Comparison of the Survival Outcomes of Minimally Invasive Surgery with Open Surgery in Patients with Uterine-Confined and Node-Negative Cervical Cancer: A Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
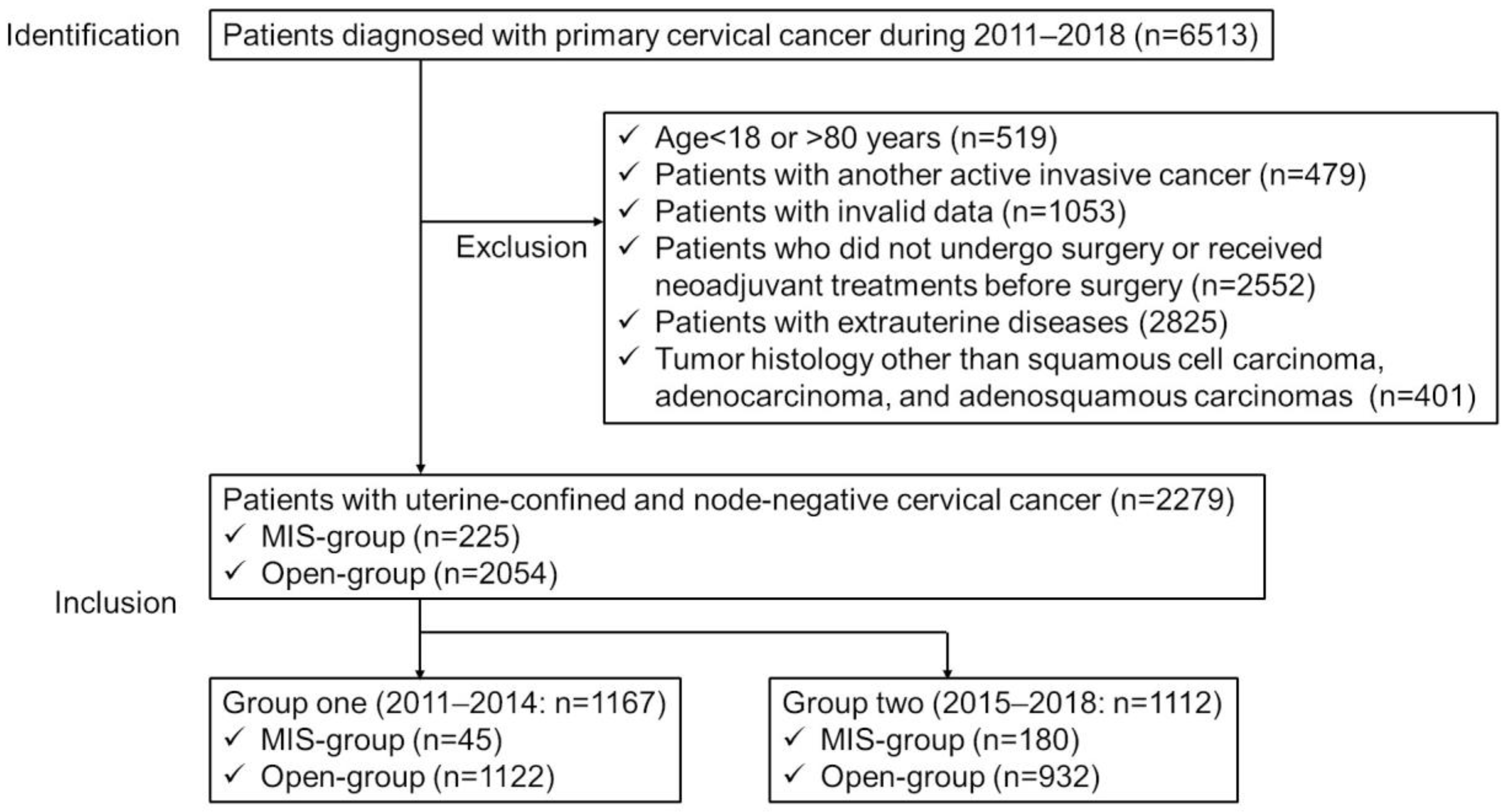
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Investigations Involving All Uterine Confined and Node-Negative Cervical Cancer Patients
3.2. Investigations According to Year of Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, S.; Okazawa, M.; Isohashi, F.; Matsuo, K.; Ohta, Y.; Suzuki, O.; Yoshioka, Y.; Enomoto, T.; Kamiura, S.; Kimura, T. Radical hysterectomy with adjuvant radiotherapy versus definitive radiotherapy alone for FIGO stage IIB cervical cancer. Gynecol. Oncol. 2011, 123, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A Randomized Trial of Pelvic Radiation Therapy versus No Further Therapy in Selected Patients with Stage IB Carcinoma of the Cervix after Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Muaddi, H.; Hafid, M.E.; Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann. Surg. 2021, 273, 467–473. [Google Scholar] [CrossRef]
- Nitecki, R.; Ramirez, P.T.; Frumovitz, M.; Krause, K.J.; Tergas, A.I.; Wright, J.D.; Rauh-Hain, J.A.; Melamed, A. Survival After Minimally Invasive vs. Open Radical Hysterectomy for Early-Stage Cervical Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1019–1027. [Google Scholar] [CrossRef]
- Dinoi, G.; Ghoniem, K.; Murad, M.H.; Segarra-Vidal, B.; Zanfagnin, V.; Coronado, P.J.; Kyrgiou, M.; Perrone, A.M.; Zola, P.; Weaver, A.; et al. Minimally Invasive Compared With Open Surgery in High-Risk Endometrial Cancer: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2023, 141, 59–68. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Cervical Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 19 December 2018).
