PLA2R1 Inhibits Differentiated Thyroid Cancer Proliferation and Migration via the FN1-Mediated ITGB1/FAK Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Tissue Samples
2.3. Immunohistochemical Staining
2.4. Cell Culture
2.5. Plasmid Synthesis and Transfection
2.6. Real-Time Polymerase Chain Reaction (RT–PCR)
2.7. Western Blot
2.8. CCK-8 Assay to Evaluate Cell Proliferation
2.9. Wound Healing Assay
2.10. Subcutaneous Xenograft Mouse Model
2.11. Coimmunoprecipitation (Co-IP) Assay
2.12. Statistical Analysis
3. Results
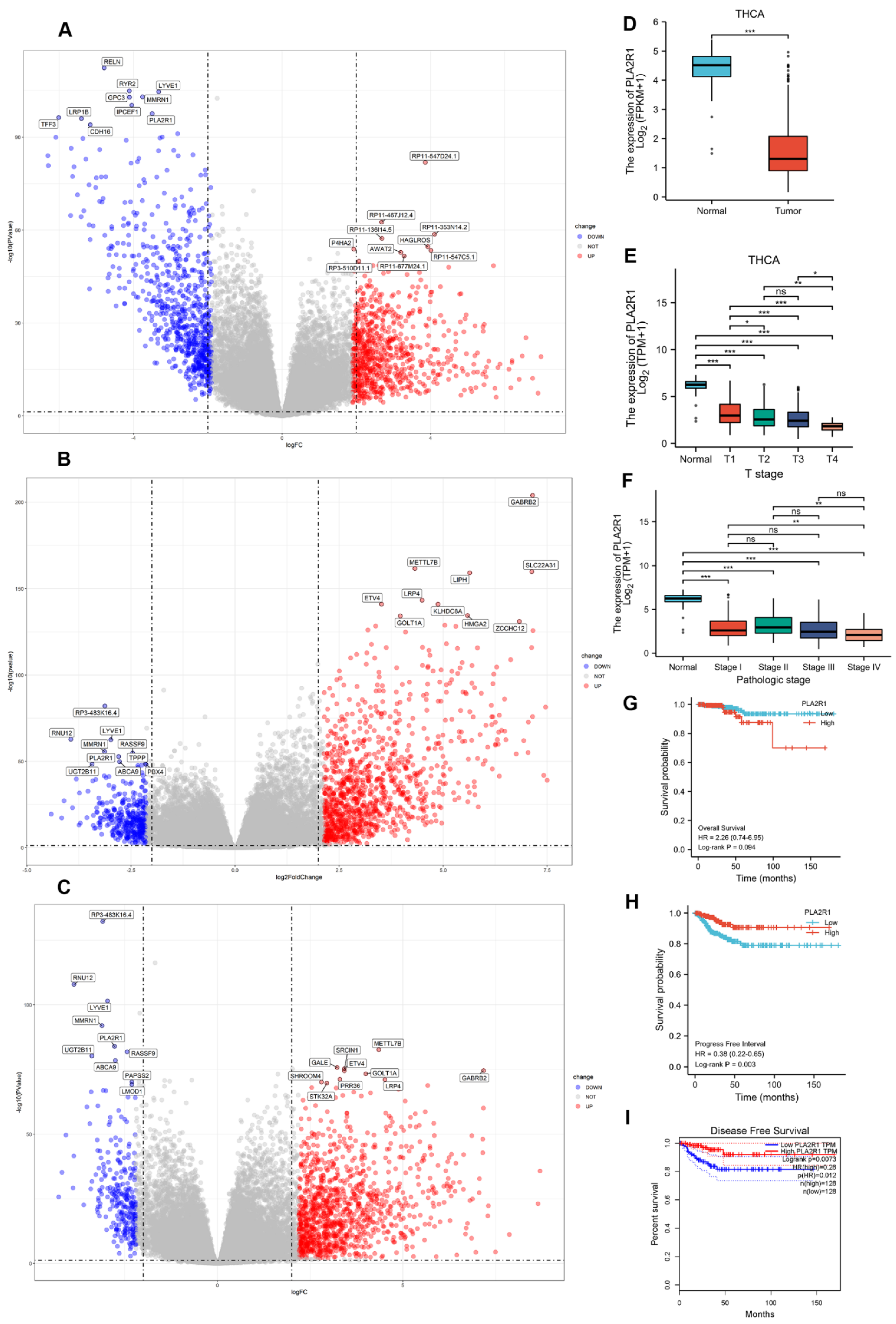
3.1. The Expression of PLA2R1 Is Associated with Tumor Stage and Patient Survival
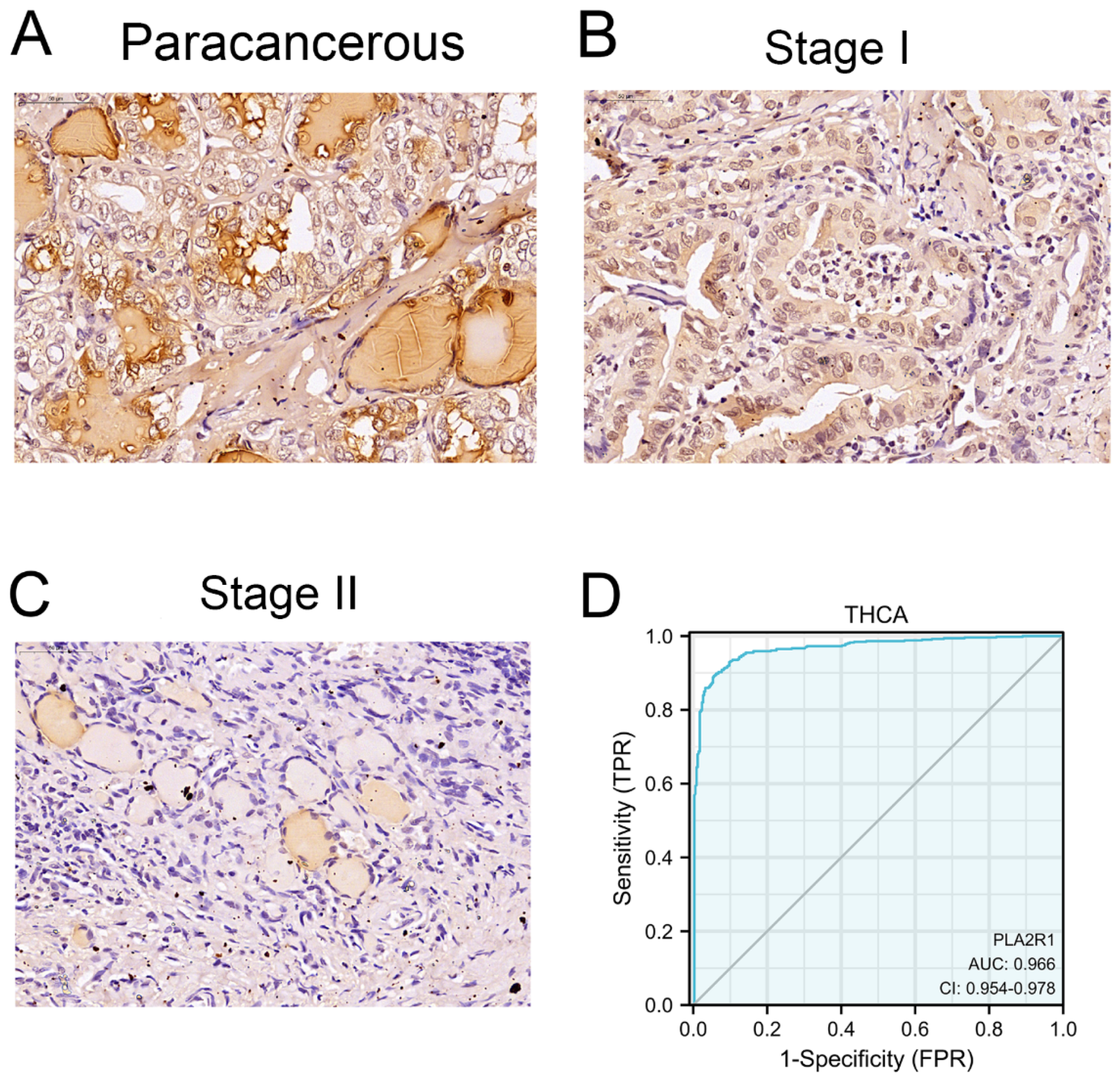
3.2. Clinical Characteristics and PLA2R1 Expression in Clinical Specimens
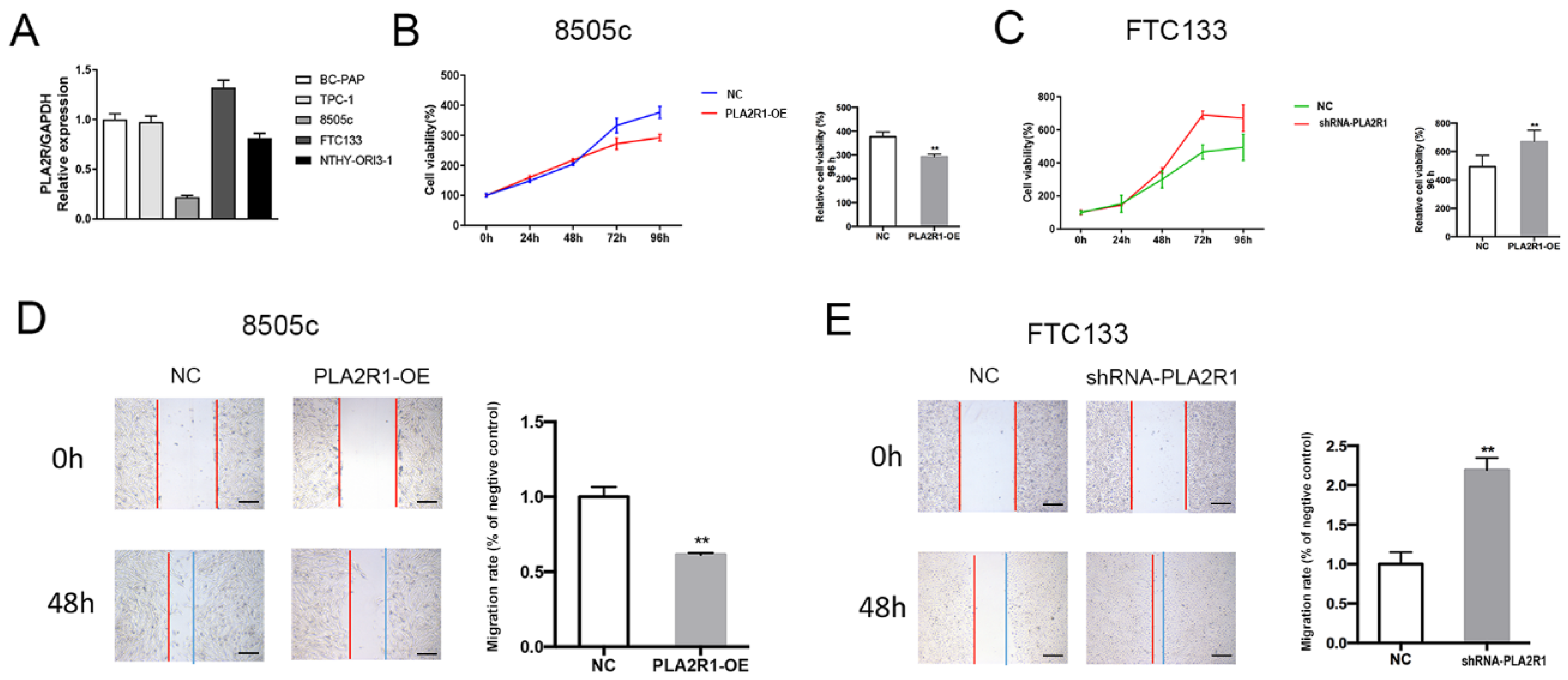
3.3. Thyroid Cell Lines with Stable Overexpression or Silencing of PLA2R1 Were Established
3.4. PLA2R1 Inhibits Thyroid Cancer Cell Proliferation and Migration
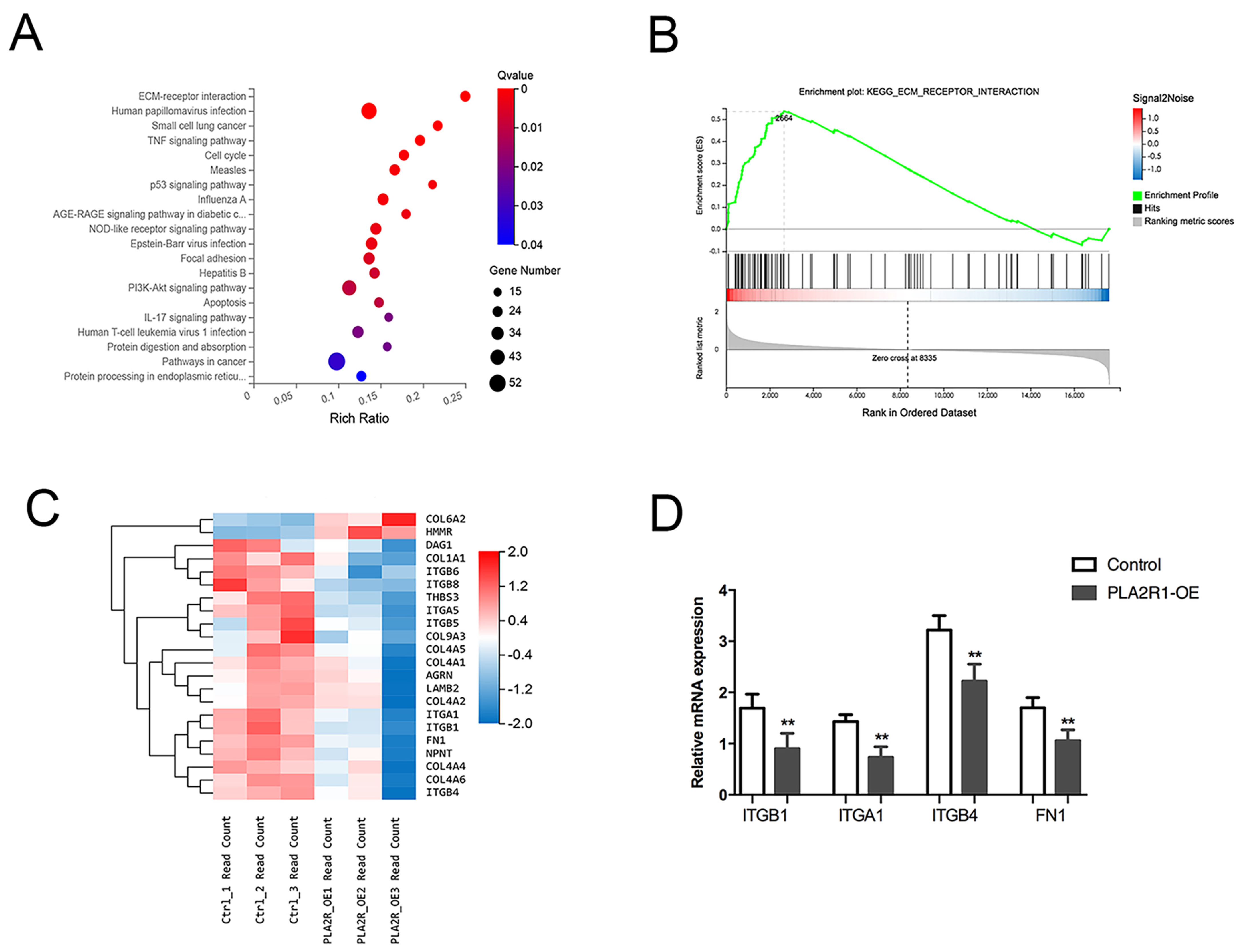
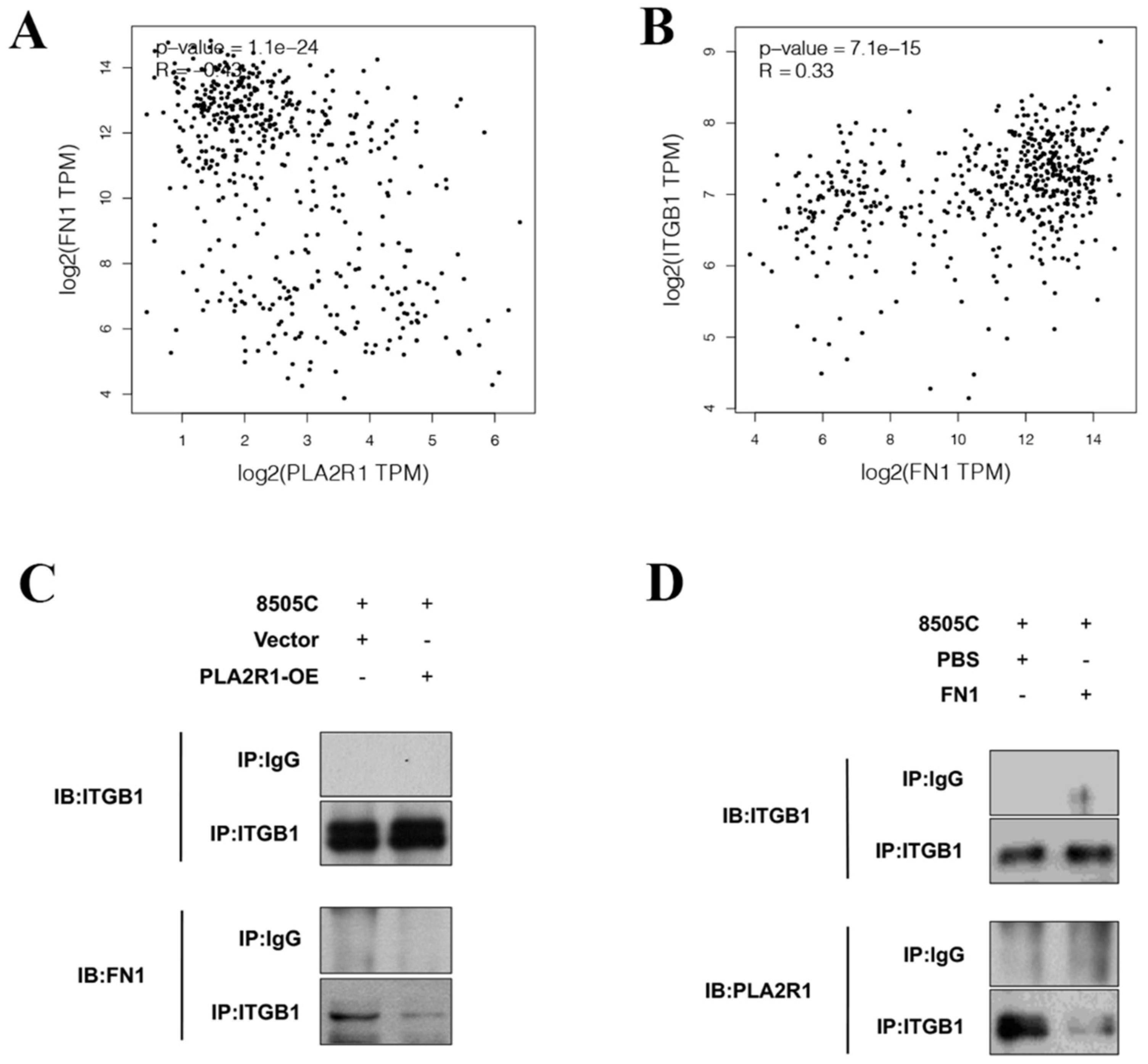
3.5. PLA2R1 Competes with FN1 to Interact with ITGB1
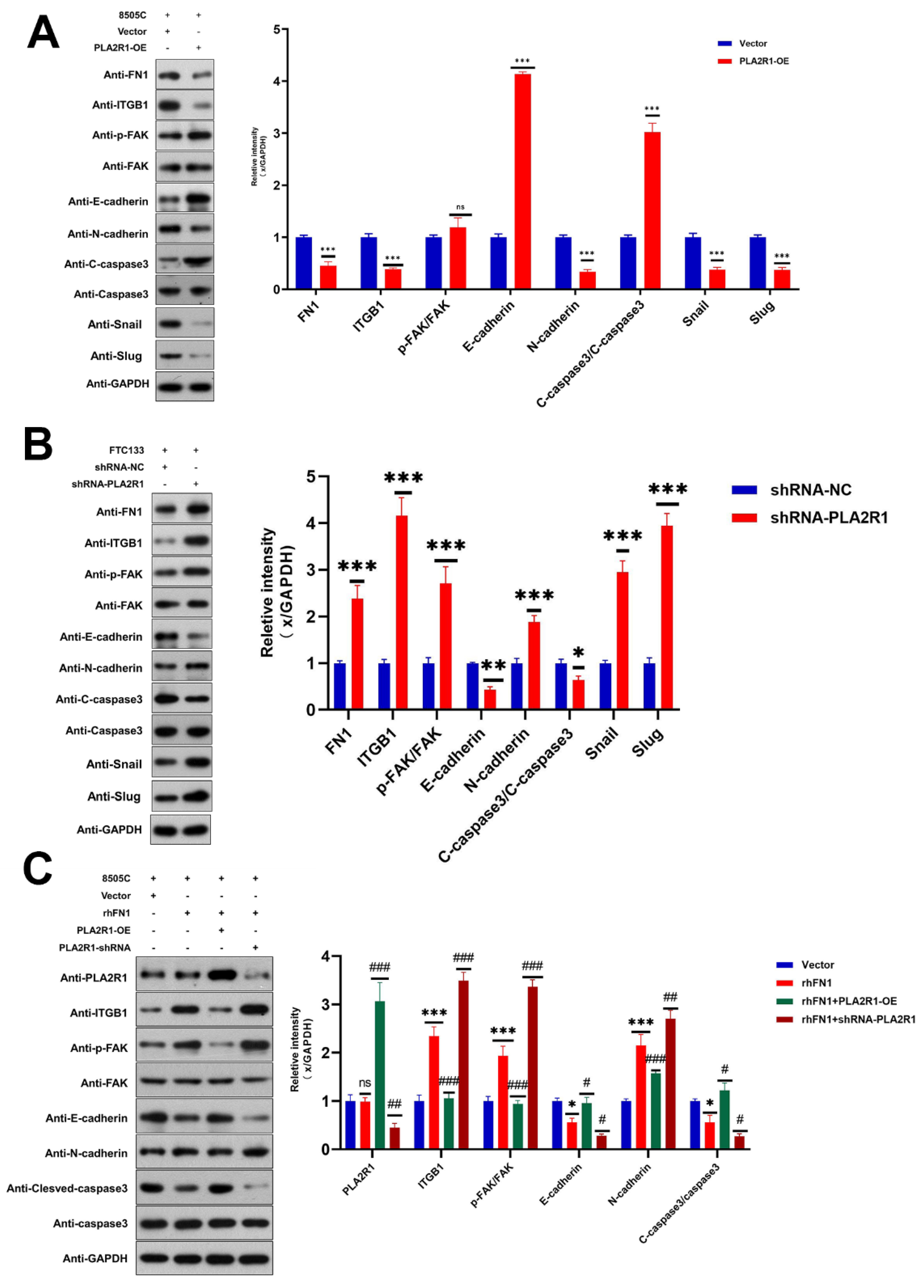
3.6. PLA2R1 Regulates the ITGB1/FAK Axis and Inhibits EMT
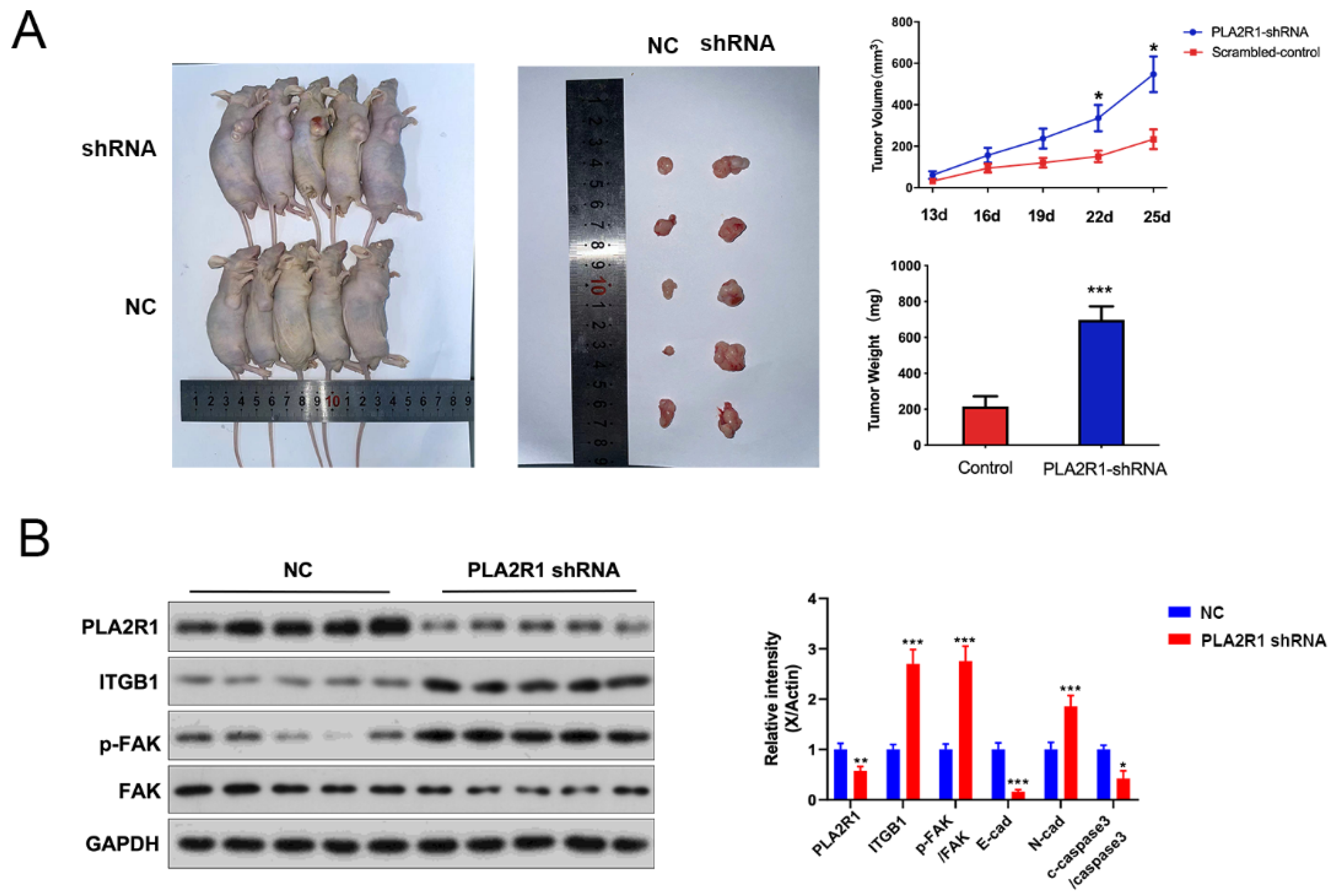
3.7. The Effect of PLA2R1 on Tumorigenic Ability of Thyroid Cancer Cells Was Detected by the Tumor Formation Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherella, C.E.; Richman, D.M.; Liu, E.J.; Frates, M.C.; Modi, B.P.; Zendejas, B.; Smith, J.R.; Barletta, J.A.; Hollowell, M.L.; Wassner, A.J. Predictors of Bilateral Disease in Pediatric Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, E4242–E4250. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.L.; Li, S.P.; Tseng, C.H.; Kim, J.; Nguyen, D.T.; Dawood, N.B.; Livhits, M.J.; Yeh, M.W.; Leung, A.M. Rising Incidence and Incidence-Based Mortality of Thyroid Cancer in California, 2000–2017. J. Clin. Endocrinol. Metab. 2020, 105, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-A Cancer J. Clin. 2018, 68, 394, Erratum in Ca-A Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- Liu, T.R.; Men, Q.Q.; Su, X.; Chen, W.C.; Zou, L.; Li, Q.L.; Song, M.; Ouyang, D.; Chen, Y.F.; Li, Z.Q.; et al. Downregulated expression of TSHR is associated with distant metastasis in thyroid cancer. Oncol. Lett. 2017, 14, 7506–7512. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Lee, S.; Bae, J.S.; Lim, D.J. Late-onset distant metastases confer poor prognosis in patients with well-differentiated thyroid cancer. Gland Surg. 2020, 9, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Chandran, U.R.; Medvedeva, O.P.; Barmada, M.M.; Blood, P.D.; Chakka, A.; Luthra, S.; Ferreira, A.; Wong, K.F.; Lee, A.V.; Zhang, Z.; et al. TCGA Expedition: A Data Acquisition and Management System for TCGA Data. PLoS ONE 2016, 11, e0165395. [Google Scholar] [CrossRef]
- Ancian, P.; Lambeau, G.; Lazdunski, M. Multifunctional Activity of the Extracellular Domain of the M-Type (180 kda) Membrane-Receptor for Secretory Phospholipases A(2). Biochemistry 1995, 34, 13146–13151. [Google Scholar] [CrossRef]
- Menschikowski, M.; Platzbecker, U.; Hagelgans, A.; Vogel, M.; Thiede, C.; Schonefeldt, C.; Lehnert, R.; Eisenhofer, G.; Siegert, G. Aberrant methylation of the M-type phospholipase A(2) receptor gene in leukemic cells. BMC Cancer 2012, 12, 1–10. [Google Scholar] [CrossRef]
