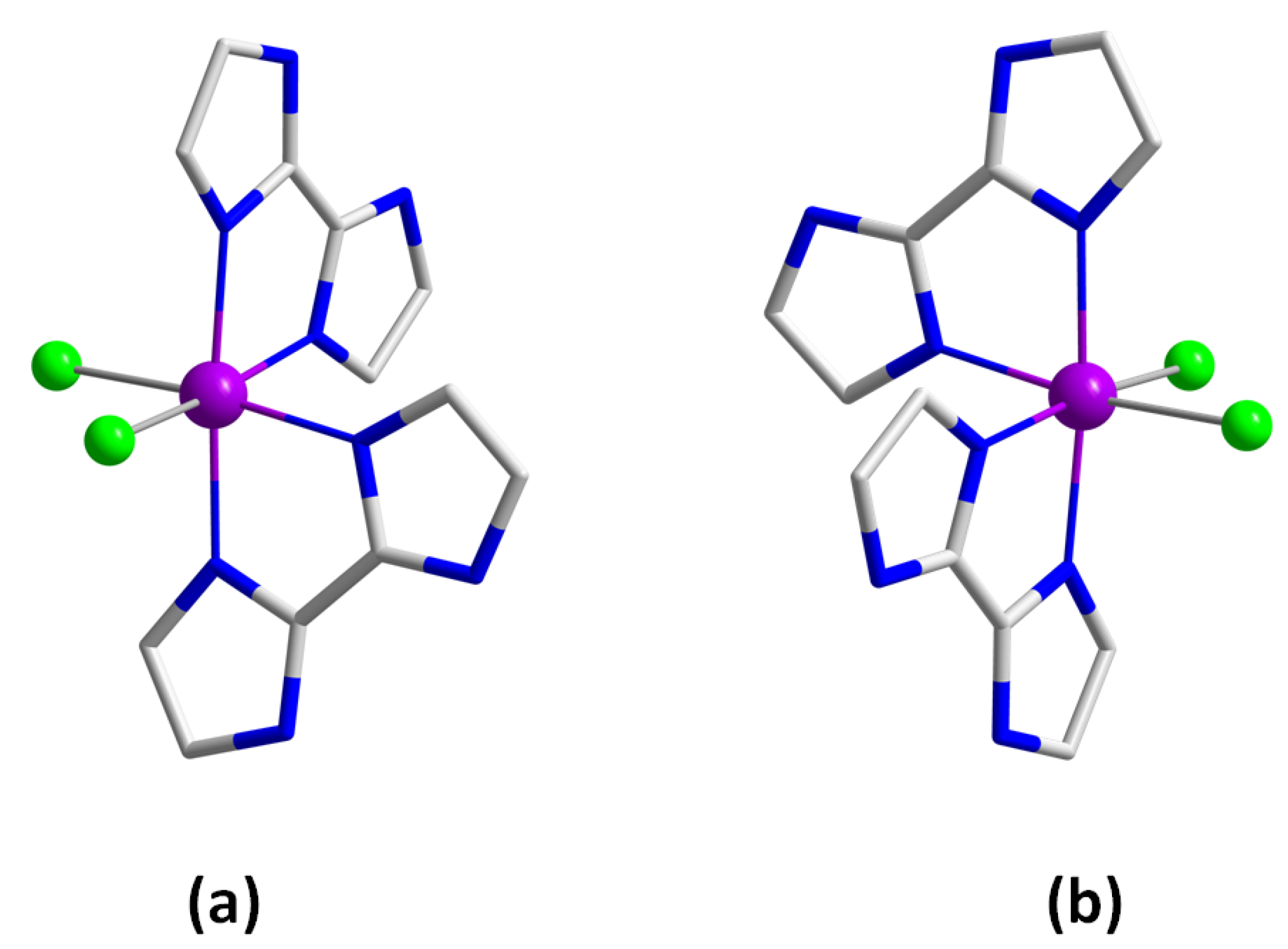
3.1. Preparation of Runat-BI
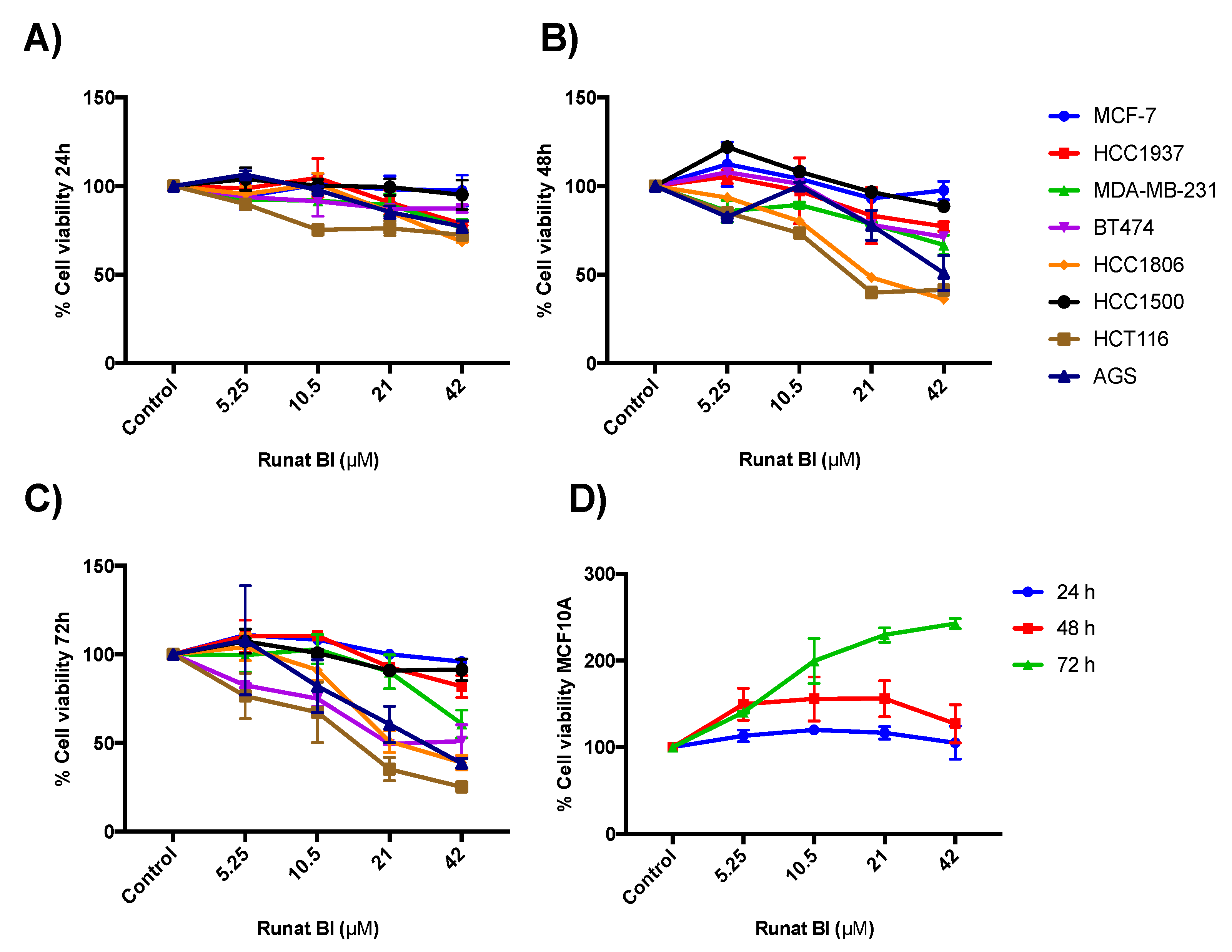
3.2. Runat-BI Reduces Proliferation in Cancer Cell Lines
3.3. Isomer 1 of Runat-BI Showed No Effect on Viability in the Cell Lines Studied
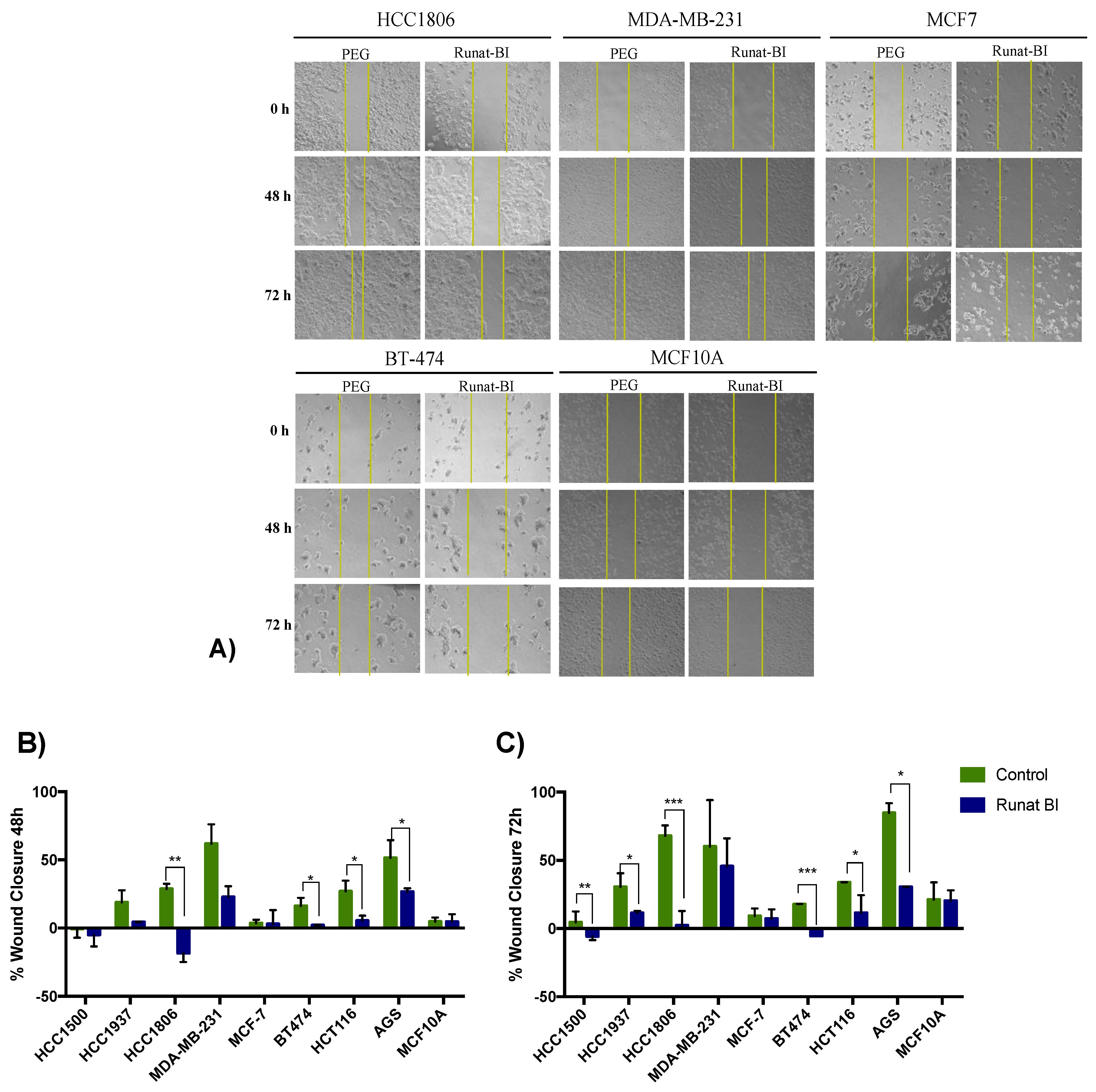
3.4. Migration Reduction in Cell Lines Treated with Runat-BI Measured by Wound Healing Assay
3.5. Relationship between Runat-BI Effect and Cell Growth Rate
3.6. Apoptosis Induced by Runat-BI Treatment
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Zielonke, N.; Kregting, L.M.; Heijnsdijk, E.A.M.; Veerus, P.; Heinävaara, S.; McKee, M.; de Kok, I.M.C.M.; de Koning, H.J.; van Ravesteyn, N.T.; EU-TOPIA Collaborators. The potential of breast cancer screening in Europe. Int. J. Cancer 2021, 148, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montani, M.; Pazmay, G.V.B.; Hysi, A.; Lupidi, G.; Pettinari, R.; Gambini, V.; Tilio, M.; Marchetti, F.; Pettinari, C.; Ferraro, S.; et al. The water soluble ruthenium(II) organometallic compound [Ru(p-cymene)(bis(3,5 dimethylpyrazol-1-yl)methane)Cl]Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016, 107, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Hongthong, K.; Ratanaphan, A. BRCA1-Associated Triple-Negative Breast Cancer and Potential Treatment for Ruthenium-Based Compounds. Curr. Cancer Drug Targets 2016, 16, 606–617. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-activity relationships for ruthenium and osmium anticancer agents-towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Gałczyńska, K.; Drulis-Kawa, Z.; Arabski, M. Antitumor activity of Pt(II), Ru(III) and Cu(II) complexes. Molecules 2020, 25, 3492. