Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Estrogen Receptor β (ERβ)
2.1. ERβ in Ovarian Cancer
2.1.1. ERβ Protein and mRNA Expression in Ovarian Cancer and Their Influence on Patients’ Survival
2.1.2. Influence of the Subcellular Localization of ERβ on Its Action in Ovarian Cancer
2.1.3. ERβ Splice Variants and Their Distinct Actions in Ovarian Cancer
2.1.4. In Vitro Studies on ERβ Action in Ovarian Cancer
| Cell Lines | Experimental Strategy and Results | Suggested Function of ERβ in OC | Reference |
|---|---|---|---|
| SK-OV-3 | Overexpression of ERβ1
| tumor-suppressive | [29] |
| OVCAR-3 and OAW-42 | ERβ-KD
| tumor-suppressive | [15] |
| Kuramochi, OVCAR4, OVCAR3, PEO1, and OV2008 | Treatment with ERβ agonist OSU-ERb-12
| tumor-suppressive | [77] |
| OCSCs from ES2, OV90, SKOV3, OVSAHO, and A2780 cells | Treatment with ERβ agonist LY500307
| tumor-suppressive | [79] |
2.2. ERβ in Endometrial Cancer
2.2.1. ERβ Protein and mRNA Expression in Endometrial Cancer and Their Influence on Patients’ Survival
2.2.2. ERβ Splice Variants and Their Actions in Endometrial Cancer
2.2.3. Impact of the ERα/ERβ Ratio on Endometrial Carcinogenesis
2.2.4. In Vitro Studies on ERβ Action in Endometrial Cancer
| Cell Lines | Experimental Strategy and Results | Suggested Function of ERβ in EC | Reference |
|---|---|---|---|
| Ishikawa | ERβ-KD
| tumor-suppressive | [88] |
| HEC-1A and RL95/2 | ERβ-KD
| tumor-suppressive | [16] |
3. G-Protein Coupled Estrogen Receptor (GPER1)
3.1. GPER1 in Ovarian Cancer
3.1.1. GPER1 Protein and mRNA Expression in Ovarian Cancer, and Their Prognostic Relevance
3.1.2. Influence of the Subcellular Localization of GPER1 on Its Action in Ovarian Cancer
3.1.3. In Vitro Studies on GPER1 Action in Ovarian Cancer
| Cell Lines | Experimental Strategy and Results | Suggested Function of GPER1 in OC | Reference |
|---|---|---|---|
| OVCAR-3 and OAW-42 | GPER1-KD
| tumor-suppressive | [115] |
| SKOV-3 and OVCAR-3 | Treatment with GPER1 agonist G-1
| tumor-suppressive | [114] |
| IGROV-1 and SKOV-3 | Treatment with GPER1 agonist G-1
| tumor-suppressive | [124] |
| KF and UWB1.289 | GPER1-KD or treatment with antagonist G15
| oncogenic | [122] |
| OVCAR5 | Treatment with GPER1 agonist G-1
| oncogenic | [113] |
| OVCAR5 | Treatment with GPER1 agonist G-1
| oncogenic | [125] |
| BG-1 | Treatment with GPER1 agonist G-1
| oncogenic | [126] |
3.1.4. Omega-3 Polyunsaturated Fatty Acids and Shikonin Act via GPER1 in Ovarian Cancer
3.2. GPER1 in Endometrial Cancer
3.2.1. GPER1 Protein and mRNA Expression in Endometrial Cancer
3.2.2. In Vitro and In Vivo Studies on GPER1 Action in Endometrial Cancer
| Cell Lines | Experimental Strategy and Results | Suggested Function of GPER1 in EC | Reference |
|---|---|---|---|
| RL-95-2, HEC-1A, and HEC-1B | Treatment with GPER1 agonist G-1