- Bernard, D.; Vindrieux, D. PLA2R1: Expression and function in cancer. Biochim. Et Biophys. Acta-Rev. Cancer 2014, 1846, 40–44. [Google Scholar] [CrossRef]
- Vindrieux, D.; Augert, A.; Girard, C.A.; Gitenay, D.; Lallet-Daher, H.; Wiel, C.; Le Calve, B.; Gras, B.; Ferrand, M.; Verbeke, S.; et al. PLA2R1 Mediates Tumor Suppression by Activating JAK2. Cancer Res. 2013, 73, 6334–6345. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Z.J.; Tang, X.Y.; Liu, R.; Wu, M.W.; Wu, J.Q.; Liu, Z.W. Comprehensive analysis of tissue proteomics in patients with papillary thyroid microcarcinoma uncovers the underlying mechanism of lymph node metastasis and its significant sex disparities. Front. Oncol. 2022, 12, 4580. [Google Scholar] [CrossRef] [PubMed]
- Vindrieux, D.; Devailly, G.; Augert, A.; Le Calve, B.; Ferrand, M.; Pigny, P.; Payen, L.; Lambeau, G.; Perrais, M.; Aubert, S.; et al. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget 2014, 5, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Menschikowski, M.; Hagelgans, A.; Nacke, B.; Jandeck, C.; Sukocheva, O.; Siegert, G. Epigenetic control of phospholipase A(2) receptor expression in mammary cancer cells. BMC Cancer 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhan, N.; Honardoost, M.; Blighe, K.; Moore, C.B.T.; Khamseh, M.E. Comprehensive transcriptomic analysis of papillary thyroid cancer: Potential biomarkers associated with tumor progression. J. Endocrinol. Investig. 2020, 43, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Holsinger, C.; Dosiou, C.; Sunwoo, J.B.; Akatsu, H.; Haile, R.; Gevaert, O. Development of prognostic signatures for intermediate-risk papillary thyroid cancer. BMC Cancer 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Quach, N.D.; Mock, J.N.; Scholpa, N.E.; Eggert, M.W.; Payré, C.; Lambeau, G.; Arnold, R.D.; Cummings, B.S. Role of the Phospholipase A(2) Receptor in Liposome Drug Delivery in Prostate Cancer Cells. Mol. Pharm. 