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Naves, M.A.; Graminha, A.E.; Vegas, L.C.; Luna-Dulcey, L.; Honorato, J.; Menezes, A.C.S.; Batista, A.A.; Cominetti, M.R. Transport of the Ruthenium Complex [Ru(GA)(dppe)2]PF6 into Triple-Negative Breast Cancer Cells Is Facilitated by Transferrin Receptors. Mol. Pharm. 2019, 16, 1167–1183. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Van Beijnum, J.R.; Casini, A.; Nazarov, A.A.; Wagnieres, G.; van den Bergh, H.; Dyson, P.J.; Griffioen, A.W. Organometallic ruthenium(II) arene compounds with antiangiogenic activity. J. Med. Chem. 2011, 54, 3895–3902. [Google Scholar] [CrossRef]
- Kapitza, S.; Pongratz, M.; Jakupec, M.A.; Heffeter, P.; Berger, W.; Lackinger, L.; Keppler, B.K.; Marian, B. Heterocyclic complexes of ruthenium(III) induce apoptosis in colorectal carcinoma cells. J. Cancer Res. Clin. Oncol. 2005, 131, 101–110. [Google Scholar] [CrossRef]
- Lizardo, M.M.; Morrow, J.J.; Miller, T.E.; Hong, E.S.; Ren, L.; Mendoza, A.; Halsey, C.H.; Scacheri, P.C.; Helman, L.J.; Khanna, C. Upregulation of Glucose-Regulated Protein 78 in Metastatic Cancer Cells Is Necessary for Lung Metastasis Progression. Neoplasia 2016, 18, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Brabec, V.; Kasparkova, J. Ruthenium coordination compounds of biological and biomedical significance. DNA binding agents. Coord. Chem. Rev. 2018, 376, 75–94. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Jiang, H.; Zhang, Y.; Jiang, C.; Ma, X. Design, synthesis and pharmacological evaluation of three novel dehydroabietyl piperazine dithiocarbamate ruthenium (II) polypyridyl complexes as potential antitumor agents: DNA damage, cell cycle arrest and apoptosis induction. Molecules 2021, 26, 1453. [Google Scholar] [CrossRef]
