Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
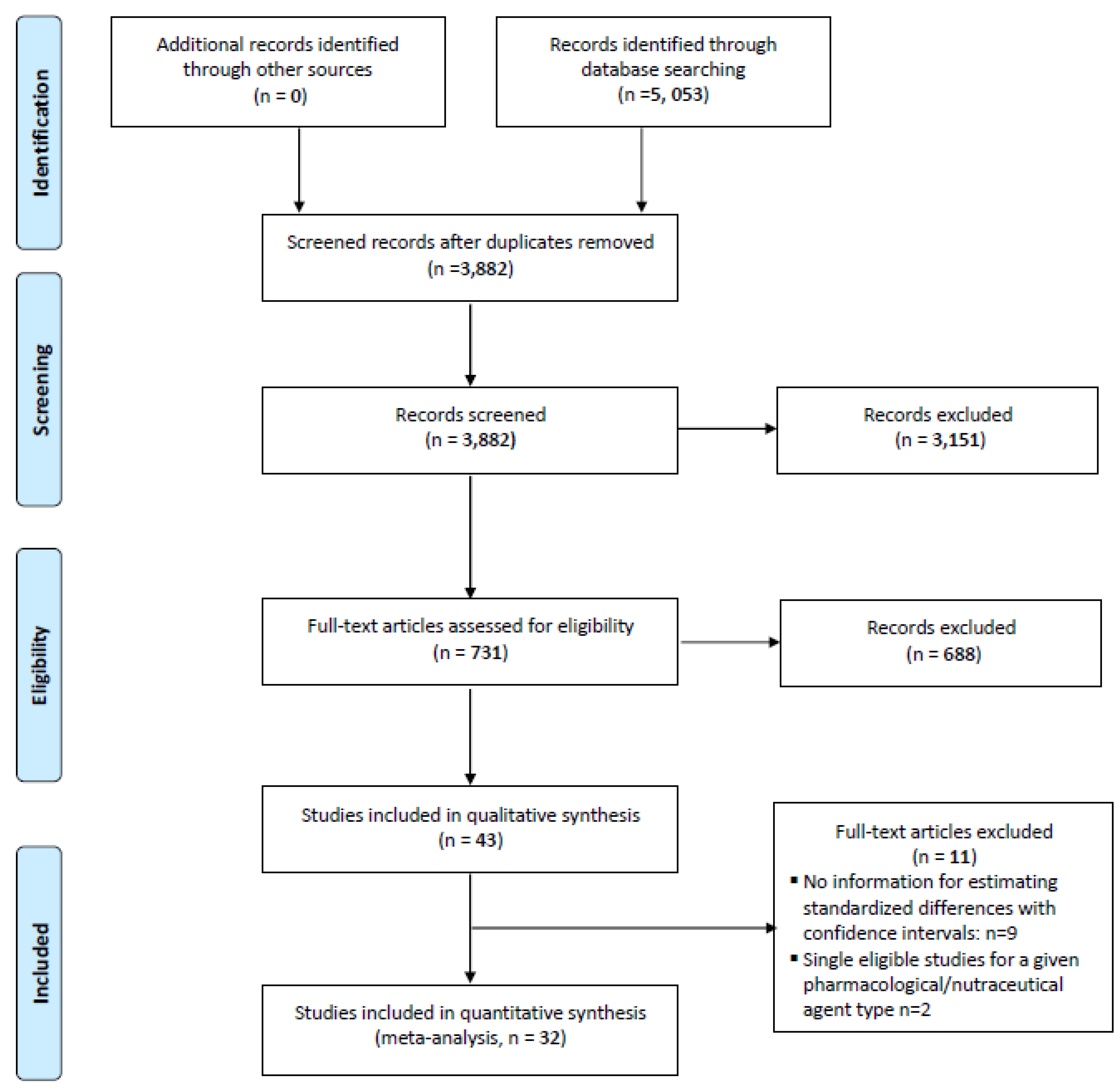
3. Study Selection
4. Collection and Analysis
4.1. Search Terms Used
- Fatigue And Cancer (Primary/Broad)
- “cancer” “neoplasm”, “tumor”, “oncology”, “fatigue”, “tiredness”, “weary”, “weariness”, “exhaustion”, “exhausted”, “lackluster”, “asthenia”, “lassitude”, “lack of energy”, “drug therapy”, “diet therapy”, “central nervous system stimulants”, ”methylphenidate”, “dextroamphetamine”, “dexmethylphenidate”, “psychostimulants”, “psychotropic”, “modafinil”, “armodafinil”, “pemoline”, “donepezil”, “amantadine”, “etanercept”, “antidepressive agents”, “serotonin uptake inhibitors”, “sertraline”, “paroxetine”, “fluoxetine“, “acetylsalicylic acid”, “aspirin”, “adrenal cortex hormones”, “glucocorticoids”, “corticosteroids”, “steroids”, “dexamethasone”, “methylprednisolone”, “progestins”, “progestational steroids”, “testosterone”, “thyrotropin-releasing hormone”, “erythropoietin”, “darbepoetin”, “adenosine triphosphate”, “thyroliberin”, “fish oils”, “docosahexaenoic acids”, “vitamin D”, “carnitine”, “levocarnitine”, “anticytokine”, “antineoplastic agents”, “medicinal plant”, “herbal medicine”, “phytotherapy”, “mistletoe”, “ginseng”, “paullinia”, “astragalus” and “placebo” etc.
4.2. Data Extraction
4.3. Statistical Analysis
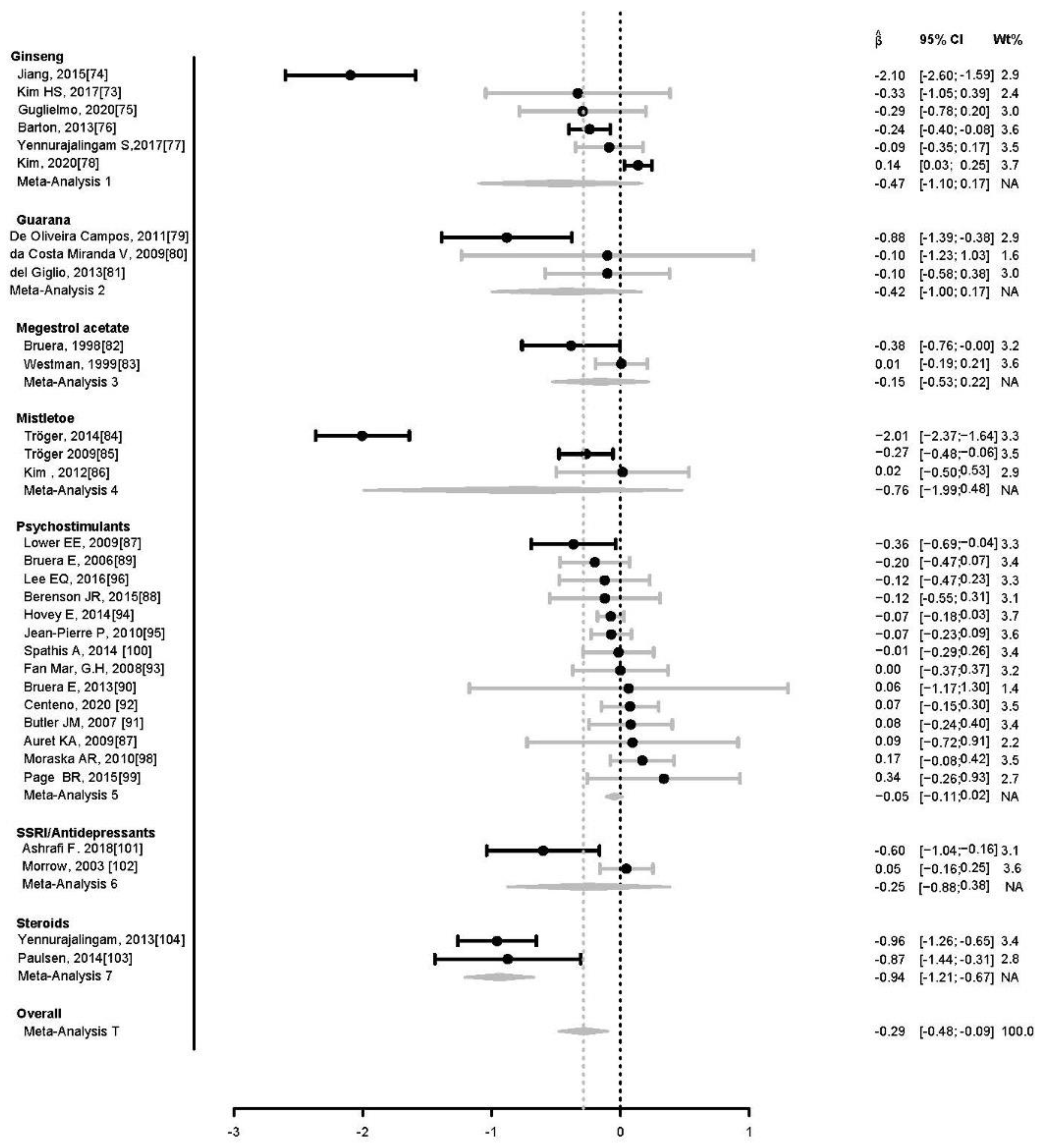
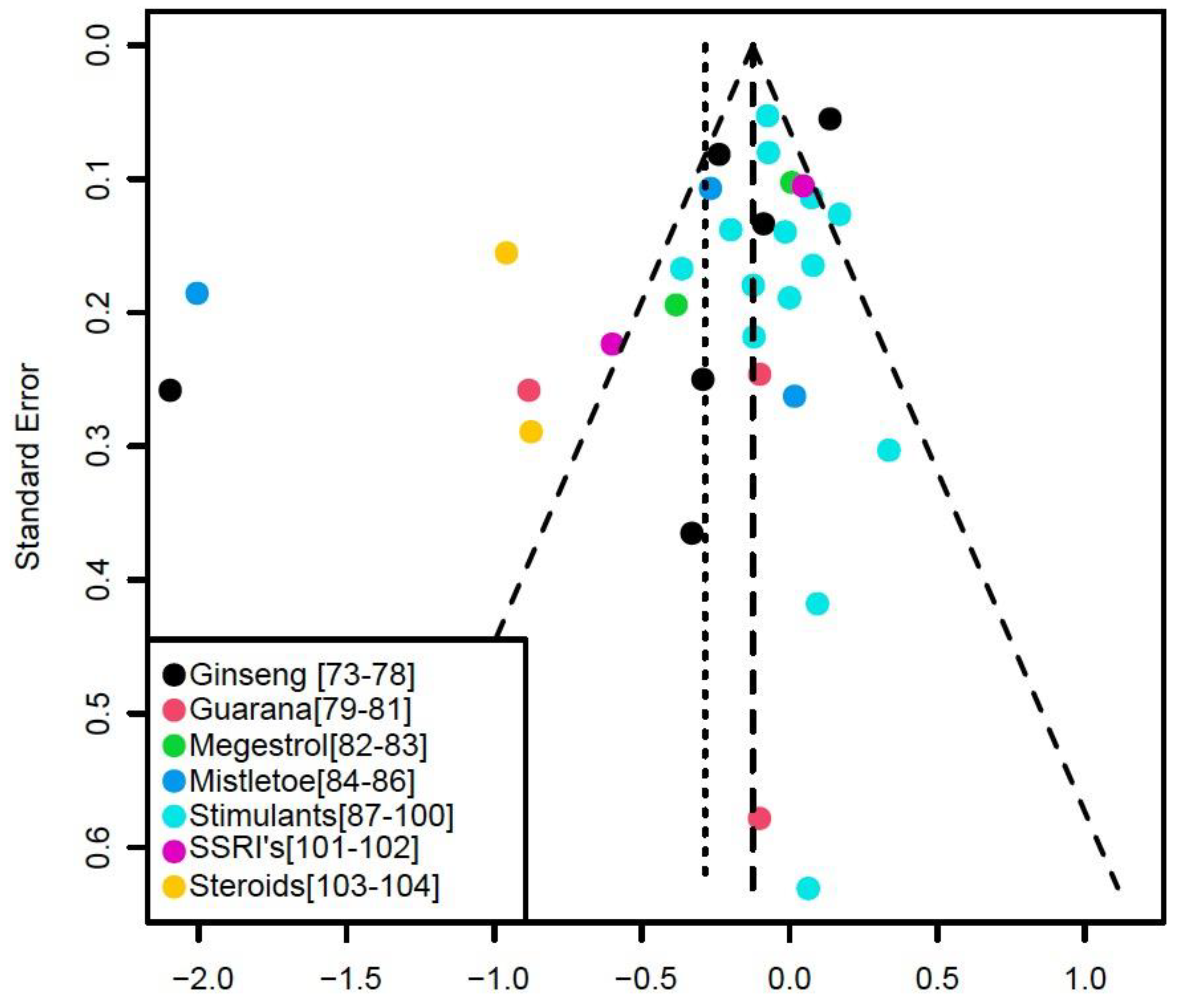
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- NIH. State-of-the-Science Statement on symptom management in cancer: Pain, depression, and fatigue. NIH Consens State Sci. Statements 2002, 19, 77–97. [Google Scholar]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Peterman, A.; Passik, S.; Jacobsen, P.; Breitbart, W. Progress toward guidelines for the management of fatigue. Oncology 1998, 12, 369–377. [Google Scholar] [PubMed]
- Minasian, L.M.; O’Mara, A.M.; Reeve, B.B.; Denicoff, A.M.; Kelaghan, J.; Rowland, J.H.; Trimble, E.L. Health-Related Quality of Life and Symptom Management Research Sponsored by the National Cancer Institute: Patient-reported outcomes assessment in cancer trials. J. Clin. Oncol. 2007, 25, 5128–5132. [Google Scholar] [CrossRef]
- Stone, P.; Richardson, A.; Ream, E.; Smith, A.G.; Kerr, D.J.; Kearney, N. Cancer-related fatigue: Inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Ann. Oncol. 2000, 11, 971–976. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist. 2000, 5, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Pollack, L.A.; Rowland, J.H.; Crammer, C.; Stefanek, M. Introduction: Charting the landscape of cancer survivors’ health-related outcomes and care. Cancer 2009, 115, 4265–4269. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Kupelnick, B.; Miller, K.; Devine, D.; Lau, J. Evidence Report on the Occurrence, Assessment, and Treatment of Fatigue in Cancer Patients. JNCI Monogr. 2004, 2004, 40–50. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten public health impacts of cancer—An overview. Arch. Ind. Hyg. Toxicol. 2017, 68, 287–297. [Google Scholar] [CrossRef] [Green Version]
- De Raaf, P.J.; de Klerk, C.; Timman, R.; Hinz, A.; van der Rijt, C.C. Differences in fatigue experiences among patients with advanced cancer, cancer survivors, and the general population. J. Pain Symptom Manag. 2012, 44, 823–830. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; O’Brien, K. Cancer and Cancer-Related Fatigue and the Interrelationships with Depression, Stress, and Inflammation. J. Evid.-Based Complement. Altern. Med. 2016, 22, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Bohlius, J.; Tonia, T.; Nüesch, E.; Jüni, P.; Fey, M.F.; Egger, M.; Bernhard, J. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: Systematic review and meta-analyses of published and unpublished data. Br. J. Cancer 2014, 111, 33–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruera, E.; El Osta, B.; Valero, V.; Driver, L.C.; Pei, B.-L.; Shen, L.; Poulter, V.A.; Palmer, J.L. Donepezil for Cancer Fatigue: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2007, 25, 3475–3481. [Google Scholar] [CrossRef] [PubMed]
- McGovern, K.A.; Durham, W.J.; Wright, T.J.; Dillon, E.L.; Randolph, K.M.; Danesi, C.P.; Urban, R.J.; Sheffield-Moore, M. Impact of Adjunct Testosterone on Cancer-Related Fatigue: An Ancillary Analysis from a Controlled Randomized Trial. Curr. Oncol. 2022, 29, 8340–8356. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E.; Garcia, J.M.; Dev, R.; Hui, D.; Williams, J.; Engineer, D.; Palmer, J.L.; Schover, L.; Bruera, E. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: A preliminary double-blind placebo-controlled trial. Support. Care Cancer 2013, 21, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Inglis, J.E.; Mustian, K.M.; Heckler, C.E.; A Padula, G.D.; Mohile, S.G.; Kamen, C.S.; Culakova, E.; Lin, P.-J.; Kerns, S.L.; et al. Multicenter Randomized Controlled Trial of Omega-3 Fatty Acids versus Omega-6 Fatty Acids for the Control of Cancer-Related Fatigue among Breast Cancer Survivors. JNCI Cancer Spectr. 2019, 3, pkz005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, Y.; Tsuji, K.; Ochi, E. Polyunsaturated Fatty Acids, Exercise, and Cancer-Related Fatigue in Breast Cancer Survivors. Front. Physiol. 2021, 12, 759280. [Google Scholar] [CrossRef]
- Bruera, E.; Strasser, F.; Palmer, J.L.; Willey, J.; Calder, K.; Amyotte, G.; Baracos, V. Effect of Fish Oil on Appetite and Other Symptoms in Patients with Advanced Cancer and Anorexia/Cachexia: A Double-Blind, Placebo-Controlled Study. J. Clin. Oncol. 2003, 21, 129–134. [Google Scholar] [CrossRef]