- Querleu, D.; Cibula, D.; Concin, N.; Fagotti, A.; Ferrero, A.; Fotopoulou, C.; Knapp, P.; Kurdiani, D.; Ledermann, J.A.; Mirza, M.R.; et al. Laparoscopic radical hysterectomy: A European Society of Gynaecological Oncology (ESGO) statement. Int. J. Gynecol. Cancer 2019, 30, 15. [Google Scholar] [CrossRef]
- Wenzel, H.H.; Smolders, R.G.; Beltman, J.J.; Lambrechts, S.; Trum, H.W.; Yigit, R.; Zusterzeel, P.L.; Zweemer, R.P.; Mom, C.H.; Bekkers, R.L.; et al. Survival of patients with early-stage cervical cancer after abdominal or laparoscopic radical hysterectomy: A nationwide cohort study and literature review. Eur. J. Cancer 2020, 133, 14–21. [Google Scholar] [CrossRef]
- Brandt, B.; Sioulas, V.; Basaran, D.; Kuhn, T.; LaVigne, K.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Long Roche, K.C.; Mueller, J.J.; et al. Minimally invasive surgery versus laparotomy for radical hysterectomy in the management of early-stage cervical cancer: Survival outcomes. Gynecol. Oncol. 2020, 156, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Köhler, C.; De Franciscis, P.; La Verde, M.; Mosca, L.; Solazzo, M.C.; Colacurci, N. Laparo-assisted vaginal radical hysterectomy as a safe option for Minimal Invasive Surgery in early stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 166, 188–195. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, M.; Lee, S.; Suh, D.H.; Kim, H.S.; Kim, K.; Chung, H.H.; No, J.H.; Kim, J.-W.; Park, N.H.; et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: A matching study of two institutional hospitals in Korea. Gynecol. Oncol. 2019, 155, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, F.; Wada-Hiraike, O.; Hirata, T.; Tajima, H.; Masuda, H.; Kitade, M.; Kumakiri, J.; Uchiide, I.; Saito, J.; Kurose, K.; et al. A nationwide survey on gynecologic endoscopic surgery in Japan, 2014–2016. J. Obstet. Gynaecol. Res. 2018, 44, 2067–2076. [Google Scholar] [CrossRef]
- Gota, T.; Tomio, K.; Kurose, T.; Saito, R.; Nara, R.; Kin, S.; Hoshiba, M.; Ogata, Y.; Nakanishi, M.; Takamoto, M.; et al. The current status of robotic surgery for endometrial cancer in Japan. Glob. Health Med. 2022, 4, 21–25. [Google Scholar] [CrossRef]
- Ohta, T.; Nagase, S.; Okui, Y.; Enomoto, T.; Yamagami, W.; Mikami, M.; Tokunaga, H.; Ino, K.; Ushijima, K.; Shozu, M.; et al. Surveillance of radical hysterectomy for early-stage cervical cancer in the early experienced period of minimally invasive surgery in Japan. Int. J. Clin. Oncol. 2021, 26, 2318–2330. [Google Scholar] [CrossRef]
- Kobayashi, E.; Kanao, H.; Takekuma, M.; Nishio, S.; Kojima-Chiba, A.; Tozawa, A.; Yamaguchi, S.; Takeshima, N.; Nakatani, E.; Mikami, M. A retrospective assessment of the safety and efficacy of laparoscopic radical hysterectomy in Japan during the early years following its introduction: A Japanese Gynecologic Oncology Group study (JGOG1081S). Int. J. Clin. Oncol. 2021, 26, 417–428. [Google Scholar] [CrossRef]
