TIM-3 Qualifies as a Potential Immunotherapeutic Target in Specific Subsets of Patients with High-Risk Soft Tissue Sarcomas (HR-STS)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Histopathology and Tissue Microarray Construction
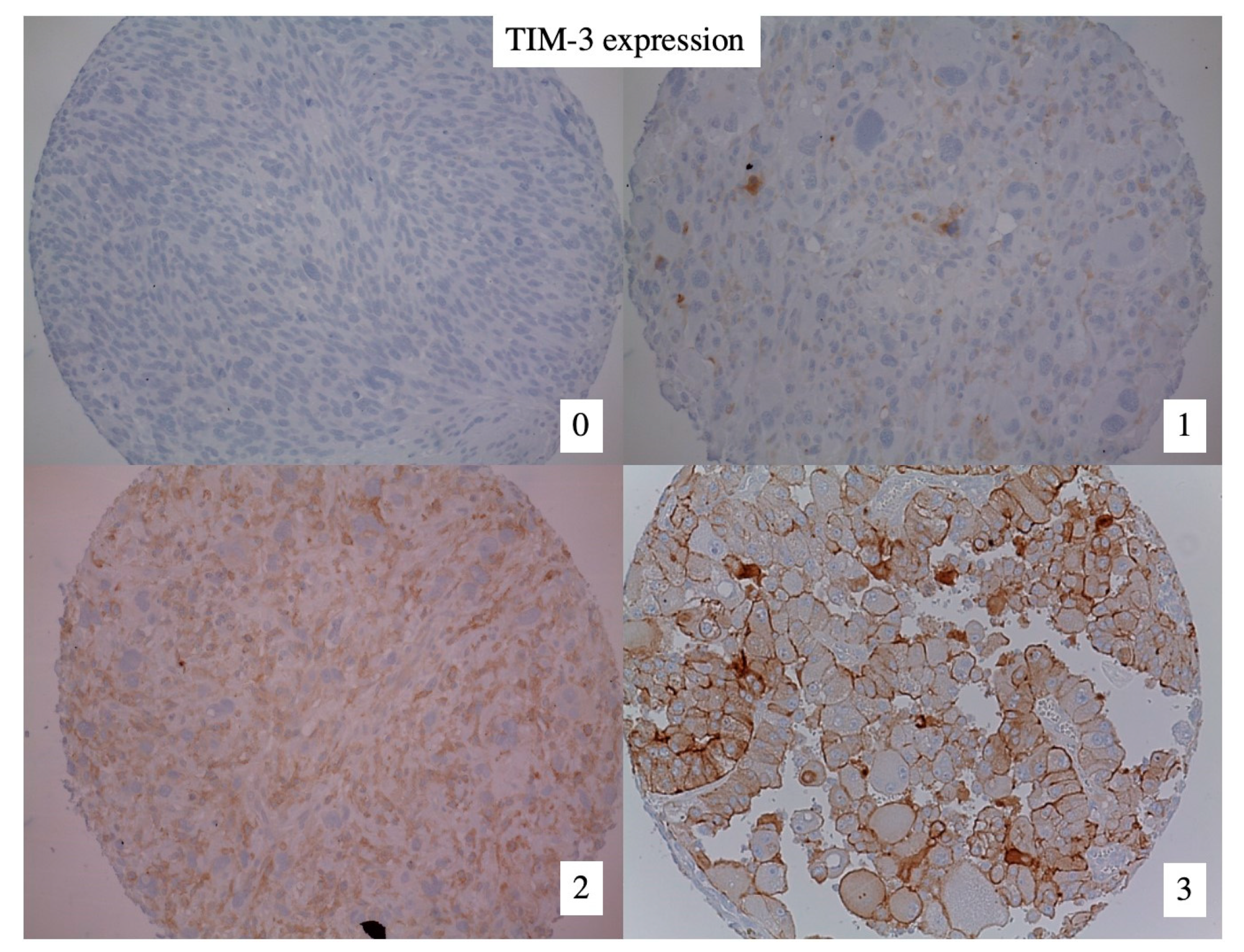
2.3. TIM-3 Immunohistochemistry
2.4. TILs, PD-1 and PD-L1
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. TIM-3 Expression in High-Risk Soft Tissue Sarcomas (HR-STS)
3.3. TIM-3 Expression Is Associated with TILs, PD-1 and PD-L1 Expression Status
3.4. TIM-3 Expression and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Le Pechoux, C.; et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol. 2016, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Magliocco, A.; Zhang, Q.; Wang, D.; Klimowicz, A.; Harris, J.; Simko, J.; Delaney, T.; Kraybill, W.; Kirsch, D.G. Correlation of High-Risk Soft Tissue Sarcoma Biomarker Expression Patterns with Outcome following Neoadjuvant Chemoradiation. Sarcoma 2018, 2018, 8310950. [Google Scholar] [CrossRef]
- In, G.K.; Hu, J.S.; Tseng, W.W. Treatment of advanced, metastatic soft tissue sarcoma: Latest evidence and clinical considerations. Ther. Adv. Med. Oncol. 2017, 9, 533–550. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, O.; Judson, I.; van Hoesel, Q.; le Cesne, A.; Keizer, H.; Blay, J.; van Oosterom, A.; Radford, J.; Svancárová, L.; Krzemienlecki, K.; et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2000, 36, 61–67. [Google Scholar] [CrossRef]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared with Gemcitabine Alone in Patients with Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research Through Collaboration Study 002. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef]
- Leahy, M.; del Muro, X.G.; Reichardt, P.; Judson, I.; Staddon, A.; Verweij, J.; Baffoe-Bonnie, A.; Jönsson, L.; Musayev, A.; Justo, N.; et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Ann. Oncol. 2012, 23, 2763–2770. [Google Scholar] [CrossRef]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with Single Agent Nivolumab for Advanced Leiomyosarcoma of the Uterus: Results of a Phase 2 Study. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef] [Green Version]
- Paoluzzi, L.; Cacavio, A.; Ghesani, M.; Karambelkar, A.; Rapkiewicz, A.; Weber, J.; Rosen, G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin. Sarcoma Res. 2016, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2019, 20, 173–185. [Google Scholar] [CrossRef]
- Clayton, K.L.; Haaland, M.S.; Douglas-Vail, M.B.; Mujib, S.; Chew, G.M.; Ndhlovu, L.C.; Ostrowski, M.A. T Cell Ig and Mucin Domain–Containing Protein 3 Is Recruited to the Immune Synapse, Disrupts Stable Synapse Formation, and Associates with Receptor Phosphatases. J. Immunol. 2014, 192, 782–791. [Google Scholar] [CrossRef] [Green Version]