- Matsui, H.; Einama, T.; Shichi, S.; Kanazawa, R.; Shibuya, K.; Suzuki, T.; Matsuzawa, F.; Hashimoto, T.; Homma, S.; Yamamoto, J.; et al. L-Carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Mol. Clin. Oncol. 2018, 8, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, R.A.; Dvorkin, E.; Homel, P.; Culliney, B.; Malamud, S.; Lapin, J.; Portenoy, R.K.; Esteban-Cruciani, N. L-Carnitine Supplementation in Patients with Advanced Cancer and Carnitine Deficiency: A Double-Blind, Placebo-Controlled Study. J. Pain Symptom Manag. 2009, 37, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Teleni, L.; Opie, R.S.; Kelly, J.; Marshall, S.; Itsiopoulos, C.; Isenring, E. Efficacy and Effectiveness of Carnitine Supplementation for Cancer-Related Fatigue: A Systematic Literature Review and Meta-Analysis. Nutrients 2017, 9, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.W.; Lin, I.H.; Chen, Y.J.; Chang, K.H.; Wu, M.H.; Su, W.H.; Huang, G.C.; Lai, Y.L. A novel infusible botanically-derived drug, PG2, for cancer-related fatigue: A phase II double-blind, randomized placebo-controlled study. Clin. Investig. Med. 2012, 35, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswal, B.M.; Sulaiman, S.A.; Ismail, H.C.; Zakaria, H.; Musa, K.I. Effect of Withania somnifera (Ashwagandha) on the Development of Chemotherapy-Induced Fatigue and Quality of Life in Breast Cancer Patients. Integr. Cancer Ther. 2012, 12, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, J.; Feinn, R.; Winokur, A. Thyrotropin-releasing hormone as a treatment for cancer-related fatigue: A randomized controlled study. Support. Care Cancer 2011, 20, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the gut microbiome and fatigue in cancer patients. Sci. Rep. 2021, 11, 5847. [Google Scholar] [CrossRef]
- Lesser, G.J.; Case, D.; Stark, N.; Williford, S.; Giguere, J.; Garino, L.A.; Naughton, M.J.; Vitolins, M.Z.; Lively, M.O.; Shaw, E.G. A Randomized, Double-Blind, Placebo-Controlled Study of Oral Coenzyme Q10 to Relieve Self-Reported Treatment-Related Fatigue in Newly Diagnosed Patients with Breast Cancer. J. Support. Oncol. 2012, 11, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; A Castillo, D.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.S.; Chen, Y.; Shi, Q.; Chen, T.H.; Li, P. A Phase II Randomized Controlled Trial of Renshen Yangrong Tang Herbal Extract Granules for Fatigue Reduction in Cancer Survivors. J. Pain Symptom Manag. 2019, 59, 966–973. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Cheung, W.K.; Wu, I.X.; Xiao, F.; Chung, V.C. Pharmacological Interventions for the Management of Cancer-Related Fatigue Among Cancer Survivors: Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2021, 20, 15347354211038008. [Google Scholar] [CrossRef]
- Ramasamy, V.; Lazim, N.B.M.; Abdullah, B.; Singh, A. Effects of Tualang Honey on Cancer Related Fatigue: A Multicenter Open-label Trial of H&N Cancer Patients. Gulf J. Oncolog. 2019, 1, 43–51. [Google Scholar] [PubMed]
- Pereira, P.T.V.T.; Reis, A.D.; Diniz, R.R.; Lima, F.A.; Leite, R.D.; da Silva, M.C.P.; Guerra, R.N.M.; Vieira, B.D.M.; Garcia, J.B.S. Dietary supplements and fatigue in patients with breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saligan, L.N.; Olson, K.; Filler, K.; Larkin, D.; Cramp, F.; Yennurajalingam, S.; Escalante, C.P.; del Giglio, A.; Kober, K.M.; Kamath, J.; et al. Multinational Association of Supportive Care in Cancer Fatigue Study Group-Biomarker Working, G., The biology of cancer-related fatigue: A review of the literature. Support Care Cancer 2015, 23, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Kimko, H.C.; Cross, J.T.; Abernethy, D.R. Pharmacokinetics and Clinical Effectiveness of Methylphenidate. Clin. Pharmacokinet. 1999, 37, 457–470. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M.; Andrzejewski, M.E.; Arnsten, A.F.; Kelley, A.E.; Schmeichel, B.; Hamilton, C.; Spencer, R.C. Methylphenidate Preferentially Increases Catecholamine Neurotransmission within the Prefrontal Cortex at Low Doses that Enhance Cognitive Function. Biol. Psychiatry 2006, 60, 1111–1120. [Google Scholar] [CrossRef]
- Gerrard, P.; Malcolm, R. Mechanisms of modafinil: A review of current research. Neuropsychiatr. Dis. Treat. 2007, 3, 349–364. [Google Scholar]
- Argilés, J.M.; Anguera, A.; Stemmler, B. A new look at an old drug for the treatment of cancer cachexia: Megestrol acetate. Clin. Nutr. 2013, 32, 319–324. [Google Scholar] [CrossRef]
- House, L.; Seminerio, M.J.; Mirkov, S.; Ramirez, J.; Skor, M.; Sachleben, J.R.; Isikbay, M.; Singhal, H.; Greene, G.L.; Griend, D.V.; et al. Metabolism of megestrol acetate in vitro and the role of oxidative metabolites. Xenobiotica 2017, 48, 973–983. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Owens, M.J. Pharmacologic differences among the SSRIs: Focus on monoamine transporters and the HPA axis. CNS Spectr. 2004, 9, 23–31. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Liu, L.; Qiao, D.; Baldwin, D.S.; Hou, R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2019, 79, 24–38. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yennurajalingam, S.; Bruera, E. Palliative Management of Fatigue at the Close of Life. JAMA 2007, 297, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lemke, E.A. Ginseng for the Management of Cancer-Related Fatigue: An Integrative Review. J. Adv. Pract. Oncol. 2021, 12, 406–414. [Google Scholar] [PubMed]