| tumor-suppressive | [134] |
| Ishikawa and KLE | Treatment with GPER1 agonist G-1
| oncogenic | [138] |
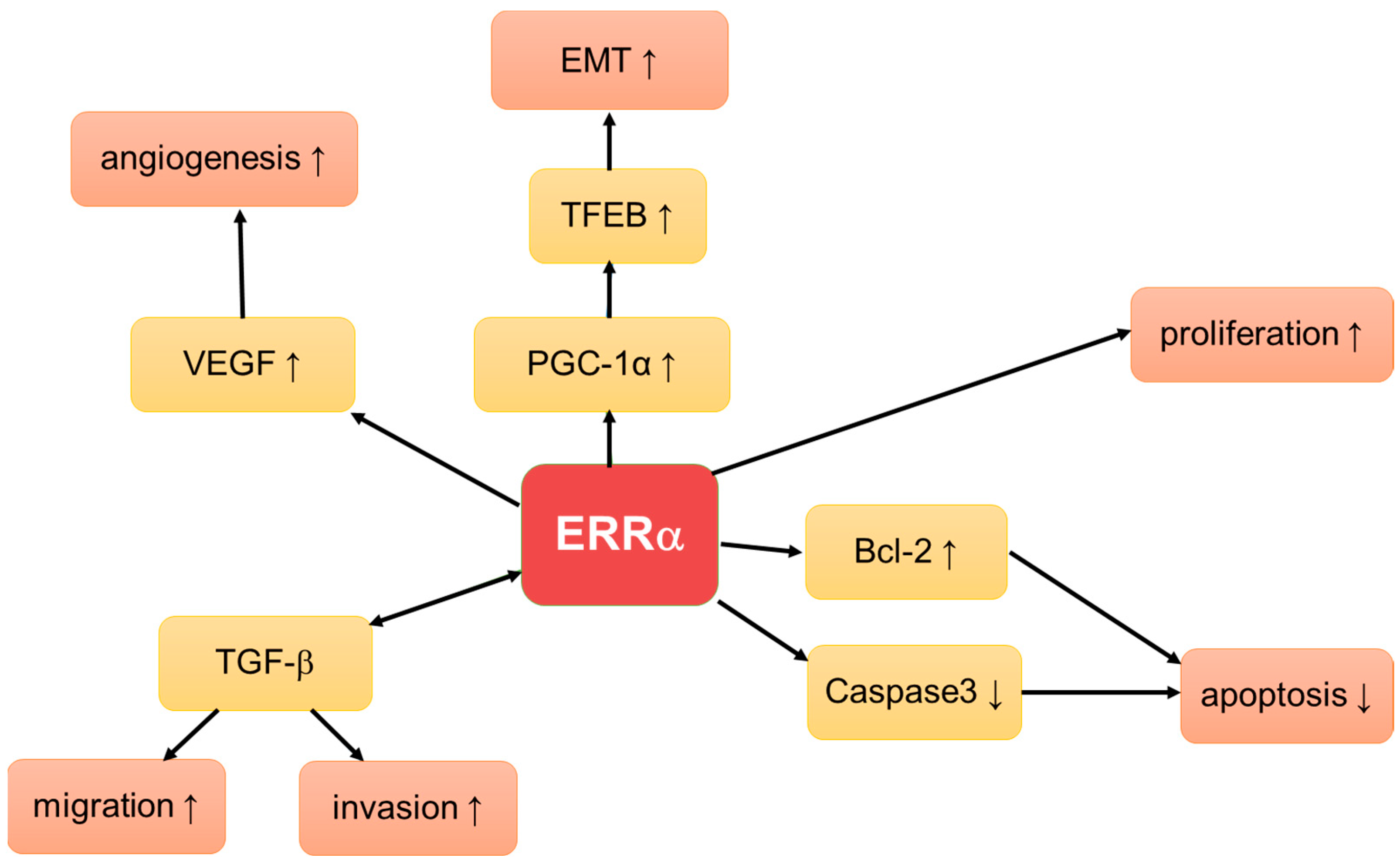
4. Estrogen Related Receptors (ERRs)
4.1. ERRs in Ovarian Cancer
4.1.1. ERRα Protein and mRNA Expression in Ovarian Cancer and Its Prognostic Relevance
4.1.2. In Vitro Studies on ERRα Action in Ovarian Cancer
4.1.3. Role of ERRβ in Ovarian Cancer
4.1.4. Role of ERRγ in Ovarian Cancer
4.2. ERRs in Endometrial Cancer
4.2.1. ERRα Protein and mRNA Expression in Endometrial Cancer
4.2.2. In Vitro Studies on ERRα Action in Endometrial Cancer
4.2.3. ERRγ Expression in Endometrial Cancer
4.2.4. In Vitro Studies on ERRγ Action in Endometrial Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, G.G.; Zeng, Q.; Tse, G.M. Estrogen and its receptors in cancer. Med. Res. Rev. 2008, 28, 954–974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen Signaling via Estrogen Receptor β. J. Biol. Chem. 2010, 285, 39575–39579. [Google Scholar] [CrossRef] [PubMed]
- Crandall, D.L.; Busler, D.E.; Novak, T.J.; Weber, R.V.; Kral, J.G. Identification of Estrogen Receptor β RNA in Human Breast and Abdominal Subcutaneous Adipose Tissue. Biochem. Biophys. Res. Commun. 1998, 248, 523–526. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Shughrue, P.J.; Merchenthaler, I.; Gustafsson, J. The Estrogen Receptor β Subtype: A Novel Mediator of Estrogen Action in Neuroendocrine Systems. Front. Neuroendocr. 1998, 19, 253–286. [Google Scholar] [CrossRef] [PubMed]
- Balla, B.; Sárvári, M.; Kósa, J.P.; Kocsis-Deák, B.; Tobiás, B.; Árvai, K.; Takács, I.; Podani, J.; Liposits, Z.; Lakatos, P. Long-term selective estrogen receptor-beta agonist treatment modulates gene expression in bone and bone marrow of ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2019, 188, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.H.; Lin, G.; Fang, M.; Rudd, J.A. Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen. Comp. Endocrinol. 2019, 272, 63–75. [Google Scholar] [CrossRef]
- Tamer, S.A.; Yıldırım, A.; Arabacı, Ş.; Çiftçi, S.; Akın, S.; Sarı, E.; Köroğlu, M.K.; Ercan, F.; Yüksel, M.; Çevik, Ö.; et al. Treatment with estrogen receptor agonist ERβ improves torsion-induced oxidative testis injury in rats. Life Sci. 2019, 222, 203–211. [Google Scholar] [CrossRef]
- Le Moëne, O.; Stavarache, M.; Ogawa, S.; Musatov, S.; Ågmo, A. Estrogen receptors α and β in the central amygdala and the ventromedial nucleus of the hypothalamus: Sociosexual behaviors, fear and arousal in female rats during emotionally challenging events. Behav. Brain Res. 2019, 367, 128–142. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Leem, H.; Cho, M.; Yoon, J.-H.; Maeng, H.-J.; Lee, Y. Rhododendrin-Induced RNF146 Expression via Estrogen Receptor β Activation is Cytoprotective Against 6-OHDA-Induced Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 1772. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Du, M.; Shan, J.; Feng, A.; Schmull, S.; Gu, J.; Xue, S. Oestrogen Receptor β Activation Protects Against Myocardial Infarction via Notch1 Signalling. Cardiovasc. Drugs Ther. 2020, 34, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, W.A.; Mohamed, D.; Corcoran, O.; Ojo, O.O. The Role of Oestrogen Receptor Beta (ERβ) in the Aetiology and Treatment of Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2019, 15, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, C.; Anupama, D.; Pratibha, N.; Venkateshwari, A. Impact of Genetic Variants in Estrogen Receptor-β Gene in the Etiology of Uterine Leiomyomas. J. Reprod. Infertil. 2019, 20, 151–160. [Google Scholar] [PubMed]
- Lattrich, C.; Stegerer, A.; Häring, J.; Schüler, S.; Ortmann, O.; Treeck, O. Estrogen receptor β agonists affect growth and gene expression of human breast cancer cell lines. Steroids 2013, 78, 195–202. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Moehle, C.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effect of estrogen receptor β agonists on proliferation and gene expression of ovarian cancer cells. BMC Cancer 2017, 17, 319. [Google Scholar] [CrossRef]
- Treeck, O.; Diepolder, E.; Skrzypczak, M.; Schüler-Toprak, S.; Ortmann, O. Knockdown of estrogen receptor β increases proliferation and affects the transcriptome of endometrial adenocarcinoma cells. BMC Cancer 2019, 19, 745. [Google Scholar] [CrossRef]
- Majumdar, S.; Rinaldi, J.C.; Malhotra, N.R.; Xie, L.; Hu, D.-P.; Gauntner, T.D.; Grewal, H.S.; Hu, W.-Y.; Kim, S.H.; Katzenellenbogen, J.A.; et al. Differential Actions of Estrogen Receptor α and β via Nongenomic Signaling in Human Prostate Stem and Progenitor Cells. Endocrinology 2019, 160, 2692–2708. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hugerth, L.W.; Hases, L.; Saxena, A.; Seifert, M.; Thomas, Q.; Gustafsson, J.; Engstrand, L.; Williams, C. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int. J. Cancer 2019, 144, 3086–3098. [Google Scholar] [CrossRef]
- Santti, K.; Ihalainen, H.; Rönty, M.; Msc, C.K.; Haglund, C.; Sampo, M.; Tarkkanen, M.; Blomqvist, C. Estrogen receptor beta expression correlates with proliferation in desmoid tumors. J. Surg. Oncol. 2019, 119, 873–879. [Google Scholar] [CrossRef]
- Liang, L.; Williams, M.D.; Bell, D. Expression of PTEN, Androgen Receptor, HER2/neu, Cytokeratin 5/6, Estrogen Receptor-Beta, HMGA2, and PLAG1 in Salivary Duct Carcinoma. Head Neck Pathol. 2019, 13, 529–534. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X. The role of estrogen receptor beta in breast cancer. Biomark. Res. 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Rey, J.M.; Nirde, P.; Roger, P.; Gastaldi, M.; Laffargue, F.; Rochefort, H.; Maudelonde, T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998, 58, 5367–5373. [Google Scholar] [PubMed]