- Popolin, C.P.; Cominetti, M.R. A Review of Ruthenium Complexes Activities on Breast Cancer Cells. Mini Rev. Med. Chem. 2017, 17, 1435–1441. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Misso, G.; Maione, F.; Montesarchio, D.; Caraglia, M.; Paduano, L.; Santamaria, R.; Irace, C. Breast Cancer Chemotherapeutic Options: A General Overview on the Preclinical Validation of a Multi-Target Ruthenium(III) Complex Lodged in Nucleolipid Nanosystems. Cells 2020, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent–Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. A Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.; Lupidi, G.; Pettinari, R.; Marchetti, F.; Di Nicola, C.; Falcioni, G.; Marchini, C.; et al. Evaluation of anticancer role of a novel ruthenium(II)-based compound compared with NAMI-A and cisplatin in impairing mitochondrial functionality and promoting oxidative stress in triple negative breast cancer models. Mitochondrion 2021, 56, 25–34. [Google Scholar] [CrossRef]
- Gaspari, A.P.S.; da Silva, R.S.; Carneiro, Z.A.; de Carvalho, M.R.; Carvalho, I.; Pernomian, L.; Ferreira, L.P.; Ramos, L.C.B.; de Souza, G.A.; Formiga, A. Improving Cytotoxicity against Breast Cancer Cells by Using Mixed-Ligand Ruthenium(II) Complexes of 2,2′-Bipyridine, Amino Acid, and Nitric Oxide Derivatives as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1602–1611. [Google Scholar] [CrossRef]