- Sadeghian, M.; Rahmani, S.; Zendehdel, M.; Hosseini, S.A.; Javid, A.Z. Ginseng and Cancer-Related Fatigue: A Systematic Review of Clinical Trials. Nutr. Cancer 2020, 73, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, J.; Robertson, H.; Hagg, T.; Drobitch, R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 2003, 184, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Newmark, H.; Zhou, R. Neuroprotective Effects of Ginseng Total Saponin and Ginsenosides Rb1 and Rg1 on Spinal Cord Neurons in Vitro. Exp. Neurol. 2002, 173, 224–234. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, K.; Oh, T.H.; Nah, S.-Y.; Rhim, H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 296, 247–254. [Google Scholar] [CrossRef]
- Itoh, T.; Zang, Y.F.; Murai, S.; Saito, H. Effects of Panax ginseng Root on the Vertical and Horizontal Motor Activities and on Brain Monoamine-Related Substances in Mice. Planta Med. 1989, 55, 429–433. [Google Scholar] [CrossRef]
- Pannacci, M.; Lucini, V.; Colleoni, F.; Martucci, C.; Grosso, S.; Sacerdote, P.; Scaglione, F. Panax ginseng C.A. Mayer G115 modulates pro-inflammatory cytokine production in mice throughout the increase of macrophage toll-like receptor 4 expression during physical stress. Brain Behav. Immun. 2006, 20, 546–551. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Wargovich, M.J. Inflammation, Cancer, and Targets of Ginseng. J. Nutr. 2007, 137, 183S–185S. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.A.F.S.; Pinaffi-Langley, A.C.d.C.; Figueira, M.D.S.; Cordeiro, K.S.; Negrão, L.D.; Soares, M.J.; da Silva, C.P.; Alfino, M.C.Z.; Sampaio, G.R.; Camargo, A.C. Effects of the consumption of guarana on human health: A narrative review. Compr. Rev. Food Sci. Food Saf. 2021, 21, 272–295. [Google Scholar] [CrossRef] [PubMed]
- Schimpl, F.C.; Kiyota, E.; Mayer, J.L.S.; Gonçalves, J.F.D.C.; da Silva, J.F.; Mazzafera, P. Molecular and biochemical characterization of caffeine synthase and purine alkaloid concentration in guarana fruit. Phytochemistry 2014, 105, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Schimpl, F.C.; da Silva, J.F.; Gonçalves, J.F.D.C.; Mazzafera, P. Guarana: Revisiting a highly caffeinated plant from the Amazon. J. Ethnopharmacol. 2013, 150, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.L.M.; Ferreira, E.D.F.; de Paula, M.N.; Klein, T.; de Mello, J.C.P. Paullinia cupana: A multipurpose plant—A review. Rev. Bras. De Farm. 2018, 29, 77–110. [Google Scholar] [CrossRef]
- Kennedy, D.; Haskell, C.; Robertson, B.; Reay, J.; Brewster-Maund, C.; Luedemann, J.; Maggini, S.; Ruf, M.; Zangara, A.; Scholey, A. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added guaraná (Paullinia cupana). Appetite 2008, 50, 506–513. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Oei, S.L.; Thronicke, A.; Schad, F. Mistletoe and Immunomodulation: Insights and Implications for Anticancer Therapies. Evid.-Based Complement. Altern. Med. 2019, 2019, 5893017. [Google Scholar] [CrossRef]
- Klasson, C.; Frankling, M.H.; Hagelin, C.L.; Björkhem-Bergman, L. Fatigue in Cancer Patients in Palliative Care—A Review on Pharmacological Interventions. Cancers 2021, 13, 985. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef] [Green Version]
- Yennurajalingam, S.; Bruera, E. Review of Clinical Trials of Pharmacologic Interventions for Cancer-Related Fatigue: Focus on Psychostimulants and Steroids. Cancer J. 2014, 20, 319–324. [Google Scholar] [CrossRef]
- Chow, R.; Bruera, E.; Sanatani, M.; Chiu, L.; Prsic, E.; Boldt, G.; Lock, M. Cancer-related fatigue—Pharmacological interventions: Systematic review and network meta-analysis. BMJ Support. Palliat. Care 2021. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Mochamat, M.; Cuhls, H.; Peuckmann-Post, V.; Minton, O.; Stone, P.; Radbruch, L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst. Rev. 2015, 2015, Cd006788. [Google Scholar] [PubMed]
- Tomlinson, D.; Robinson, P.D.; Oberoi, S.; Cataudella, D.; Culos-Reed, N.; Davis, H.; Duong, N.; Gibson, F.; Götte, M.; Hinds, P.; et al. Pharmacologic Interventions for Fatigue in Cancer and Transplantation: A Meta-Analysis. Curr. Oncol. 2018, 25, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, J.E.; Bak, K.; Berger, A.; Breitbart, W.; Escalante, C.P.; Ganz, P.A.; Schnipper, H.H.; Lacchetti, C.; Ligibel, J.A.; Lyman, G.H.; et al. Screening, Assessment, and Management of Fatigue in Adult Survivors of Cancer: An American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J. Clin. Oncol. 2014, 32, 1840–1850. [Google Scholar] [CrossRef] [Green Version]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Wang, X.S.; Woodruff, J.F. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015, 136, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. Cochrane Statistical Methods, G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Pashaki, A.S.; Mohammadian, K.; Afshar, S.; Gholami, M.H.; Moradi, A.; Javadinia, S.A.; Amlashi, Z.K. A Randomized, Controlled, Parallel-Group, Trial on the Effects of Melatonin on Fatigue Associated with Breast Cancer and Its Adjuvant Treatments. Integr. Cancer Ther. 2021, 20, 1534735420988343. [Google Scholar] [CrossRef]