- van de Weyer, P.S.; Muehlfeit, M.; Klose, C.; Bonventre, J.V.; Walz, G.; Kuehn, E.W. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006, 351, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.F.; Dougan, S.K.; Petersen, B.-S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiener, Z.; Kohalmi, B.; Pocza, P.; Jeager, J.; Tolgyesi, G.; Toth, S.; Gorbe, E.; Papp, Z.; Falus, A. TIM-3 Is Expressed in Melanoma Cells and Is Upregulated in TGF-Beta Stimulated Mast Cells. J. Investig. Dermatol. 2007, 127, 906–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Zhang, X.; Xia, X.; Zhang, C.; Liang, X.; Gao, L.; Zhang, X.; Ma, C. Ectopic Expression of TIM-3 in Lung Cancers. Am. J. Clin. Pathol. 2012, 137, 978–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Zhu, C.; Sakuishi, K.; Xiao, S.; Sun, Z.; Zaghouani, S.; Gu, G.; Wang, C.; Tan, D.J.; Wu, C.; Rangachari, M.; et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat. Commun. 2015, 6, 6072. [Google Scholar] [CrossRef] [Green Version]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Chauvin, J.-M.; Sander, C.; Janjic, B.; Tarhini, A.A.; Tawbi, H.A.; Kirkwood, J.M.; Moschos, S.; et al. PD-1 and Tim-3 Regulate the Expansion of Tumor Antigen–Specific CD8+ T Cells Induced by Melanoma Vaccines. Cancer Res. 2014, 74, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, P.; Liang, T.; Wang, L.; Hu, L. TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 31705–31713. [Google Scholar] [CrossRef] [Green Version]
- Dancsok, A.R.; Setsu, N.; Gao, D.; Blay, J.-Y.; Thomas, D.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod. Pathol. 2019, 32, 1772–1785. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.-C.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef]
- Harding, J.J.; Patnaik, A.; Moreno, V.; Stein, M.; Jankowska, A.M.; de Mendizabal, N.V.; Liu, Z.T.; Koneru, M.; Calvo, E. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): Interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J. Clin. Oncol. 2019, 37, 12. [Google Scholar] [CrossRef]
- Ahn, M. MS28.02 Combination IO + IO. J. Thorac. Oncol. 2018, 13, S299–S300. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, A.; Laken, H.; Correia, J.D.S.; McNeeley, P.; Altobell, L.; Zhang, J.; Vancutsem, P.; Wilcoxen, K.; Jenkins, D. Discovery of TSR-022, a novel, potent anti-human TIM-3 therapeutic antibody. Eur. J. Cancer 2016, 69, S102. [Google Scholar] [CrossRef]
- Burugu, S.; Dancsok, A.R.; Nielsen, T.O. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2017, 52, 39–52. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [Green Version]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.L.; Smyth, M.J. Anti-TIM3 Antibody Promotes T Cell IFN-γ–Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef] [Green Version]