2014, 11, 3443–3451. [Google Scholar] [CrossRef]
- Mitwally, N.; Yousef, E.; Aziz, A.A.; Taha, M. Clinical Significance of Expression Changes and Promoter Methylation of PLA2R1 in Tissues of Breast Cancer Patients. Int. J. Mol. Sci. 2020, 21, 5453. [Google Scholar] [CrossRef]
- Griveau, A.; Devailly, G.; Eberst, L.; Navaratnam, N.; Le Calve, B.; Ferrand, M.; Faull, P.; Augert, A.; Dante, R.; Vanacker, J.M.; et al. The PLA2R1-JAK2 pathway upregulates ERR alpha and its mitochondrial program to exert tumor-suppressive action. Oncogene 2016, 35, 5033–5042. [Google Scholar] [CrossRef]
- Sukocheva, O.; Menschikowski, M.; Hagelgans, A.; Yarla, N.S.; Siegert, G.; Reddanna, P.; Bishayee, A. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: More questions than answers. Semin. Cancer Biol. 2019, 56, 116–127. [Google Scholar] [CrossRef]
- Augert, A.; Vindrieux, D.; Girard, C.A.; Le Calve, B.; Gras, B.; Ferrand, M.; Bouchet, B.P.; Puisieux, A.; de Launoit, Y.; Simonnet, H.; et al. PLA2R1 kills cancer cells by inducing mitochondrial stress. Free Radic. Biol. Med. 2013, 65, 969–977. [Google Scholar] [CrossRef]
- Augert, A.; Payre, C.; de Launoit, Y.; Gil, J.; Lambeau, G.; Bernard, D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.M.; Shi, Q.P.; Li, W.J.; Wu, W.R.; Zha, Z.G. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesenchymal stem cells by activating the ERK signaling. J. Mol. Histol. 