- Wang, X.S.; Shi, Q.; Mendoza, T.; Lin, S.; Chang, J.Y.; Bokhari, R.H.; Lin, H.-K.; Garcia-Gonzalez, A.; Kamal, M.; Cleeland, C.S.; et al. Minocycline Reduces Chemoradiation-Related Symptom Burden in Patients with Non-Small Cell Lung Cancer: A Phase 2 Randomized Trial. Int. J. Radiat. Oncol. 2019, 106, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 November 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. EÉvid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin Ginseng (Panax quinquefolius) to Improve Cancer-Related Fatigue: A Randomized, Double-Blind Trial, N07C2. Gynecol. Oncol. 2013, 105, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-L.; Liu, H.-J.; Liu, Z.-C.; Liu, N.; Liu, R.; Kang, Y.-R.; Ji, J.-G.; Zhang, C.; Hua, B.-J.; Kang, S.-J. Adjuvant effects of fermented red ginseng extract on advanced non-small cell lung cancer patients treated with chemotherapy. Chin. J. Integr. Med. 2015, 23, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.-K.; Lee, M.; Kwon, B.-S.; Suh, D.H.; Song, Y.S. Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial. Nutrients 2017, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Yennurajalingam, S.; Tannir, N.M.; Williams, J.L.; Lu, Z.; Hess, K.R.; Frisbee-Hume, S.; House, H.L.; Lim, Z.D.; Lim, K.H.; Lopez, G.; et al. A Double-Blind, Randomized, Placebo-Controlled Trial of Panax Ginseng for Cancer-Related Fatigue in Patients with Advanced Cancer. J. Natl. Compr. Canc. Netw. 2017, 15, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Guglielmo, M.; Di Pede, P.; Alfieri, S.; Bergamini, C.; Platini, F.; Ripamonti, C.I.; Orlandi, E.; Iacovelli, N.A.; Licitra, L.; Maddalo, M.; et al. A randomized, double-blind, placebo controlled, phase II study to evaluate the efficacy of ginseng in reducing fatigue in patients treated for head and neck cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 2479–2487. [Google Scholar] [CrossRef]
- Kim, J.W.; Han, S.W.; Cho, J.Y.; Chung, I.-J.; Kim, J.G.; Lee, K.H.; Park, K.U.; Baek, S.K.; Oh, S.C.; Lee, M.A.; et al. Korean red ginseng for cancer-related fatigue in colorectal cancer patients with chemotherapy: A randomised phase III trial. Eur. J. Cancer 2020, 130, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Miranda, V.D.C.; Trufelli, D.C.; Santos, J.; Campos, M.P.; Nobuo, M.; Miranda, M.D.C.; Schlinder, F.; Riechelmann, R.; del Giglio, A. Effectiveness of Guaraná (Paullinia cupana) for Postradiation Fatigue and Depression: Results of a Pilot Double-Blind Randomized Study. J. Altern. Complement. Med. 2009, 15, 431–433. [Google Scholar] [CrossRef]
- Campos, M.P.D.O.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.A.; Del Giglio, A. Guarana (Paullinia cupana) Improves Fatigue in Breast Cancer Patients Undergoing Systemic Chemotherapy. J. Altern. Complement. Med. 2011, 17, 505–512. [Google Scholar] [CrossRef]
- Del Giglio, A.B.; Cubero, D.D.I.G.; Lerner, T.G.; Guariento, R.T.; de Azevedo, R.G.S.; Paiva, H.; Goldman, C.; Carelli, B.; Cruz, F.M.; Schindler, F.; et al. Purified Dry Extract of Paullinia cupana (Guaraná) (PC-18) for Chemotherapy-Related Fatigue in Patients with Solid Tumors: An Early Discontinuation Study. J. Diet. Suppl. 2013, 10, 325–334. [Google Scholar] [CrossRef]
- Bruera, E.; Ernst, S.; Hagen, N.; Spachynski, K.; Belzile, M.; Hanson, J.; Summers, N.; Brown, B.; Dulude, H.; Gallant, G. Effectiveness of megestrol acetate in patients with advanced cancer: A randomized, double-blind, crossover study. Cancer Prev. Control 1998, 2, 74–78. [Google Scholar] [PubMed]
- Westman, G.; Bergman, B.; Albertsson, M.; Kadar, L.; Gustavsson, G.; Thaning, L.; Andersson, M.; Straumits, A.; Jeppson, B.; Lindén, C.-J.; et al. Megestrol acetate in advanced, progressive, hormone-insensitive cancer. Effects on the quality of life: A placebo-controlled, randomised, multicentre trial. Eur. J. Cancer 1999, 35, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Yook, J.H.; Eisenbraun, J.; Kim, B.S.; Huber, R. Quality of life, immunomodulation and safety of adjuvant mistletoe treatment in patients with gastric carcinoma—A randomized, controlled pilot study. BMC Complement. Altern. Med. 2012, 12, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tröger, W.; Jezdić, S.; Ždrale, Z.; Tišma, N.; Hamre, H.J.; Matijašević, M. Quality of Life and Neutropenia in Patients with Early Stage Breast Cancer: A Randomized Pilot Study Comparing Additional Treatment with Mistletoe Extract to Chemotherapy Alone. Breast Cancer Basic Clin. Res. 2009, 3, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: A randomized controlled trial. Dtsch. Arztebl. Int. 2014, 111, 493–502. [Google Scholar]
- Auret, K.A.; Schug, S.A.; Bremner, A.P.; Bulsara, M. A Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Impact of Dexamphetamine on Fatigue in Patients with Advanced Cancer. J. Pain Symptom Manag. 2009, 37, 613–621. [Google Scholar] [CrossRef]
- Berenson, J.R.; Yellin, O.; Shamasunder, H.K.; Chen, C.-S.; Charu, V.; Woliver, T.B.; Sanani, S.; Schlutz, M.; Nassir, Y.; Swift, R.A.; et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support. Care Cancer 2014, 23, 1503–1512. [Google Scholar] [CrossRef]
- Bruera, E.; Valero, V.; Driver, L.; Shen, L.; Willey, J.; Zhang, T.; Palmer, J.L. Patient-Controlled Methylphenidate for Cancer Fatigue: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2006, 24, 2073–2078. [Google Scholar] [CrossRef]
- Bruera, E.; Yennurajalingam, S.; Palmer, J.L.; Perez-Cruz, P.E.; Frisbee-Hume, S.; Allo, J.A.; Williams, J.L.; Cohen, M.Z. Methylphenidate and/or a Nursing Telephone Intervention for Fatigue in Patients with Advanced Cancer: A Randomized, Placebo-Controlled, Phase II Trial. J. Clin. Oncol. 2013, 31, 2421–2427. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.M.; Case, L.D.; Atkins, J.; Frizzell, B.; Sanders, G.; Griffin, P.; Lesser, G.; McMullen, K.; McQuellon, R.; Naughton, M.; et al. A Phase III, Double-Blind, Placebo-Controlled Prospective Randomized Clinical Trial of d-Threo-Methylphenidate HCl in Brain Tumor Patients Receiving Radiation Therapy. Int. J. Radiat. Oncol. 2007, 69, 1496–1501. [Google Scholar] [CrossRef]