- Knösel, T.; Emde, A.; Schlüns, K.; Chen, Y.; Jürchott, K.; Krause, M.; Dietel, M.; Petersen, I. Immunoprofiles of 11 Biomarkers Using Tissue Microarrays Identify Prognostic Subgroups in Colorectal Cancer. Neoplasia 2005, 7, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Orth, M.F.; Buecklein, V.L.; Kampmann, E.; Subklewe, M.; Noessner, E.; Cidre-Aranaz, F.; Romero-Pérez, L.; Wehweck, F.S.; Lindner, L.; Issels, R.; et al. A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol. Immunother. 2020, 69, 1353–1362. [Google Scholar] [CrossRef]
- Albertsmeier, M.; Altendorf-Hofmann, A.; Lindner, L.H.; Issels, R.D.; Kampmann, E.; Dürr, H.-R.; Angele, M.K.; Klauschen, F.; Werner, J.; Jungbluth, A.A.; et al. VISTA in Soft Tissue Sarcomas: A Perspective for Immunotherapy? Cancers 2022, 14, 1006. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [Green Version]
- Klaver, Y.; Rijnders, M.; Oostvogels, A.; Wijers, R.; Smid, M.; Grünhagen, D.; Verhoef, K.; Sleijfer, S.; Lamers, C.; Debets, R. Differential quantities of immune checkpoint-expressing CD8 T cells in soft tissue sarcoma subtypes. J. Immunother. Cancer 2020, 8, e000271. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Lucchesi, C.; Verbeke, S.; Crombe, A.; Adam, J.; Geneste, D.; Chaire, V.; Laroche-Clary, A.; Perret, R.; Bertucci, F.; et al. High throughput profiling of undifferentiated pleomorphic sarcomas identifies two main subgroups with distinct immune profile, clinical outcome and sensitivity to targeted therapies. Ebiomedicine 2020, 62, 103131. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, R.D.; Cadena, R.H.; Nijhof, S.H.; Abdulahad, W.H.; Huitema, M.G.; Paap, D.; Brouwer, E.; Boots, A.M.H.; Heeringa, P. Effect of age and sex on immune checkpoint expression and kinetics in human T cells. Immun. Ageing 2020, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Kugel, C.H., III; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [Green Version]
- Daste, A.; Domblides, C.; Gross-Goupil, M.; Chakiba, C.; Quivy, A.; Cochin, V.; de Mones, E.; Larmonier, N.; Soubeyran, P.; Ravaud, A. Immune checkpoint inhibitors and elderly people: A review. Eur. J. Cancer 2017, 82, 155–166. [Google Scholar] [CrossRef]
- Jin, H.-T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.-J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, S.; Hu, Y.; Yang, Z.; Li, J.; Liu, X.; Deng, L.; Wang, Y.; Zhang, X.; Jiang, T.; et al. Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. J. Immunother. 2016, 39, 171–180. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chen, Y.; Long, Q.; Li, Q.; Tian, J.; Liu, T.; Wu, Y.; Ding, Z. Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J. Immunother. Cancer 2021, 9, e002836. [Google Scholar] [CrossRef]

| Factor | Strata | n | % |
|---|---|---|---|
| Total | 179 | 100 | |
| Sex | Male | 92 | 51 |
| Female | 87 | 49 | |
| Histological subtype | UPS | 59 | 33 |
| Liposarcoma | 40 | 22 | |
| Leiomyosarcoma | 31 | 17 | |