2020, 51, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qu, X.J.; Lu, W.Q.; Wang, Y.Z.; Jin, Y.; Hou, K.Z.; Yang, B.W.; Li, C.; Qi, J.F.; Xiao, J.W.; et al. N-6-Methyladenosine RNA Demethylase FTO Promotes Gastric Cancer Metastasis by Down-Regulating the m6A Methylation of ITGB1. Front. Oncol. 2021, 11, 681280. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chang, L.L. Maspin suppresses cell invasion and migration in gastric cancer through inhibiting EMT and angiogenesis via ITGB1/FAK pathway. Hum. Cell 2020, 33, 663–675. [Google Scholar] [CrossRef]
- Shu, C.; Han, S.M.; Hu, C.; Chen, C.; Qu, B.; He, J.; Dong, S.; Xu, P. Integrin beta 1 regulates proliferation, apoptosis, and migration of trophoblasts through activation of phosphoinositide 3 kinase/protein kinase B signaling. J. Obstet. Gynaecol. Res. 2021, 47, 2406–2416. [Google Scholar] [CrossRef]
- Ren, L.L.; Mo, W.J.; Wang, L.L.; Wang, X.J. Matrine suppresses breast cancer metastasis by targeting ITGB1 and inhibiting epithelial-to-mesenchymal transition. Exp. Ther. Med. 2020, 19, 367–374. [Google Scholar] [CrossRef]
- Meng, X.L.; Liu, P.; Wu, Y.H.; Liu, X.L.; Huang, Y.P.; Yu, B.Q.; Han, J.H.; Jin, H.Y.; Tan, X.D. Integrin beta 4 (ITGB4) and its tyrosine-1510 phosphorylation promote pancreatic tumorigenesis and regulate the MEK1-ERK1/2 signaling pathway. Bosn. J. Basic Med. Sci. 2020, 20, 106–116. [Google Scholar] [CrossRef]
- Krishn, S.R.; Salem, I.; Quaglia, F.; Naranjo, N.M.; Agarwal, E.; Liu, Q.; Sarker, S.; Kopenhaver, J.; McCue, P.A.; Weinreb, P.H.; et al. The alpha v beta 6 integrin in cancer cell-derived small extracellular vesicles enhances angiogenesis. J. Extracell. Vesicles 2020, 9, 1763594. [Google Scholar] [CrossRef]
