Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of [18F]FAPI-74 with [18F]FDG in Patients with PDAC: A Prospective Exploratory Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study Design and Patient Cohort
2.2. Radiochemistry and Image Acquisition
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort
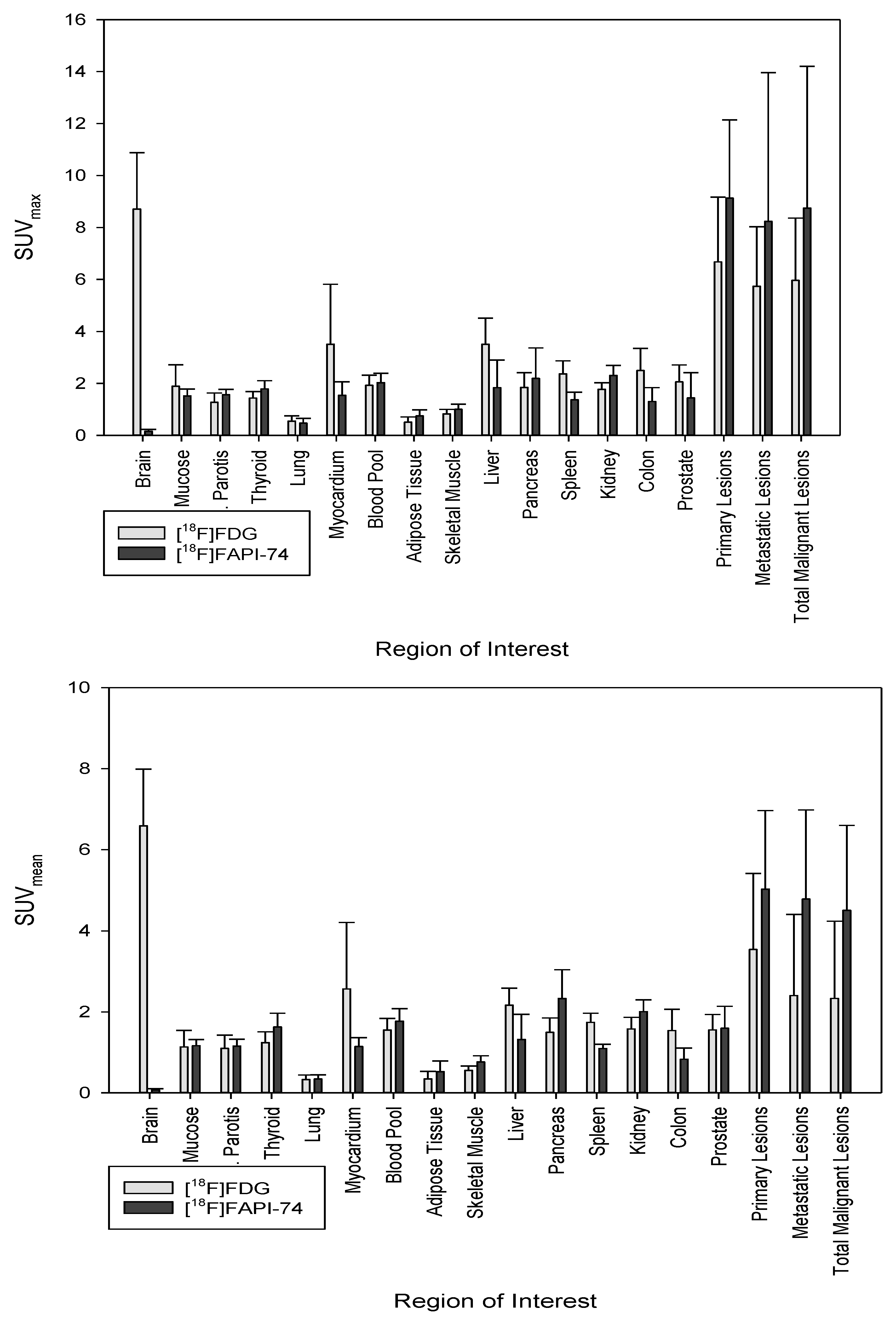
3.2. Biodistribution of [18F]FAPI-74 in Normal Tissue
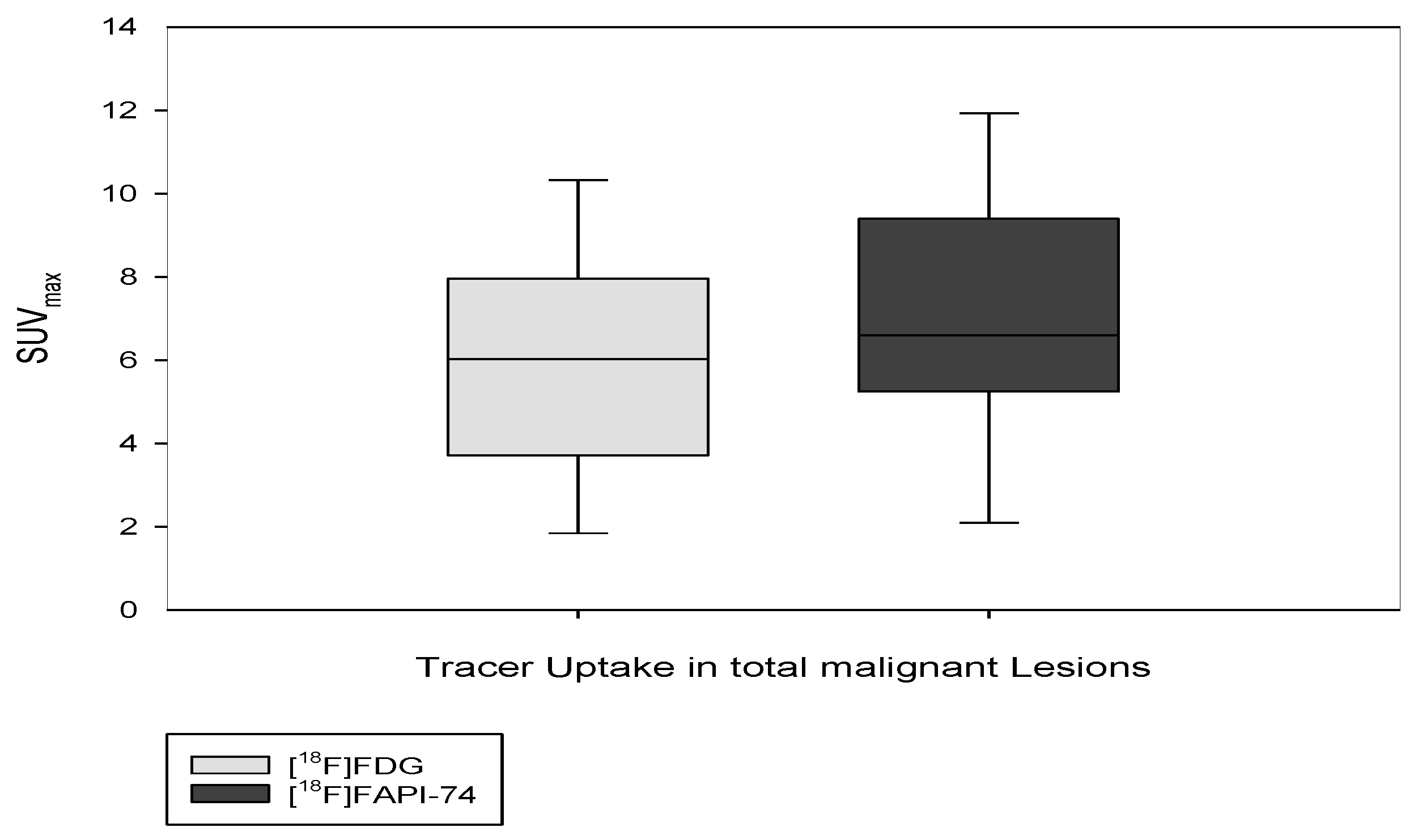
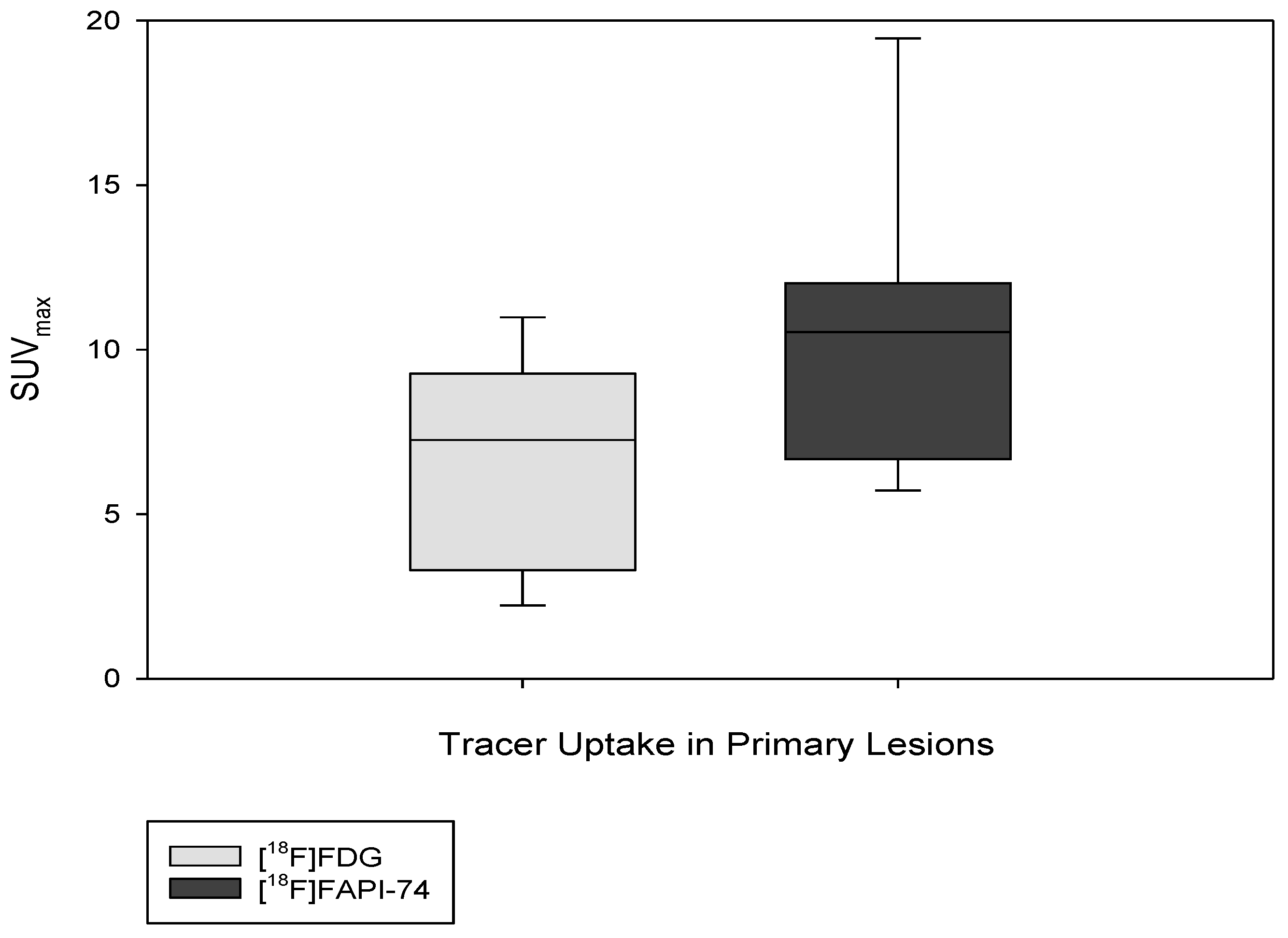
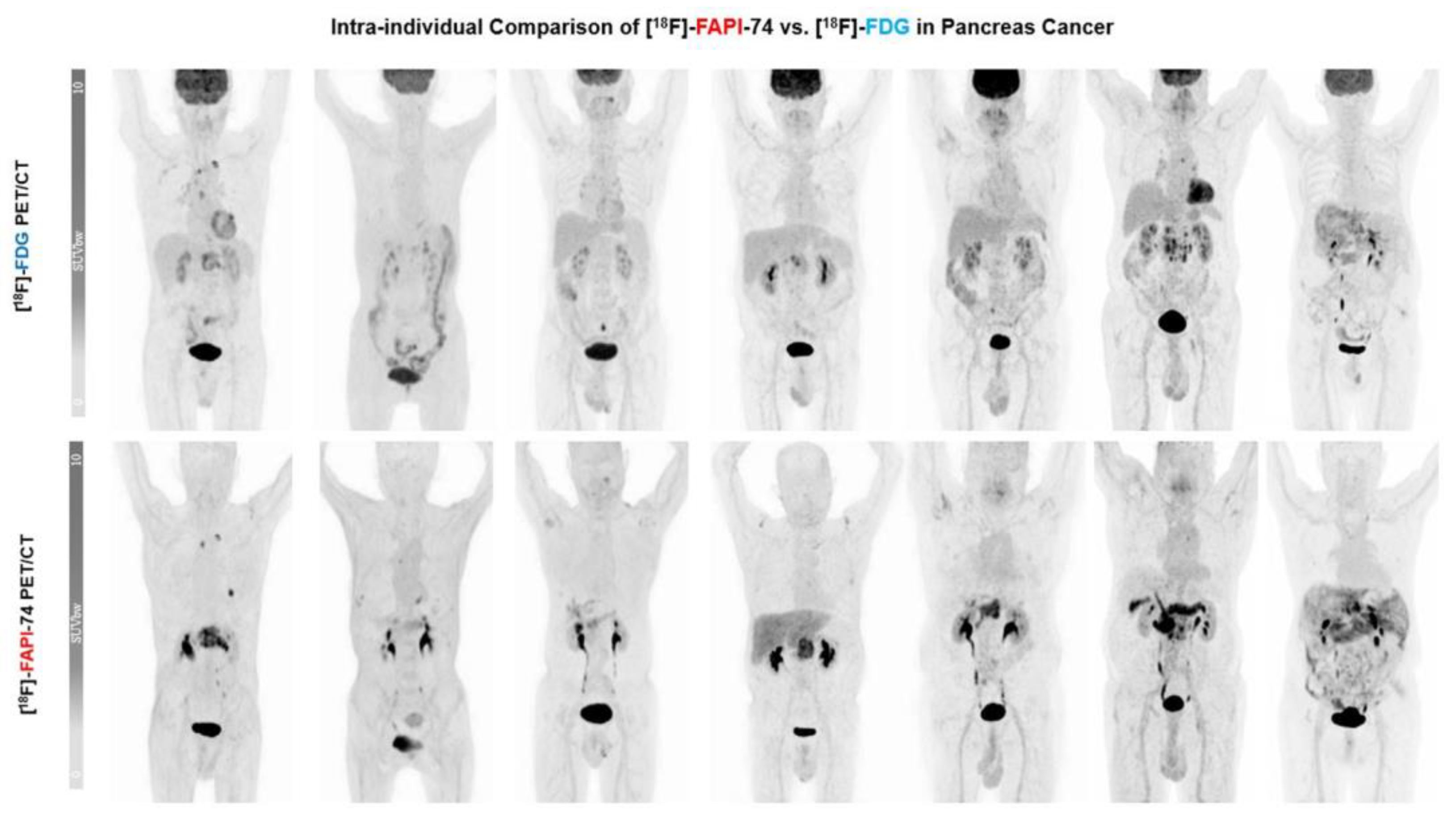
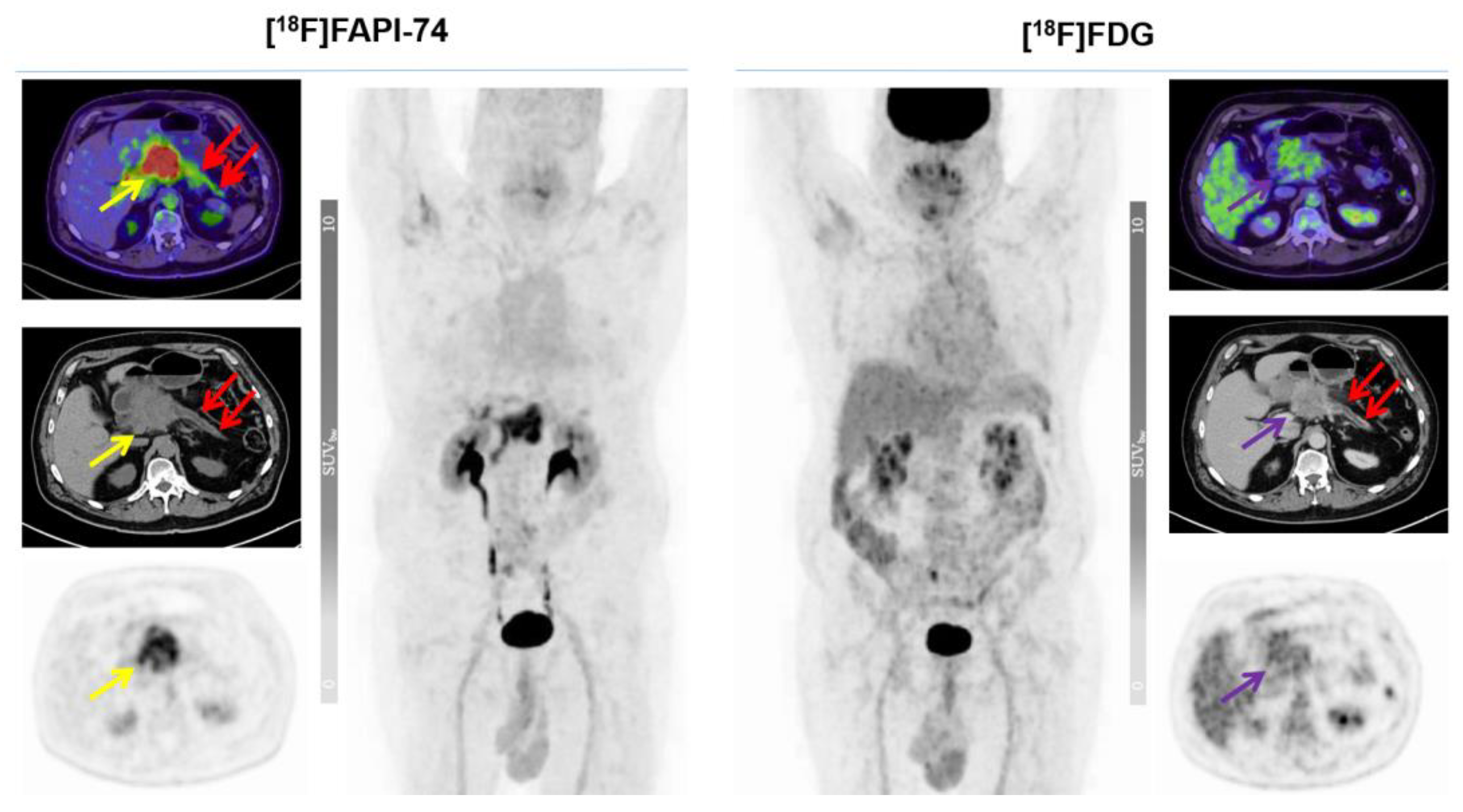
3.3. [18F]FAPI-74 Uptake in Tumor Lesions
3.4. [18F]FAPI-74 in PDAC with Confounding Pancreatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, Y.; Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 2021, 172, 37–51. [Google Scholar] [CrossRef]
- Cohen, R.; Neuzillet, C.; Tijeras-Raballand, A.; Faivre, S.; De Gramont, A.; Raymond, E. Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget 2015, 6, 16832–16847. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Vennin, C.; Papanicolaou, M.; Chambers, C.R.; Herrmann, D.; Morton, J.P.; Cox, T.R.; Timpson, P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 2019, 5, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and Kinetic Analysis of the Substrate Specificity of Human Fibroblast Activation Protein alpha. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, 220749. [Google Scholar] [CrossRef]
- Shi, M.; Yu, D.-H.; Chen, Y.; Zhao, C.-Y.; Zhang, J.; Liu, Q.-H.; Ni, C.-R.; Zhu, M.-H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Arnone, A.; Laudicella, R.; Caobelli, F.; Guglielmo, P.; Spallino, M.; Abenavoli, E.; Martini, A.L.; Filice, R.; Comis, A.D.; Cuzzocrea, M.; et al. Clinical Impact of 18F-FDG PET/CT in the Diagnostic Workup of Pancreatic Ductal Adenocarcinoma: A Systematic Review. Diagnostics 2020, 10, 1042. [Google Scholar] [CrossRef]
- Daamen, L.A.; Groot, V.P.; Goense, L.; Wessels, F.J.; Rinkes, I.H.B.; Intven, M.P.; Van Santvoort, H.C.; Molenaar, I.Q. The diagnostic performance of CT versus FDG PET-CT for the detection of recurrent pancreatic cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2018, 106, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Hirmas, N.; Hamacher, R.; Sraieb, M.; Ingenwerth, M.; Kessler, L.; Pabst, K.M.; Barbato, F.; Lueckerath, K.; Kasper, S.; Nader, M.; et al. Fibroblast activation protein positron emission tomography and histopathology in a single-center database of 324 patients and 21 tumor entities. J. Nucl. Med. 2022, 64, 711–716. [Google Scholar] [CrossRef]