- Scolaro, C.; Geldbach, T.J.; Rochat, S.; Dorcier, A.; Gossens, C.; Bergamo, A.; Cocchietto, M.; Tavernelli, I.; Sava, G.; Rothlisberger, U.; et al. Influence of hydrogen-bonding substituents on the cytotoxicity of RAPTA compounds. Organometallics 2006, 25, 756–765. [Google Scholar] [CrossRef]
- Popolin, C.P.; Reis, J.P.B.; Becceneri, A.B.; Graminha, A.E.; Almeida, M.A.P.; Corrêa, R.S.; Colina-Vegas, L.A.; Ellena, J.; Batista, A.A.; Cominetti, M.R. Cytotoxicity and anti-tumor effects of new ruthenium complexes on triple negative breast cancer cells. PLoS ONE 2017, 12, e0183275. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; He, J.; Mei, W.; Zhang, Z.; Wu, X.; Sun, F. Arene ruthenium(II) complex, a potent inhibitor against proliferation, migration and invasion of breast cancer cells, reduces stress fibers, focal adhesions and invadopodia. Metallomics 2014, 6, 2204–2212. [Google Scholar] [CrossRef]
- Bergamo, A.; Masi, A.; Dyson, P.; Sava, G. Modulation of the metastatic progression of breast cancer with an organometallic ruthenium compound. Int. J. Oncol. 2008, 33, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Hartinger, C.G.; Nazarov, A.A.; Dyson, P.J. Organometallic antitumour agents with alternative modes of action. Med. Organomet. Chem. 2010, 32, 57–80. [Google Scholar] [CrossRef]
- Kladnik, J.; Coverdale, J.P.C.; Kljun, J.; Burmeister, H.; Lippman, P.; Ellis, F.G.; Jones, A.M.; Ott, I.; Romero-Canelón, I.; Turel, I. Organoruthenium complexes with benzo-fused pyrithiones overcome platinum resistance in ovarian cancer cells. Cancers 