- Huaman, J.; Ogunwobi, O.O. Circulating Tumor Cell Migration Requires Fibronectin Acting through Integrin B1 or SLUG. Cells 2020, 9, 1594. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.Z.; Wei, Y.; Ell, B.; Sheng, X.; Esposito, M.; Kang, J.; Hang, X.; Zheng, H.; Rowicki, M.; et al. Tinagl1 Suppresses Triple-Negative Breast Cancer Progression and Metastasis by Simultaneously Inhibiting Integrin/FAK and EGFR Signaling. Cancer Cell 2019, 35, 64–80.e7. [Google Scholar] [CrossRef]
- Yang, H.; Messina-Pacheco, J.; Corredor, A.L.G.; Gregorieff, A.; Liu, J.L.; Nehme, A.; Najafabadi, H.S.; Riazalhosseini, Y.; Gao, B.; Gao, Z.H. An integrated model of acinar to ductal metaplasia-related N7-methyladenosine regulators predicts prognosis and immunotherapy in pancreatic carcinoma based on digital spatial profiling. Front. Immunol. 2022, 13, 961457. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.J.; Huo, Q.Y.; Yuan, Q.; Xu, C. Fibronectin 1: A Potential Biomarker for Ovarian Cancer. Dis. Markers 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nana, F.A.; Hoton, D.; Ambroise, J.; Lecocq, M.; Vanderputten, M.; Sibille, Y.; Vanaudenaerde, B.; Pilette, C.; Bouzin, C.; Ocak, S. Increased Expression and Activation of FAK in Small-Cell Lung Cancer Compared to Non-Small-Cell Lung Cancer. Cancers 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.Y.; Zhang, W.J.; Zhao, G.N.; Chen, Z.W.; Dong, P.X.; Watari, H.; Narayanan, R.; Tillmanns, T.D.; Pfeffer, L.M.; Yue, J.M. FAK PROTAC Inhibits Ovarian Tumor Growth and Metastasis by Disrupting Kinase Dependent and Independent Pathways. Front. Oncol. 2022, 12, 851065. [Google Scholar] [CrossRef]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, A.; Tasso, B.; Russo, E.; Villa, C.; Brullo, C. The Development of FAK Inhibitors: A Five-Year Update. Int. J. Mol. Sci. 2022, 23, 6381. [Google Scholar] [CrossRef]