- Li, A.J.; Baldwin, R.L.; Karlan, B.Y. Estrogen and progesterone receptor subtype expression in normal and malignant ovarian epithelial cell cultures. Am. J. Obstet. Gynecol. 2003, 189, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafsson, J.A. Estrogen Signaling: A Subtle Balance Between ER alpha and ER beta. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef]
- Fujimuraa, T.; Takahashi, S.; Uranob, T.; Ogawab, S.; Ouchib, Y.; Kitamuraa, T.; Muramatsuc, M.; Inouebcd, S. Differential Expression of Estrogen Receptor β (ERβ) and Its C-Terminal Truncated Splice Variant ERβcx as Prognostic Predictors in Human Prostatic Cancer. Biochem. Biophys. Res. Commun. 2001, 289, 692–699. [Google Scholar] [CrossRef]
- Park, B.-W.; Kim, K.-S.; Heo, M.-K.; Yang, W.-I.; Kim, S.I.; Kim, J.-H.; Kim, G.E.; Lee, K.S. The changes of estrogen receptor-β variants expression in breast carcinogenesis: Decrease of estrogen receptor-β2 expression is the key event in breast cancer development. J. Surg. Oncol. 2006, 93, 504–510. [Google Scholar] [CrossRef]
- Esslimani-Sahla, M.; Simony-Lafontaine, J.; Kramar, A.; Lavaill, R.; Mollevi, C.; Warner, M.; Gustafsson, J.-A.; Rochefort, H. Estrogen Receptor β (ERβ) Level but Not Its ERβcx Variant Helps to Predict Tamoxifen Resistance in Breast Cancer. Clin. Cancer Res. 2004, 10, 5769–5776. [Google Scholar] [CrossRef]
- Häring, J.; Skrzypczak, M.; Stegerer, A.; Lattrich, C.; Weber, F.; Görse, R.; Ortmann, O.; Treeck, O. Estrogen receptor β transcript variants associate with oncogene expression in endometrial cancer. Int. J. Mol. Med. 2012, 29, 1127–1136. [Google Scholar] [CrossRef]
- Treeck, O.; Pfeiler, G.; Mitter, D.; Lattrich, C.; Piendl, G.; Ortmann, O. Estrogen receptor β1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells. J. Endocrinol. 2007, 193, 421–433. [Google Scholar] [CrossRef]
- Springwald, A.; Lattrich, C.; Skrzypczak, M.; Goerse, R.; Ortmann, O.; Treeck, O. Identification of novel transcript variants of estrogen receptor α, β and progesterone receptor gene in human endometrium. Endocrine 2010, 37, 415–424. [Google Scholar] [CrossRef]
- Zhou, R.; Treeck, O.; Horn, F.; Ortmann, O. Effects of prolonged tamoxifen treatment on receptor expression and apoptosis of ovarian cancer cells. Gynecol. Oncol. 2005, 96, 678–683. [Google Scholar] [CrossRef]
- Häring, J.; Schüler, S.; Lattrich, C.; Ortmann, O.; Treeck, O. Role of estrogen receptor β in gynecological cancer. Gynecol. Oncol. 2012, 127, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Silva, C.D.; Villegas-Pineda, J.C.; Pereira-Suárez, A.L. Expression and Role of the G Protein-Coupled Estrogen Receptor (GPR30/GPER) in the Development and Immune Response in Female Reproductive Cancers. Front. Endocrinol. 2020, 11, 544. [Google Scholar] [CrossRef]