- Fusegi, A.; Kanao, H.; Ishizuka, N.; Nomura, H.; Tanaka, Y.; Omi, M.; Aoki, Y.; Kurita, T.; Yunokawa, M.; Omatsu, K.; et al. Oncologic Outcomes of Laparoscopic Radical Hysterectomy Using the No-Look No-Touch Technique for Early Stage Cervical Cancer: A Propensity Score-Adjusted Analysis. Cancers 2021, 13, 6097. [Google Scholar] [CrossRef]
- Tanaka, T.; Ueda, S.; Miyamoto, S.; Hashida, S.; Terada, S.; Konishi, H.; Kogata, Y.; Taniguchi, K.; Komura, K.; Ohmichi, M. Comparison of Prognosis between Minimally Invasive and Abdominal Radical Hysterectomy for Patients with Early-Stage Cervical Cancer. Curr. Oncol. 2022, 29, 2272–2283. [Google Scholar] [CrossRef]
- Kondo, E.; Yoshida, K.; Kubo-Kaneda, M.; Nii, M.; Okamoto, K.; Magawa, S.; Nimua, R.; Okumura, A.; Okugawa, T.; Yamawaki, T.; et al. Does Vaginal Cuff Creation and Avoidance of a Uterine Manipulator Improve the Prognosis of Total Laparoscopic Radical Hysterectomy for Early Cervical Cancer? A Retrospective Multicenter Study. Cancers 2022, 14, 4389. [Google Scholar] [CrossRef] [PubMed]
- Cancer Control Center, Osaka International Cancer Institute. The History of Osaka Cancer Registry; Osaka International Cancer Institute: Osaka, Japan, 2018; Available online: https://oici.jp/ocr/history/history.html (accessed on 15 October 2022).
- Mabuchi, S.; Morishige, K.-I.; Isohashi, F.; Yoshioka, Y.; Takeda, T.; Yamamoto, T.; Yoshino, K.; Enomoto, T.; Inoue, T.; Kimura, T. Postoperative concurrent nedaplatin-based chemoradiotherapy improves survival in early-stage cervical cancer patients with adverse risk factors. Gynecol. Oncol. 2009, 115, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Touhami, O.; Plante, M. Minimally Invasive Surgery for Cervical Cancer in Light of the LACC Trial: What Have We Learned? Curr. Oncol. 2022, 29, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, L.; Ni, Y.; Liu, J.; Li, D.; Guo, J.; Liu, Z.; Jin, S.; Xu, Y.; Li, Z.; et al. Comparison between laparoscopic and abdominal radical hysterectomy for stage IB1 and tumor size < 2 cm cervical cancer with visible or invisible tumors: A multicentre retrospective study. J. Gynecol. Oncol. 2021, 32, e17. [Google Scholar] [CrossRef]
- Kobayashi, E.; Nakatani, E.; Tanaka, T.; Yosuke, K.; Kanao, H.; Shiki, Y.; Kotani, Y.; Hoshiba, T.; Minami, R.; Yoshida, H.; et al. Surgical skill and oncological outcome of laparoscopic radical hysterectomy: JGOG1081s-A1, an ancillary analysis of the Japanese Gynecologic Oncology Group Study JGOG1081. Gynecol. Oncol. 2022, 165, 293–301. [Google Scholar] [CrossRef]
- Brajcich, B.C.; Stulberg, J.J.; Palis, B.E.; Chung, J.W.; Huang, R.; Nelson, H.; Bilimoria, K.Y. Association Between Surgical Technical Skill and Long-term Survival for Colon Cancer. JAMA Oncol. 2021, 7, 127–129. [Google Scholar] [CrossRef]
- Markar, S.R.; Lagergren, J. Surgical and Surgeon-Related Factors Related to Long-Term Survival in Esophageal Cancer: A Review. Ann. Surg. Oncol. 2020, 27, 718–723. [Google Scholar] [CrossRef]
- Osaka Prefectural Government Census 2010; Osaka Prefectural Government: Osaka, Japan, 2011; Available online: https://www.stat.go.jp/english/data/kokusei/pdf/census.pdf (accessed on 15 January 2023).