- Liermann, J.; Syed, M.; Ben-Josef, E.; Schubert, K.; Schlampp, I.; Sprengel, S.D.; Ristau, J.; Weykamp, F.; Röhrich, M.; Koerber, S.A.; et al. Impact of FAPI-PET/CT on Target Volume Definition in Radiation Therapy of Locally Recurrent Pancreatic Cancer. Cancers 2021, 13, 796. [Google Scholar] [CrossRef]
- Mona, C.E.; Benz, M.R.; Hikmat, F.; Grogan, T.R.; Lueckerath, K.; Razmaria, A.; Riahi, R.; Slavik, R.; Girgis, M.D.; Carlucci, G.; et al. Correlation of 68Ga-FAPi-46 PET Biodistribution with FAP Expression by Immunohistochemistry in Patients with Solid Cancers: Interim Analysis of a Prospective Translational Exploratory Study. J. Nucl. Med. 2021, 63, 1021–1026. [Google Scholar] [CrossRef]
- Röhrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2020, 62, 779–786. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, S.; Liu, S.; Xu, X.; Hu, S.; Zhang, J.; Wang, C.; Yu, X.; Song, S. The added value of [68Ga]Ga-DOTA-FAPI-04 PET/CT in pancreatic cancer: A comparison to [18F]F-FDG. Eur. Radiol. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Giesel, F.; Kratochwil, C.; Kleist, C.; Krämer, S.; Mier, W.; Cardinale, J.; Kauczor, H.