- Tanida, T. Molecular dynamics of estrogen-related receptors and their regulatory proteins: Roles in transcriptional control for endocrine and metabolic signaling. Anat. Sci. Int. 2022, 97, 15–29. [Google Scholar] [CrossRef]
- Crevet, L.; Vanacker, J.-M. Regulation of the expression of the estrogen related receptors (ERRs). Cell Mol. Life Sci. 2020, 77, 4573–4579. [Google Scholar] [CrossRef]
- Yeramian, A.; Moreno-Bueno, G.; Dolcet, X.; Catasus, L.; Abal, M.; Colas, E.; Matias-Guiu, X. Endometrial carcinoma: Molecular alterations involved in tumor development and progression. Oncogene 2012, 2, 403–413. [Google Scholar] [CrossRef]
- Sivridis, E.; Giatromanolaki, A. The pathogenesis of endometrial carcinomas at menopause: Facts and figures. J. Clin. Pathol. 2011, 64, 553–560. [Google Scholar] [CrossRef]
- Šmuc, T.; Rižner, T.L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol. Cell Endocrinol. 2009, 301, 74–82. [Google Scholar] [CrossRef]
- Collins, F.; MacPherson, S.; Brown, P.; Bombail, V.; Williams, A.R.; Anderson, R.A.; Jabbour, H.N.; Saunders, P.T. Expression of oestrogen receptors, ERα, ERβ, and ERβ variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERα. BMC Cancer 2009, 9, 330. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; ISBN 9789283245049. [Google Scholar]
- Fixemer, T.; Remberger, K.; Bonkhoff, H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate 2003, 54, 79–87. [Google Scholar] [CrossRef]
- Foley, E.F.; Jazaeri, A.A.; Shupnik, M.A.; Jazaeri, O.; Rice, L.W. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000, 60, 245–248. [Google Scholar]
- Park, B.-W.; Kim, K.-S.; Heo, M.-K.; Ko, S.-S.; Lee, K.S.; Hong, S.; Yang, W.-I.; Kim, J.-H.; Kim, G.E. Expression of Estrogen Receptor-β in Normal Mammary and Tumor Tissues: Is it Protective in Breast Carcinogenesis? Breast Cancer Res. Treat. 2003, 80, 79–85. [Google Scholar] [CrossRef]
- Roger, P.; Sahla, M.E.; Mäkelä, S.; Gustafsson, J.A.; Baldet, P.; Rochefort, H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001, 61, 2537–2541. [Google Scholar]
- Skliris, G.P.; Munot, K.; Bell, S.M.; Carder, P.J.; Lane, S.; Horgan, K.; Lansdown, M.R.J.; Parkes, A.T.; Hanby, A.M.; Markham, A.F.; et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J. Pathol. 2003, 201, 213–220. [Google Scholar] [CrossRef]
- Brandenberger. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: Down-regulation of ER-beta in neoplastic tissues. J. Clin. Endocrinol. Metab. 1998, 83, 1025. [Google Scholar]
- Chan, K.K.L.; Wei, N.; Liu, S.S.; Xiao-Yun, L.; Cheung, A.N.; Ngan, H.Y.S. Estrogen receptor subtypes in ovarian cancer: A clinical correlation. Obstet. Gynecol. 2008, 111, 144–151. [Google Scholar] [CrossRef]