- Centeno, C.; Rojí, R.; Portela, M.A.; De Santiago, A.; Cuervo, M.A.; Ramos, D.; Gandara, A.; Salgado, E.; Gagnon, B.; Sanz, A. Improved cancer-related fatigue in a randomised clinical trial: Methylphenidate no better than placebo. BMJ Support. Palliat. Care 2020, 12, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.G.M.; Clemons, M.; Xu, W.; Chemerynsky, I.; Breunis, H.; Braganza, S.; Tannock, I.F. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support. Care Cancer 2007, 16, 577–583. [Google Scholar] [CrossRef]
- Hovey, E.; de Souza, P.; Marx, G.; Parente, P.; Rapke, T.; Hill, A.; Bonaventura, A.; Michele, A.; Craft, P.; Abdi, E.; et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support. Care Cancer 2013, 22, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, P.; Morrow, G.R.; Roscoe, J.A.; Heckler, C.; Mohile, S.; Janelsins, M.; Peppone, L.; Hemstad, A.; Esparaz, B.T.; Hopkins, J.O. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: A University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010, 116, 3513–3520. [Google Scholar] [PubMed]
- Lee, E.Q.; Muzikansky, A.; Drappatz, J.; Kesari, S.; Wong, E.T.; Fadul, C.E.; Reardon, D.A.; Norden, A.D.; Nayak, L.; Rinne, M.L.; et al. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro-Oncol. 2016, 18, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Lower, E.E.; Fleishman, S.; Cooper, A.; Zeldis, J.; Faleck, H.; Yu, Z.; Manning, D. Efficacy of Dexmethylphenidate for the Treatment of Fatigue After Cancer Chemotherapy: A Randomized Clinical Trial. J. Pain Symptom Manag. 2009, 38, 650–662. [Google Scholar] [CrossRef]
- Moraska, A.R.; Sood, A.; Dakhil, S.R.; Sloan, J.A.; Barton, D.; Atherton, P.J.; Suh, J.J.; Griffin, P.C.; Johnson, D.B.; Ali, A.; et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Long-Acting Methylphenidate for Cancer-Related Fatigue: North Central Cancer Treatment Group NCCTG-N05C7 Trial. J. Clin. Oncol. 2010, 28, 3673–3679. [Google Scholar] [CrossRef] [Green Version]
- Page, B.R.; Shaw, E.G.; Lu, L.; Bryant, D.; Grisell, D.; Lesser, G.J.; Monitto, D.C.; Naughton, M.J.; Rapp, S.R.; Savona, S.R.; et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation-induced fatigue. Neuro-Oncol. 2015, 17, 1393–1401. [Google Scholar] [CrossRef]
- Spathis, A.; Fife, K.; Blackhall, F.; Dutton, S.; Bahadori, R.; Wharton, R.; O’Brien, M.; Stone, P.; Benepal, T.; Bates, N.; et al. Modafinil for the Treatment of Fatigue in Lung Cancer: Results of a Placebo-Controlled, Double-Blind, Randomized Trial. J. Clin. Oncol. 2014, 32, 1882–1888. [Google Scholar] [CrossRef]
- Morrow, G.R.; Hickok, J.T.; Roscoe, J.A.; Raubertas, R.F.; Andrews, P.L.; Flynn, P.J.; Hynes, H.E.; Banerjee, T.K.; Kirshner, J.J.; King, D.K. Differential Effects of Paroxetine on Fatigue and Depression: A Randomized, Double-Blind Trial From the University of Rochester Cancer Center Community Clinical Oncology Program. J. Clin. Oncol. 2003, 21, 4635–4641. [Google Scholar] [CrossRef]
- Ashrafi, F.; Mousavi, S.; Karimi, M. Potential Role of Bupropion Sustained Release for Cancer-Related Fatigue: A Double-Blind, Placebo-Controlled Study. Asian Pac. J. Cancer Prev. 2018, 19, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, O.; Klepstad, P.; Rosland, J.H.; Aass, N.; Albert, E.; Fayers, P.; Kaasa, S. Efficacy of Methylprednisolone on Pain, Fatigue, and Appetite Loss in Patients with Advanced Cancer Using Opioids: A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Oncol. 2014, 32, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Frisbee-Hume, S.; Palmer, J.L.; Delgado-Guay, M.O.; Bull, J.; Phan, A.T.; Tannir, N.M.; Litton, J.K.; Reddy, A.; Hui, D.; et al. Reduction of Cancer-Related Fatigue with Dexamethasone: A Double-Blind, Randomized, Placebo-Controlled Trial in Patients with Advanced Cancer. J. Clin. Oncol. 2013, 31, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Roji, R.; Stone, P.; Ricciardi, F.; Candy, B. Placebo response in trials of drug treatments for cancer-related fatigue: A systematic review, meta-analysis and meta-regression. BMJ Support. Palliat. Care 2020, 10, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Junior, P.N.A.; Barreto, C.M.N.; Cubero, D.D.I.G.; del Giglio, A. The efficacy of placebo for the treatment of cancer-related fatigue: A systematic review and meta-analysis. Support. Care Cancer 2019, 28, 1755–1764. [Google Scholar] [CrossRef]
- Qu, D.; Zhang, Z.; Yu, X.; Zhao, J.; Qiu, F.; Huang, J. Psychotropic drugs for the management of cancer-related fatigue: A systematic review and meta-analysis. Eur. J. Cancer Care 2015, 25, 970–979. [Google Scholar] [CrossRef]
- Minton, O.; Richardson, A.; Sharpe, M.; Hotopf, M.; Stone, P.C. Psychostimulants for the Management of Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2011, 41, 761–767. [Google Scholar] [CrossRef]
- Gong, S.; Sheng, P.; Jin, H.; He, H.; Qi, E.; Chen, W.; Dong, Y.; Hou, L. Effect of Methylphenidate in Patients with Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e84391. [Google Scholar] [CrossRef]
- Dicato, M.; Plawny, L. Erythropoietin in cancer patients: Pros and cons. Curr. Opin. Oncol. 2010, 22, 307–311. [Google Scholar] [CrossRef]
- Hedley, B.D.; Allan, A.L.; Xenocostas, A. The Role of Erythropoietin and Erythropoiesis-Stimulating Agents in Tumor Progression. Clin. Cancer Res. 2011, 17, 6373–6380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruera, E.; Roca, E.; Cedaro, L.; Carraro, S.; Chacon, R. Action of oral methylprednisolone in terminal cancer patients: A prospective randomized double-blind study. Cancer Treat. Rep. 1985, 69, 751–754. [Google Scholar] [PubMed]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 1974, 33, 1607–1609. [Google Scholar] [CrossRef]