| Synovial sarcoma | 18 | 10 | |
| MPNST | 12 | 7 | |
| Angiosarcoma | 5 | 3 | |
| Malignant SFT | 2 | 1 | |
| Dediff. chondrosarcoma | 3 | 2 | |
| Myxofibrosarcoma | 5 | 3 | |
| Other | 4 | 2 | |
| Location | Extremities | 71 | 40 |
| Non-Extremities | 108 | 60 | |
| Size of primary tumor (cm) | 50–79 mm | 46 | 26 |
| 80–120 mm | 62 | 35 | |
| >120 mm | 71 | 40 | |
| Presence of metastases | No | 167 | 93 |
| Yes | 12 | 7 | |
| FNCLCC Grade | Intermediate (G2) | 89 | 50 |
| High (G3) | 90 | 50 | |
| TIM-3 expression (Grades 0–3) | 0 | 78 | 44 |
| 1 | 56 | 31 | |
| 2 | 37 | 21 | |
| 3 | 8 | 4 | |
| Follow-up status 5 years after initial diagnosis | Alive | 108 | 60 |
| Dead | 71 | 40 | |
| Local recurrence within 5 years after R0/R1 resection | No local recurrence | 91 | 60 |
| Local recurrence | 61 | 40 | |
| Distant recurrence within 5 years after R0/R1 resection | No distant recurrence | 103 | 68 |
| Distant recurrence | 49 | 32 |
| Factor | Strata | Total | TIM-3 > 0 | p-Value | |
|---|---|---|---|---|---|
| n | n | % | |||
| All Patients | 179 | 101 | 56 | -- | |
| Sex | Male | 92 | 59 | 64 | 0.036 |
| Female | 87 | 42 | 48 | ||
| Age at initial diagnosis (years) | <55 | 92 | 43 | 47 | 0.010 |
| ≥55 | 87 | 58 | 67 | ||
| Histological subtype | UPS | 59 | 44 | 75 | <0.001 |
| Liposarcoma | 31 | 11 | 35 | ||
| Leiomyosarcoma | 40 | 26 | 65 | ||
| Other | 49 | 20 | 41 | ||
| Tumor Location | Extremities | 71 | 47 | 66 | 0.045 |
| Non-extremities | 108 | 54 | 50 | ||
| FNCLCC Grade | Intermediate (G2) | 89 | 46 | 52 | 0.229 |
| High (G3) | 90 | 55 | 61 | ||
| Surgical margins | R0 | 69 | 48 | 70 | 0.011 |
| R1 | 83 | 41 | 49 | ||
| R2 | 14 | 4 | 29 | ||
| No resection | 13 | 8 | 62 | ||
| Chemotherapy | Yes | 134 | 80 | 60 | 0.164 |
| No | 45 | 21 | 47 | ||
| Radiotherapy | Yes | 30 | 16 | 53 | 0.535 |
| No | 106 | 48 | 45 | ||
| Missing | 43 | ||||
| Regional Hyperthermia (RHT) | Yes | 139 | 86 | 62 | 0.007 |
| No | 40 | 15 | 38 | ||
| TIL counts (cells/50HPF) | 0–5 | 108 | 46 | 43 | <0.001 |
| ≥6 | 70 | 54 | 77 | ||
| Missing | 1 | ||||
| PD-1 expression | 0 | 61 | 18 | 30 | <0.001 |
| ≥0 | 77 | 46 | 60 | ||
| Missing | 41 | ||||
| PD-L1 expression | 0 | 139 | 66 | 47 | <0.001 |
| ≥0 | 34 | 31 | 91 | ||
| Factor | Strata | Significance | Hazard Ratio | 95.0% CI |
|---|---|---|---|---|
| Sex | Male vs. Female | 0.026 | 2.289 | (1.106–4.737) |
| Age | <55 vs. ≥55 | 0.027 | 1.030 | (1.003–1.056) |
| TIL counts | 0–5 vs. ≥6 | 0.002 | 3.499 | (1.565–7.823) |
| PD-L1 expression | 0 vs. >0 | 0.001 | 9.173 | (2.420–34.772) |
| Histology | UPS vs. other subtypes | 0.038 | 2.316 | (1.046–5.128) |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Factor | Strata | Sig. | Hazard Ratio | Sig. | Hazard Ratio |
| Sex | Male vs. Female | 0.366 | 0.806 (0.505–1.287) | ||
| Age | 1 year step | 0.678 | 1.003 (0.987–1.020) | ||