- Wang, S.Y.; Basson, M.D. Akt directly regulates focal adhesion kinase through association and serine phosphorylation: Implication for pressure-induced colon cancer metastasis. Am. J. Physiol. Cell Physiol. 2011, 300, C657–C670. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.K.; Kim, H.Y.; Gawk, H.; Bae, S.H.; Sim, H.W.; Kang, E.K.; Seoh, J.Y.; Jang, H.; Hong, K.M. FAK-Copy-Gain Is a Predictive Marker for Sensitivity to FAK Inhibition in Breast Cancer. Cancers 2019, 11, 1288. [Google Scholar] [CrossRef]
- Kong, D.B.; Chen, F.; Sima, N. Focal adhesion kinases crucially regulate TGFw beta-induced migration and invasion of bladder cancer cells via Src kinase and E-cadherin. Oncotargets Ther. 2017, 10, 1783–1792. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yao, L.W.; Zhou, W.; Tian, J.P.; Ruan, B.L.; Lu, Z.H.; Deng, Y.C.; Li, Q.; Zeng, Z.; Yang, D.M.; et al. miR-497 defect contributes to gastric cancer tumorigenesis and progression via regulating CDC42/ITGB1/FAK/PXN/AKT signaling. Mol. Ther.-Nucleic Acids 2021, 25, 567–577. [Google Scholar] [CrossRef]
- Kawahara, R.; Niwa, Y.; Simizu, S. Integrin 1 is an essential factor in vasculogenic mimicry of human cancer cells. Cancer Sci. 2018, 109, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
| Features | No. of Patients | PLA2R1 Expression | p Value | |
|---|---|---|---|---|
| All Patients | 54 | Low | High | |
| 32 | 22 | |||
| Age (years) | 0.724 | |||
| ≥55 | 23 | 13 | 10 | |
| <55 | 31 | 19 | 12 | |
| Gender | ||||