- Metz, C.A.; Della Cuna, G.R.; Pellegrini, A.; Piazzi, M. Effect of methylprednisolone sodium succinate on quality of life in preterminal cancer patients: A placebo-controlled, multicenter study. Eur. J. Cancer Clin. Oncol. 1989, 25, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.A.; Popiela, T.; Lucchi, R.; Giongo, F. Methylprednisolone as palliative therapy for female terminal cancer patients. Eur. J. Cancer Clin. Oncol. 1989, 25, 1823–1829. [Google Scholar] [CrossRef]
- De Giglio, A.; Mezquita, L.; Auclin, E.; Blanc-Durand, F.; Riudavets, M.; Caramella, C.; Martinez, G.; Benitez, J.; Martín-Romano, P.; El-Amarti, L.; et al. Impact of Intercurrent Introduction of Steroids on Clinical Outcomes in Advanced Non-Small-Cell Lung Cancer (NSCLC) Patients under Immune-Checkpoint Inhibitors (ICI). Cancers 2020, 12, 2827. [Google Scholar] [CrossRef]
- Yennu, S.; Basen-Engquist, K.; Reed, V.K.; Carmack, C.L.; Lee, A.; Mahmood, U.; Choi, S.; Hess, K.R.; Wu, J.; Williams, J.L.; et al. Multimodal therapy for cancer related fatigue in patients with prostate cancer receiving radiotherapy and androgen deprivation therapy. J. Clin. Oncol. 2017, 35, 10114. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Thompson, J.A.; Masood, N. Interferon-Induced Fatigue in Patients with Melanoma: A Pilot Study of Exercise and Methylphenidate. Oncol. Nurs. Forum 2002, 29, E85–E90. [Google Scholar] [CrossRef] [Green Version]
- Yennurajalingam, S.; Palmer, J.L.; Chacko, R.; Bruera, E. Factors Associated with Response to Methylphenidate in Advanced Cancer Patients. Oncologist 2011, 16, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Bruera, E.; Pace, E.; Zhang, K.; Reyes-Gibby, C.C. Clinically Important Improvement in the Intensity of Fatigue in Patients with Advanced Cancer. J. Palliat. Med. 2007, 10, 1068–1075. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Valero, V.; Lu, Z.; Liu, D.D.; Busaidy, N.L.; Reuben, J.M.; Fleming, C.D.; Williams, J.L.; Hess, K.R.; Basen-Engquist, K.; et al. Combination Therapy of Physical Activity and Dexamethasone for Cancer-Related Fatigue: A Phase II Randomized Double-Blind Controlled Trial. J. Natl. Compr. Cancer Netw. 2022, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Design | Treatment | Fatigue Scale | Patient Number | Population | Treatment Duration | Conclusion | PSS |
|---|---|---|---|---|---|---|---|---|
| Meta-Analysis 1: Ginseng | ||||||||
| Barton, 2013 [73] | Randomized double-blind placebo-controlled study | Wisconsin ginseng 2000 mg/day | MFSI-SF | 364 | Cancer patients diagnosed in <=2 years (except brain or CNS lymphoma) undergoing or having undergone curative intent treatment | 8 weeks | Wisconsin ginseng improves CRF after 8 weeks | 9 |
| Jiang, 2015 [74] | Randomized study | 3000 mg of fermented red ginseng extract daily for 60 days | FSI | 60 | Non-small cell lung cancer patients treated with chemotherapy | 60 days | Fermented red ginseng extract significantly improved CRF in patients who received fermented red ginseng. | 5 |
| Kim HS, 2017 [75] | Randomized, double-blind, placebo-controlled study | Red ginseng 3000 mg/day | BFI | 30 | Female patients with ovarian cancer | 12 weeks | CRF significantly improved after 12 weeks of treatment with Red ginseng | 9 |
| Yennurajalingam, 2017 [76] | Randomized double-blind placebo-controlled Study | Oral panax ginseng extract 800 mg/day | FACIT-F | 133 | Mixed Cancer patients with cancer-related fatigue | 4 weeks | Ginseng and placebo result in significant improvement in cancer related fatigue. Ginseng was not significantly superior to placebo after 4 weeks of treatment. | 9 |
| Guglielmo, 2020 [77] | Randomized, double-blind, placebo controlled, phase II trial | 1000 mg of American ginseng/day | BFI | 32 | Head & neck cancer survivors | 8 Weeks | American ginseng arm was not significantly different from placebo arm in post treatment Head & neck cancer survivors. | 9 |
| Kim, 2020 [78] | Randomized, double blinded, placebo-controlled, parallel phase III trial | 2000 mg Korean red ginseng/day | BFI | 438 | Colorectal cancer patients on mFOLFOX-6 chemotherapy regimen | 16 Weeks | Korean Red Ginseng reduced CRF compared with placebo. | 9 |
| Meta-Analysis 2: Guarana | ||||||||
| Da Costa Miranda V, 2009 [79] | Double-blind placebo controlled, randomized clinical with crossover | Guarana 75 mg daily | BFI | 36 | Breast cancer patients undergoing adjuvant radiation therapy | 2 weeks | CRF was not significantly reduced by guarana over placebo in patients with breast cancer undergoing to radiation therapy | 8 |
| De Oliveira Campos, 2011 [80] | Randomized, double-blind, placebo-controlled crossover trial | Guarana 100 mg daily | FACIT-F | 75 | Breast cancer patients to start the first cycle of systemic chemotherapy | 3 weeks | Guarana significantly improved CRF in breast cancer patients receiving systemic chemotherapy. | 9 |
| del Giglio, 2013 [81] | Randomized, placebo-controlled study | Guarana extract 75 mg daily | BFI | 40 | Solid tumors | 3 weeks | No significant differences could be seen between the placebo and Guarana arms in the randomized phase | 5 |
| Meta-Analysis 3: Megestrol | ||||||||
| Bruera, 1998 [82] | Randomized, double-blind, crossover study | Megestrol acetate 480 mg daily | PFS | 84 | Advanced, solid tumor patients not responsive to hormone therapy | 10 days | There was a significant improvement in 2 of the 3 factors measured by the Piper Fatigue Scale and in the overall fatigue score in the Megestrol group. | 8 |