| Grade | G2 vs. G3 | 0.015 | 1.812 (1.122–2.926) | 0.014 | 1.889 (1.139–3.133) |
| Surgical margins | R0/1 vs. R2 | <0.001 | 7.310 (4.339–12.318) | <0.001 | 6.866 (3.815–12.357) |
| Distant metastases | M0 vs. M1 | <0.001 | 4.187 (2.119–8.273) | 0.003 | 3.059 (1.476–6.341) |
| PD-L1 expression | 0 vs. >0 | 0.180 | 1.455 (0.840–2.520) | 0.542 | 1.227 (0.636–2.364) |
| TIL counts | 0–5 vs. ≥6 | 0.830 | 1.055 (0.649–1.713) | 0.247 | 1.406 (0.790–2.502) |
| TIM3 expression | 0 vs. >0 | 0.342 | 0.798 (0.501–1.271) | 0.246 | 1.403 (0.792–2.483) |
| Histology | UPS vs. other | 0.259 | 0.759 (0.470–1.226) | ||
| Tumor location | Extremities vs. non-Extremities | 0.285 | 1.302 (0.802–2.112) | ||
| Chemotherapy | Yes vs. no | 0.010 | 1.912 (1.168–3.129) | 0.498 | 1.212 (0.695–2.114) |
| Radiotherapy | Yes vs. no | 0.241 | 1.440 (0.783–2.647) | ||
| Regional hyperthermia | Yes vs. no | 0.749 | 1.091 (0.639–1.865) | ||
| PD1 expression | 0 vs > 0 | 0.106 | 1.521 (0.914–2.530) | ||
| TIM-3 x PDL1 | Both 0 vs. both >0 | 0.690 | 1.133 (0.613–2.095) | ||
| TIL x TIM-3 | TIL ≥ 6 and TIM-3 > 0 vs. TIL < 6 and TIM-3 = 0 | 0.599 | 0.848 (0.459–1.566) | ||
| TIM-3 x PD1 | Both 0 vs. both >0 | 0.323 | 1.372 (0.733–2.569) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berclaz, L.M.; Altendorf-Hofmann, A.; Lindner, L.H.; Burkhard-Meier, A.; Di Gioia, D.; Dürr, H.R.; Klein, A.; Albertsmeier, M.; Schmidt-Hegemann, N.-S.; Klauschen, F.; et al. TIM-3 Qualifies as a Potential Immunotherapeutic Target in Specific Subsets of Patients with High-Risk Soft Tissue Sarcomas (HR-STS). Cancers 2023, 15, 2735. https://doi.org/10.3390/cancers15102735
Berclaz LM, Altendorf-Hofmann A, Lindner LH, Burkhard-Meier A, Di Gioia D, Dürr HR, Klein A, Albertsmeier M, Schmidt-Hegemann N-S, Klauschen F, et al. TIM-3 Qualifies as a Potential Immunotherapeutic Target in Specific Subsets of Patients with High-Risk Soft Tissue Sarcomas (HR-STS). Cancers. 2023; 15(10):2735. https://doi.org/10.3390/cancers15102735
Chicago/Turabian StyleBerclaz, Luc M., Annelore Altendorf-Hofmann, Lars H. Lindner, Anton Burkhard-Meier, Dorit Di Gioia, Hans Roland Dürr, Alexander Klein, Markus Albertsmeier, Nina-Sophie Schmidt-Hegemann, Frederick Klauschen, and et al. 2023. "TIM-3 Qualifies as a Potential Immunotherapeutic Target in Specific Subsets of Patients with High-Risk Soft Tissue Sarcomas (HR-STS)" Cancers 15, no. 10: 2735. https://doi.org/10.3390/cancers15102735
APA StyleBerclaz, L. M., Altendorf-Hofmann, A., Lindner, L. H., Burkhard-Meier, A., Di Gioia, D., Dürr, H. R., Klein, A., Albertsmeier, M., Schmidt-Hegemann, N.-S., Klauschen, F., & Knösel, T. (2023). TIM-3 Qualifies as a Potential Immunotherapeutic Target in Specific Subsets of Patients with High-Risk Soft Tissue Sarcomas (HR-STS). Cancers, 15(10), 2735. https://doi.org/10.3390/cancers15102735