- De Stefano, I.; Zannoni, G.F.; Prisco, M.G.; Fagotti, A.; Tortorella, L.; Vizzielli, G.; Mencaglia, L.; Scambia, G.; Gallo, D. Cytoplasmic expression of estrogen receptor beta (ERβ) predicts poor clinical outcome in advanced serous ovarian cancer. Gynecol. Oncol. 2011, 122, 573–579. [Google Scholar] [CrossRef]
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Muñoz, A.; Mor, G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar] [CrossRef]
- Suzuki, F.; Akahira, J.-I.; Miura, I.; Suzuki, T.; Ito, K.; Hayashi, S.-I.; Sasano, H.; Yaegashi, N. Loss of estrogen receptor β isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 2008, 99, 2365–2372. [Google Scholar] [CrossRef]
- Filigheddu, N.; Sampietro, S.; Chianale, F.; Porporato, P.E.; Gaggianesi, M.; Gregnanin, I.; Rainero, E.; Ferrara, M.; Perego, B.; Riboni, F.; et al. Diacylglycerol kinase α mediates 17-β-estradiol-induced proliferation, motility, and anchorage-independent growth of Hec-1A endometrial cancer cell line through the G protein-coupled estrogen receptor GPR30. Cell Signal. 2011, 23, 1988–1996. [Google Scholar] [CrossRef]
- Lindgren, P.R.; Cajander, S.; Bäckström, T.; Gustafsson, J.; Mäkelä, S.; Olofsson, J.I. Estrogen and progesterone receptors in ovarian epithelial tumors. Mol. Cell Endocrinol. 2004, 221, 97–104. [Google Scholar] [CrossRef]
- Halon, A.; Nowak-Markwitz, E.; Maciejczyk, A.; Pudelko, M.; Gansukh, T.; Györffy, B.; Donizy, P.; Murawa, D.; Matkowski, R.; Spaczynski, M.; et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011, 31, 711–718. [Google Scholar]
- Hyder, S.M.; Chiappetta, C.; Stancel, G.M. Interaction of human estrogen receptors α and β with the same naturally occurring estrogen response elements. Biochem. Pharmacol. 1999, 57, 597–601. [Google Scholar] [CrossRef]
- Bogush, T.A.; Basharina, A.A.; Bogush, E.A.; Ryabinina, O.M.; Tjulandina, A.S.; Tjulandin, S.A. Estrogen Receptors alpha and beta in Ovarian Cancer: Expression Level and Prognosis. Dokl. Biochem. Biophys. 2018, 482, 249–251. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; Defazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Kumar, S.; Dasari, P.; Sylvia, M.T. The expression of immunohistochemical markers estrogen receptor, progesterone receptor, Her-2-neu, p53 and Ki-67 in epithelial ovarian tumors and its correlation with clinicopathologic variables. Indian J. Pathol. Microbiol. 2012, 55, 33–37. [Google Scholar] [CrossRef]
- Damião, R.D.S.; Oshima, C.F.; Stávale, J.; Gonçalves, W. Analysis of the expression of estrogen receptor, progesterone receptor and chicken ovalbumin upstream promoter-transcription factor I in ovarian epithelial cancers and normal ovaries. Oncol. Rep. 2007, 18, 25–32. [Google Scholar] [CrossRef]