| Westman, 1999 [83] | Randomized, double-blind, placebo-controlled study | Megestrol acetate 320 mg daily | EORTC QLQ-C30 | 255 | Advanced, solid tumor patients not responsive to hormone therapy | 12 weeks | Megestrol acetate does not appear to improve CRF in Megestrol group compared to placebo. | 9 |
| Meta-Analysis 4: Mistletoe | ||||||||
| Kim 2012 [84] | Randomized, controlled Trial | Mistle toe extract 20 mg three times a week | EORTC QLQ-C30 | 32 | Gastric cancer (stage Ib or II) who were waiting for oral chemotherapy | 24 weeks | No significant difference in fatigue between mistletoe and control group. | 6 |
| Tröger, 2009 [85] | Randomized controlled Trial | mistletoe extract 0.01–5 mg three times a week. | EORTC QLQ-C30 | 95 | Breast cancer during six cycles of consecutive treatment with CAF | 3 weeks | Symptoms of fatigue decreased in mistletoe group compared to the control group. | 6 |
| Tröger, 2014 [86] | Single-center, group-sequential, randomized controlled study | mistletoe extract 0.01–10 mg three times a week. | EORTC QLQ-C30 | 220 | Locally advanced or metastatic pancreatic carcinoma | 12 Months | Mistletoe treatment significantly improves the quality of life, including CRF. | 6 |
| Meta-Analysis 5: Psychostimulant | ||||||||
| Auret KA, 2009 [87] | Randomized, double-blind, placebo-controlled trial | Dexamphetamine 20 mg daily | BFI | 50 | Patients with advanced cancer receiving palliative care | 1 week | Fatigue intensity was not significantly different between the Dexamphetamine and placebo arms. | 9 |
| Berenson JR, 2015 [88] | Double-blind, randomized, placebo-controlled, crossover study | Armodafinil 150 mg once daily. | FACIT-F | 50 | Patients with multiple myeloma | 8 weeks | No significant difference between Methylphenidate and Placebo after 4 weeks. | 9 |
| Bruera E, 2006 [89] | Randomized, double blinded placebo controlled clinical trial | Methylphenidate 5 mg was given every 2 h, as needed, up to 20 mg per day. | FACIT-F | 112 | Cancer patients with fatigue | 1 week | Methylphenidate was not significantly superior to placebo after 1 week of treatment. | 9 |
| Bruera E, 2013 [90] | Randomized, double blinded placebo controlled clinical trial | Methylphenidate 5 mg every 2 h as needed up to 20 mg per day | FACIT-F | 190 | Patients with advanced cancer | 2 weeks | Methylphenidate and a nursing telephone intervention alone or combined were not superior to placebo in improving CRF. | 9 |
| Butler JM, 2007 [91] | Randomized placebo controlled double blind clinical trial | Methylphenidate 5 mg twice daily, starting by day 5 of radiation treatment, escalated by 5 mg twice daily to a maximum of 15 mg twice daily for 8 weeks | FACIT-F | 68 | Metastatic or histologic confirmed primary brain tumor receiving radiation therapy | 8 weeks | Prophylactic use of d-Methylphenidate in brain tumor patients undergoing radiation therapy did not result in an improvement in CRF. | 8 |
| Centeno, 2020 [92] | Randomized double-blind placebo- controlled clinical trial | Methylphenidate 10–25 mg/day | FACIT-F | 77 | Patients with advanced cancer | 6 Days | Methylphenidate was not significantly better than placebo to treat cancer- related fatigue. | 9 |
| Fan Mar, G.H, 2008 [93] | Randomized double-blind placebo controlled clinical trial | d-Methylphenidate 5 mg twice a day, then increased 1 week later to 10 mg bid until the end of the final cycle of chemotherapy. | FACIT-F | 57 | Women undergoing adjuvant chemotherapy for breast cancer | End of chemotherapy | There are no trends to suggest that d-Methylphenidate, taken concurrently with adjuvant chemotherapy, improves CRF or quality of life. | 8 |
| Hovey E, 2014 [94] | Randomized, double blinded placebo controlled clinical trial | Modafinil 100 mg twice daily | MDASI | 88 | Patients with metastatic prostate or breast cancer undergoing docetaxel chemotherapy | 2 weeks | Modafinil treatment did not significantly improve CRF compared to placebo. | 9 |
| Jean-Pierre P, 2010 [95] | Randomized, double blinded placebo controlled clinical trial | Modafinil 100 mg started on day 5 or day 10 of the second cycle of chemotherapy for 3 days and then increased to the full dose of 200 mg and continued on this regimen until day 7 of study cycle 4 | MDASI | 877 | Mixed cancer types who were beginning a cancer-treatment course of 4 planned cycles of chemotherapy | Day 7 of study chemotherapy cycle 4 | No significant differences in the control of cancer-related fatigue between modafinil and placebo. | 8 |
| Lee EQ, 2016 [96] | Randomized, double blinded placebo controlled clinical trial | Armodafinil 150 mg daily | FACIT-F | 81 | Patients with grade 2–4 glioma scheduled to receive radiotherapy | Day 42 | No significant differences were found between armodafinil and placebo arm in CRF improvement. | 9 |
| Lower EE, 2009 [97] | Randomized double blind, placebo-controlled, parallel group study | d-Methylphenidate 10 mg a day increasing to a maximum of 50 mg per day over 8 weeks | FACIT-F | 154 | Patients with cancer (excluding primary or metastatic brain cancer) | Week 8 | Compared with placebo, d-Methylphenidate treated subjects demonstrated a significant improvement in CRF | 9 |
| Moraska AR, 2010 [98] | Randomized, double blinded placebo controlled clinical trial | Long-acting methylphenidate 18 mg tablet; one tablet on days 1 through 7, two tablets on days 8 through 14, and three tablets on days 15 through 28. | BFI | 148 | Mixed tumor type Cancer patients | 4 weeks | Long-acting methylphenidate did not significantly decrease CRF compared to placebo. | 9 |