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Orts-Arroyo, M.; Gutiérrez, F.; Gil-Tebar, A.; Ibarrola-Villava, M.; Jiménez-Martí, E.; Silvestre-Llora, A.; Castro, I.; Ribas, G.; Martínez-Lillo, J. A novel adenine-based diruthenium(III) complex: Synthesis, crystal structure, electrochemical properties and evaluation of the anticancer activity. J. Inorg. Biochem. 2022, 232, 111812–111819. [Google Scholar] [CrossRef] [PubMed]
- Bakewell, S.; Conde, I.; Fallah, Y.; McCoy, M.; Jin, L.; Shajahan-Haq, A.N. Inhibition of DNA repair pathways and induction of ROS are potential mechanisms of action of the small molecule inhibitor bold-100 in breast cancer. Cancers 2020, 12, 2647. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanell-Fernández, M.; Oltra, S.S.; Orts-Arroyo, M.; Ibarrola-Villava, M.; Carrasco, F.; Jiménez-Martí, E.; Cervantes, A.; Castro, I.; Martínez-Lillo, J.; Ribas, G. RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines. Cancers 2023, 15, 69. https://doi.org/10.3390/cancers15010069
Albanell-Fernández M, Oltra SS, Orts-Arroyo M, Ibarrola-Villava M, Carrasco F, Jiménez-Martí E, Cervantes A, Castro I, Martínez-Lillo J, Ribas G. RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines. Cancers. 2023; 15(1):69. https://doi.org/10.3390/cancers15010069
Chicago/Turabian StyleAlbanell-Fernández, Marta, Sara S. Oltra, Marta Orts-Arroyo, Maider Ibarrola-Villava, Fany Carrasco, Elena Jiménez-Martí, Andrés Cervantes, Isabel Castro, José Martínez-Lillo, and Gloria Ribas. 2023. "RUNAT-BI: A Ruthenium(III) Complex as a Selective Anti-Tumor Drug Candidate against Highly Aggressive Cancer Cell Lines" Cancers 15, no. 1: 69. https://doi.org/10.3390/cancers15010069