| Male | 21 | 13 | 8 | 0.755 |
| Female | 33 | 19 | 14 | |
| T Infiltrate | ||||
| T1 | 33 | 20 | 13 | 0.781 |
| T2 | 19 | 11 | 8 | |
| T3 | 2 | 1 | 1 | |
| Lymphatic Metastasis (N) | ||||
| N0 | 26 | 16 | 10 | 0.867 |
| N1 | 27 | 16 | 11 | |
| AJCC stage | ||||
| I | 44 | 23 | 21 | 0.030 |
| II | 10 | 9 | 1 | |
| Tumor size | 54 | 32 | 22 | 0.790 |
| PLA2R1 | Tumor Tissue | Para-Cancerous Tissue | p Value | ||
|---|---|---|---|---|---|
| Expression | Cases | Percentage | Cases | Percentage | 0.0000 |
| Low | 32 | 59.3% | 4 | 5.6% | |
| High | 22 | 40.7% | 57 | 93.4% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Zhang, M.; Gao, D.; Zhang, X.; Cai, H.; Cui, Z.; Gao, Y.; Lv, Z. PLA2R1 Inhibits Differentiated Thyroid Cancer Proliferation and Migration via the FN1-Mediated ITGB1/FAK Axis. Cancers 2023, 15, 2720. https://doi.org/10.3390/cancers15102720
Zheng H, Zhang M, Gao D, Zhang X, Cai H, Cui Z, Gao Y, Lv Z. PLA2R1 Inhibits Differentiated Thyroid Cancer Proliferation and Migration via the FN1-Mediated ITGB1/FAK Axis. Cancers. 2023; 15(10):2720. https://doi.org/10.3390/cancers15102720
Chicago/Turabian StyleZheng, Hui, Mengyu Zhang, Dingwei Gao, Xiaoying Zhang, Haidong Cai, Zhijun Cui, Yang Gao, and Zhongwei Lv. 2023. "PLA2R1 Inhibits Differentiated Thyroid Cancer Proliferation and Migration via the FN1-Mediated ITGB1/FAK Axis" Cancers 15, no. 10: 2720. https://doi.org/10.3390/cancers15102720
APA StyleZheng, H., Zhang, M., Gao, D., Zhang, X., Cai, H., Cui, Z., Gao, Y., & Lv, Z. (2023). PLA2R1 Inhibits Differentiated Thyroid Cancer Proliferation and Migration via the FN1-Mediated ITGB1/FAK Axis. Cancers, 15(10), 2720. https://doi.org/10.3390/cancers15102720