-U.; Jäger, D.; et al. 18F-labeled tracers targeting fibroblast activation protein. EJNMMI Radiopharm. Chem. 2021, 6, 26. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2018, 60, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Glatting, F.M.; Hoppner, J.; Kauczor, H.-U.; Huber, P.E.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U.; Röhrich, M. Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74. Cancers 2022, 14, 5301. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Wang, C.; Zhao, S.; Xie, R.; Zhao, L.; Li, K.; Yang, C.; Zhang, R.; Tian, Y.; Tan, L.; et al. The clinical application of 18F-FDG PET/CT in pancreatic cancer: A narrative review. Transl. Cancer Res. 2021, 10, 3560–3575. [Google Scholar] [CrossRef]
- Tang, S.; Huang, G.; Liu, J.; Liu, T.; Treven, L.; Song, S.; Zhang, C.; Pan, L.; Zhang, T. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: A meta-analysis. Eur. J. Radiol. 2011, 78, 142–150. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Lang, M.; Spektor, A.-M.; Hielscher, T.; Hoppner, J.; Glatting, F.M.; Bicu, F.; Hackert, T.; Heger, U.; Pausch, T.; Gutjahr, E.; et al. Static and Dynamic 68Ga-FAPI PET/CT for the Detection of Malignant Transformation of Intraductal Papillary Mucinous Neoplasia of the Pancreas. J. Nucl. Med. 2022, 64, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either 18F-AlF or Cold-Kit 68Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2020, 62, 201–207. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Xu, X.; Ding, J.; Liu, S.; Hou, X.; Li, N.; Zhu, H.; Yang, Z. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4259–4271. [Google Scholar] [CrossRef]
- Dabir, M.; Novruzov, E.; Mattes-György, K.; Beu, M.; Dendl, K.; Antke, C.; Koerber, S.A.; Röhrich, M.; Kratochwil, C.; Debus, J.; et al. Distinguishing Benign and Malignant Findings on [68Ga]-FAPI PET/CT Based on Quantitative SUV Measurements. Mol. Imaging Biol. 2022, 25, 324–333. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Qin, C.; Liu, F.; Lan, X. Non-malignant findings of focal 68Ga-FAPI-04 uptake in pancreas. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2635–2641. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Pang, Y.; Fu, K.; Shang, Q.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics 2022, 12, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Fibroblast Activation Protein Inhibitor Theranostics: Early Clinical Trans-lation. PET Clin. 2023; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]


| Patient | Age | Sex | Previous Surgery | Previous Chemotherapy | Previous Radiation | Clinical Indication | Additional Findings in [18F]FAPI-74 | Staging Change |
|---|---|---|---|---|---|---|---|---|
| 1 | 57 | F | No | No | No | Primary staging | 4 | No |
| 2 | 69 | M | No | FOLFIRI | No | Therapy response control/primary staging | 1 | Yes |
| 3 | 65 | M | No | FOLFIRI | Yes | Therapy response control/re-staging | no | No |
| 4 | 66 | M | No | FOLFIRI | Yes | Therapy response control/re-staging | no | No |
| 5 | 78 | M | No | FOLFOX | Yes | Therapy response control/re-staging | no | No |
| 6 | 79 | M | No | No | No | Primary staging | no | No |
| 7 | 75 | M | No | No | No | Primary staging | 2 | No |
| VOI | Tumor/Blood Pool | Tumor/Skeletal Muscle | Tumor/Fat Tissue | |||
|---|---|---|---|---|---|---|
| Tracer | [18F]FAPI-74 | [18F]FDG | [18F]FAPI-74 | [18F]FDG | [18F]FAPI-74 | [18F]FDG |
| TBR | 2.64 | 2.64 | 5.24 | 7.01 | 9.06 | 10.13 |
| p value | 0.14 | 0.97 | 0.51 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novruzov, E.; Giesel, F.L.; Mori, Y.; Choyke, P.L.; Dabir, M.; Mamlins, E.; Schmitt, D.; Antke, C.; Pinto, C.; Soza-Ried, C.; et al. Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of [18F]FAPI-74 with [18F]FDG in Patients with PDAC: A Prospective Exploratory Study. Cancers 2023, 15, 2798. https://doi.org/10.3390/cancers15102798