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. Cancer Incidence in Five Continents; IARC Scientific Publication No. 166; International Agency for Research on Cancer: Lyon, France, 2021; Volume XI. [Google Scholar]


| MIS Group (n = 225) No. (%) | Open Group (n = 2054) No. (%) | p-Value | ||
|---|---|---|---|---|
| Age (years) | 18–39 | 50 (22.2) | 758 (36.9) | <0.0001 |
| 40–60 | 133 (59.1) | 966 (47.0) | ||
| 61–80 | 42 (18.7) | 330 (16.1) | ||
| Means ± SD | 48.0 ± 11.6 | 45.5 ± 12.5 | 0.0024 | |
| Histological type | Squamous cell carcinoma | 139 (61.8) | 1497 (72.9) | 0.0018 |
| Adenocarcinoma | 76 (33.8) | 482 (23.5) | ||
| Adenosquamous carcinoma | 10 (4.4) | 75 (3.6) | ||
| Treatments | Surgery | 176 (78.2) | 1633 (79.5) | 0.0016 |
| Surgery + Radiotherapy | 5 (2.2) | 102 (5.0) | ||
| Surgery + Chemotherapy | 17 (7.6) | 196 (9.5) | ||
| Surgery + Radiotherapy + Chemotherapy | 27 (12.0) | 123 (6.0) |
| Group One (2011–2014) | Group Two (2015–2018) | ||||||
|---|---|---|---|---|---|---|---|
| MIS Group (n = 45) | Open Group (n = 1122) | p-Value | MIS Group (n = 180) | Open Group (n = 932) | p-Value | ||
| Age (years) | 18–39 | 10 (22.2) | 394 (35.1) | 0.0569 | 40 (22.2) | 364 (39.1) | <0.0001 |
| 40–60 | 30 (66.7) | 544 (48.5) | 103 (57.2) | 422 (45.3) | |||
| 61–80 | 5 (11.1) | 184 (16.4) | 37 (20.6) | 146 (15.7) | |||
| Means ± SD | 45.9 ± 10.3 | 45.6 ± 12.4 | 0.8867 | 48.8 ± 11.8 | 45.4 ± 12.6 | 0.0019 | |
| Histological type | Squamous cell carcinoma | 27 (60.0) | 825 (73.5) | 0.0649 | 112 (62.2) | 672 (72.1) | 0.0290 |
| Adenocarcinoma | 17 (37.8) | 256 (22.8) | 59 (32.8) | 226 (24.2) | |||
| Adenosquamous carcinoma | 1 (2.2) | 41 (3.7) | 9 (5.0) | 34 (3.6) | |||
| Treatments | Surgery | 37 (82.2) | 880 (78.4) | 0.4064 | 139 (77.2) | 753 (80.8) | 0.0026 |
| Surgery + Radiotherapy | 0 (0) | 59 (5.3) | 5 (2.8) | 43 (4.6) | |||
| Surgery + Chemotherapy | 6 (13.3) | 118 (10.5) | 11 (6.1) | 78 (8.4) | |||
| Surgery + Radiotherapy + Chemotherapy | 2 (4.4) | 65 (5.8) | 25 (13.9) | 58 (6.2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabuchi, S.; Sasano, T.; Komura, N.; Maeda, M.; Matsuzaki, S.; Hisa, T.; Kamiura, S.; Morishima, T.; Miyashiro, I. Comparison of the Survival Outcomes of Minimally Invasive Surgery with Open Surgery in Patients with Uterine-Confined and Node-Negative Cervical Cancer: A Population-Based Study. Cancers 2023, 15, 2756. https://doi.org/10.3390/cancers15102756
Mabuchi S, Sasano T, Komura N, Maeda M, Matsuzaki S, Hisa T, Kamiura S, Morishima T, Miyashiro I. Comparison of the Survival Outcomes of Minimally Invasive Surgery with Open Surgery in Patients with Uterine-Confined and Node-Negative Cervical Cancer: A Population-Based Study. Cancers. 2023; 15(10):2756. https://doi.org/10.3390/cancers15102756
Chicago/Turabian StyleMabuchi, Seiji, Tomoyuki Sasano, Naoko Komura, Michihide Maeda, Shinya Matsuzaki, Tsuyoshi Hisa, Shoji Kamiura, Toshitaka Morishima, and Isao Miyashiro. 2023. "Comparison of the Survival Outcomes of Minimally Invasive Surgery with Open Surgery in Patients with Uterine-Confined and Node-Negative Cervical Cancer: A Population-Based Study" Cancers 15, no. 10: 2756. https://doi.org/10.3390/cancers15102756