| Page BR, 2015 [99] | Double-blind placebo-controlled randomized clinical trial | Armodafinil 150 mg daily | FACIT-F | 54 | Patients with primary brain tumor (malignant or benign/low grade) receiving either partial or WBRT | 4 weeks | There were no significant differences in CRF severity between armodafinil and placebo at the end-radiation therapy or 4-week post-radiation therapy. | 9 |
| Spathis A, 2014 [100] | Double-blind, placebo-controlled, randomized clinical trial | Modafinil 100 mg on days 1 to 14 and then 200 mg on days 15 to 28. | FACIT-F | 208 | Adults with advanced non-small cell lung cancer or recurrent disease after surgery or radiotherapy | 4 weeks | There was no difference of CRF between Modafinil and Placebo arms. | 9 |
| Meta-Analysis 6: SSRI/Antidepressant | ||||||||
| Morrow, 2003 [101] | Double-blind, placebo-controlled, randomized clinical trial | paroxetine 20 mg daily | MAF | 549 | Patients with solid cancer scheduled to begin chemotherapy | 8 Weeks | Paroxetine did not result in improvement of CRF in patients with solid cancer receiving chemotherapy. | 9 |
| Ashrafi F. 2018 [102] | Randomized, double-blind, placebo-controlled trial | bupropion SR tablet 150 mg daily | FACIT-F | 57 | Both solid and non-solid cancer patients | 4 weeks | Significant improvement in CRF and quality of life in the bupropion compared to placebo arm. | 9 |
| Meta-Analysis 7: Steroids | ||||||||
| Paulsen, 2014 [103] | Randomized, placebo-controlled, double-blind trial | Methylprednisolone 32 mg daily | EORTC QLQ-C30 | 50 | Advanced cancer patients | 1 week | Methylprednisolone 32 mg daily improved fatigue, appetite loss, and patient satisfaction. | 10 |
| Yennurajalingam, 2013 [104] | Double-blind, randomized, placebo-controlled trial | Dexamethasone 8 mg daily | FACIT-F | 132 | Advanced cancer patients | 2 weeks | Dexamethasone is more effective than placebo in CRF and quality of life in patients with advanced cancer. | 9 |
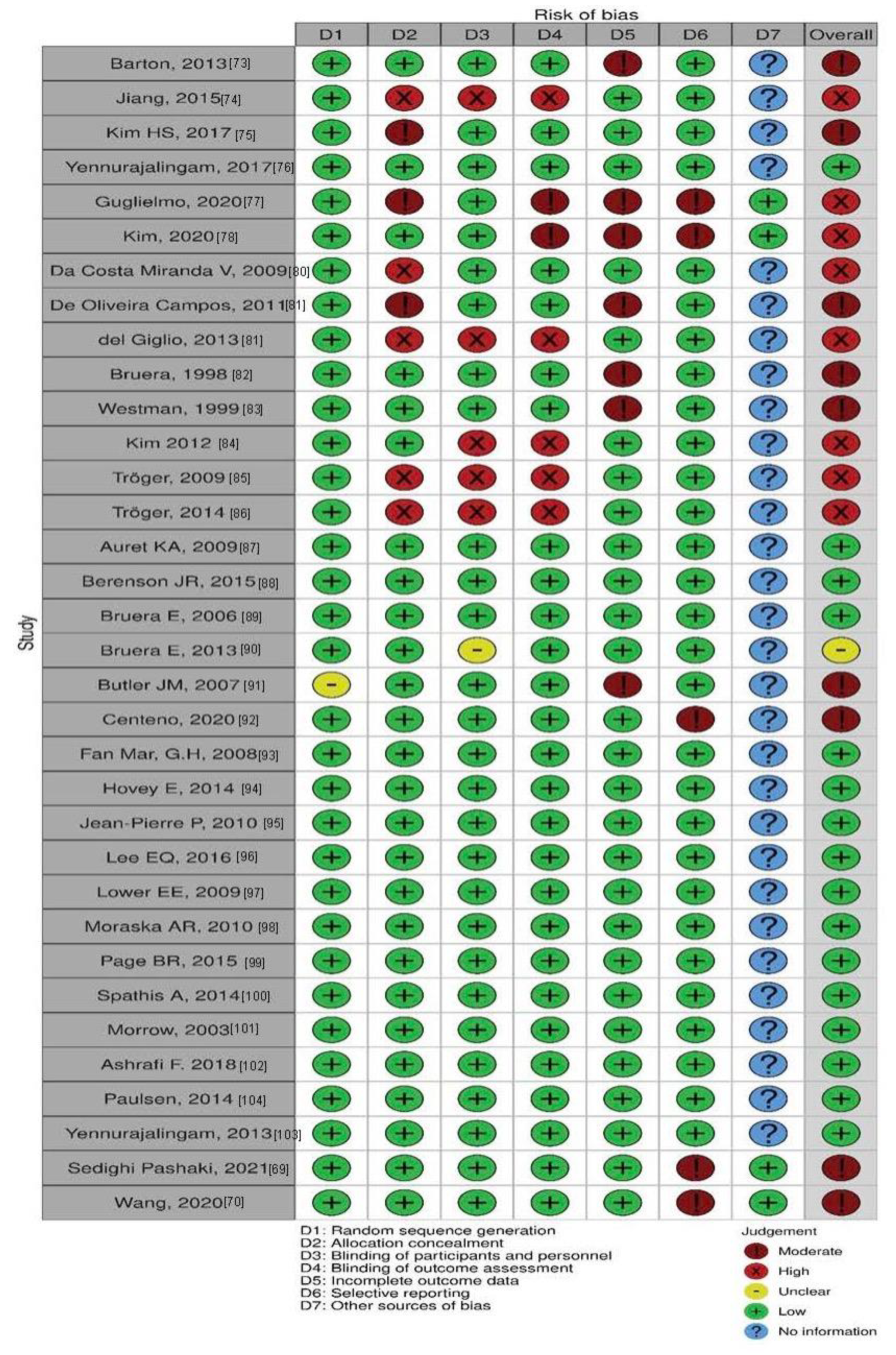
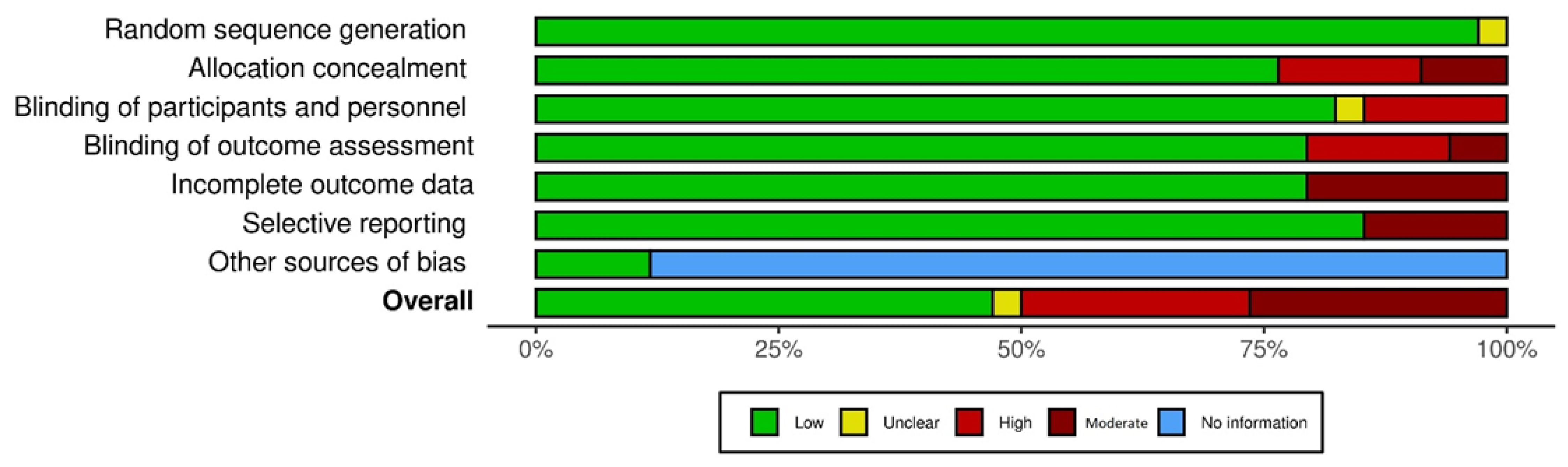
| Author, Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Sources of Bias |
|---|---|---|---|---|---|---|---|
| Meta-Analysis 1: Ginseng | |||||||
| Barton, 2013 [73] | Low | Low | Low | Low | Moderate | Low | NI |
| Jiang, 2015 [74] | Low | High | High | High | Low | Low | NI |
| Kim HS, 2017 [75] | Low | Moderate | Low | Low | Low | Low | NI |
| Yennurajalingam, 2017 [76] | Low | Low | Low | Low | Low | Low | NI |
| Guglielmo, 2020 [77] | Low | Moderate | Low | Moderate | Moderate | Moderate | Low |
| Kim, 2020 [78] | Low | Low | Low | Moderate | Moderate | Moderate | Low |
| Meta-Analysis 2: Guarana | |||||||
| Da Costa Miranda V, 2009 [79] | Low | High | Low | Low | Low | Low | NI |
| De Oliveira Campos, 2011 [80] | Low | Moderate | Low | Low | Moderate | Low | NI |
| del Giglio, 2013 [81] | Low | High | High | High | Low | Low | NI |
| Meta-Analysis 3: Megestrol | |||||||
| Bruera, 1998 [82] | Low | Low | Low | Low | Moderate | Low | NI |
| Westman, 1999 [83] | Low | Low | Low | Low | Moderate | Low | NI |
| Meta-Analysis 4: Mistletoe | |||||||
| Kim 2012 [84] | Low | Low | High | High | Low | Low | NI |
| Tröger, 2009 [85] | Low | High | High | High | Low | Low | NI |
| Tröger, 2014 [86] | Low | High | High | High | Low | Low | NI |
| Meta-Analysis 5: Psychostimulant | |||||||
| Auret KA, 2009 [87] | Low | Low | Low | Low | Low | Low | NI |
| Berenson JR, 2015 [88] | Low | Low | Low | Low | Low | Low | NI |
| Bruera E, 2006 [89] | Low | Low | Low | Low | Low | Low | NI |
| Bruera E, 2013 [90] | Low | Low | Unclear * | Low | Low | Low | NI |
| Butler JM, 2007 [91] | Unclear * | Low | Low | Low | Moderate | Low | NI |
| Centeno, 2020 [92] | Low | Low | Low | Low | Low | Moderate | NI |
| Fan Mar, G.H, 2008 [93] | Low | Low | Low | Low | Low | Low | NI |
| Hovey E, 2014 [94] | Low | Low | Low | Low | Low | Low | NI |
| Jean-Pierre P, 2010 [95] | Low | Low | Low | Low | Low | Low | NI |
| Lee EQ, 2016 [96] | Low | Low | Low | Low | Low | Low | NI |
| Lower EE, 2009 [97] | Low | Low | Low | Low | Low | Low | NI |
| Moraska AR, 2010 [98] | Low | Low | Low | Low | Low | Low | NI |
| Page BR, 2015 [99] | Low | Low | Low | Low | Low | Low | NI |
| Spathis A, 2014 [100] | Low | Low | Low | Low | Low | Low | NI |
| Meta-Analysis 6: SSRI/Antidepressant | |||||||
| Morrow, 2003 [101] | Low | Low | Low | Low | Low | Low | NI |
| Ashrafi F. 2018 [102] | Low | Low | Low | Low | Low | Low | NI |
| Meta-Analysis 7: Steroids | |||||||
| Paulsen, 2014 [103] | Low | Low | Low | Low | Low | Low | NI |
| Yennurajalingam, 2013 [104] | Low | Low | Low | Low | Low | Low | NI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yennurajalingam, S.; Lu, Z.; Rozman De Moraes, A.; Tull, N.N.; Kubiak, M.J.; Geng, Y.; Andersen, C.R.; Bruera, E. Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue. Cancers 2023, 15, 91. https://doi.org/10.3390/cancers15010091
Yennurajalingam S, Lu Z, Rozman De Moraes A, Tull NN, Kubiak MJ, Geng Y, Andersen CR, Bruera E. Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue. Cancers. 2023; 15(1):91. https://doi.org/10.3390/cancers15010091
Chicago/Turabian StyleYennurajalingam, Sriram, Zhanni Lu, Aline Rozman De Moraes, Nhu Nhu Tull, Michal J. Kubiak, Yimin Geng, Clark R. Andersen, and Eduardo Bruera. 2023. "Meta-Analysis of Pharmacological, Nutraceutical and Phytopharmaceutical Interventions for the Treatment of Cancer Related Fatigue" Cancers 15, no. 1: 91. https://doi.org/10.3390/cancers15010091