Novruzov E, Giesel FL, Mori Y, Choyke PL, Dabir M, Mamlins E, Schmitt D, Antke C, Pinto C, Soza-Ried C, et al. Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of [18F]FAPI-74 with [18F]FDG in Patients with PDAC: A Prospective Exploratory Study. Cancers. 2023; 15(10):2798. https://doi.org/10.3390/cancers15102798
Chicago/Turabian StyleNovruzov, Emil, Frederik L. Giesel, Yuriko Mori, Peter L. Choyke, Mardjan Dabir, Eduards Mamlins, Dominik Schmitt, Christina Antke, Claudio Pinto, Cristian Soza-Ried, and et al. 2023. "Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of [18F]FAPI-74 with [18F]FDG in Patients with PDAC: A Prospective Exploratory Study" Cancers 15, no. 10: 2798. https://doi.org/10.3390/cancers15102798
APA StyleNovruzov, E., Giesel, F. L., Mori, Y., Choyke, P. L., Dabir, M., Mamlins, E., Schmitt, D., Antke, C., Pinto, C., Soza-Ried, C., Fernandez, R., Amaral, H., Kramer, V., & Badinez, L. (2023). Head-to-Head Intra-Individual Comparison of Biodistribution and Tumor Uptake of [18F]FAPI-74 with [18F]FDG in Patients with PDAC: A Prospective Exploratory Study. Cancers, 15(10), 2798. https://doi.org/10.3390/cancers15102798