- Tarallo, R.; Giurato, G.; Bruno, G.; Ravo, M.; Rizzo, F.; Salvati, A.; Ricciardi, L.; Marchese, G.; Cordella, A.; Rocco, T.; et al. The nuclear receptor ERβ engages AGO2 in regulation of gene transcription, RNA splicing and RISC loading. Genome Biol. 2017, 18, 189. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Estrogen receptor β is associated with expression of cancer associated genes and survival in ovarian cancer. BMC Cancer 2018, 18, 981. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Babic, A.; Kuliszewski, M.G.; Rice, M.S.; Townsend, M.K.; Hecht, J.L.; Tworoger, S.S. Estrogen Receptor-β Expression of Ovarian Tumors and Its Association with Ovarian Cancer Risk Factors. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2211–2219. [Google Scholar] [CrossRef]
- Oturkar, C.C.; Gandhi, N.; Rao, P.; Eng, K.H.; Miller, A.; Singh, P.K.; Zsiros, E.; Odunsi, K.O.; Das, G.M. Estrogen Receptor-Beta2 (ERβ2)–Mutant p53–FOXM1 Axis: A Novel Driver of Proliferation, Chemoresistance, and Disease Progression in High Grade Serous Ovarian Cancer (HGSOC). Cancers 2022, 14, 1120. [Google Scholar] [CrossRef]
- Ciucci, A.; Zannoni, G.F.; Travaglia, D.; Petrillo, M.; Scambia, G.; Gallo, D. Prognostic significance of the estrogen receptor beta (ERβ) isoforms ERβ1, ERβ2, and ERβ5 in advanced serous ovarian cancer. Gynecol. Oncol. 2014, 132, 351–359. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Wang, Y.; Leung, T.H.Y.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S. Differential expression of estrogen receptor subtypes and variants in ovarian cancer: Effects on cell invasion, proliferation and prognosis. BMC Cancer 2017, 17, 606. [Google Scholar] [CrossRef]
- Warner, M.; Fan, X.; Strom, A.; Wu, W.; Gustafsson, J.-Å. 25 years of ERβ: A personal journey. J. Mol. Endocrinol. 2021, 68, R1–R9. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of Estrogen Action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Paruthiyil, S.; Parmar, H.; Kerekatte, V.; Cunha, G.R.; Firestone, G.L.; Leitman, D.C. Estrogen Receptor β Inhibits Human Breast Cancer Cell Proliferation and Tumor Formation by Causing a G2 Cell Cycle Arrest. Cancer Res. 2004, 64, 423–428. [Google Scholar] [CrossRef]
- Ström, A.; Hartman, J.; Foster, J.S.; Kietz, S.; Wimalasena, J.; Gustafsson, J.-A. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl. Acad. Sci. USA 2004, 101, 1566–1571. [Google Scholar] [CrossRef]
- Hayashido, Y.; Lucas, A.; Rougeot, C.; Godyna, S.; Argraves, W.S.; Rochefort, H. Estradiol and fibulin-1 inhibit motility of human ovarian-and breast-cancer cells induced by fibronectin. Int. J. Cancer 1998, 75, 654–658. [Google Scholar] [CrossRef]
- Treeck, O.; Pfeiler, G.; Horn, F.; Federhofer, B.; Houlihan, H.; Vollmer, A.; Ortmann, O. Novel estrogen receptor beta transcript variants identified in human breast cancer cells affect cell growth and apoptosis of COS-1 cells. Mol. Cell Endocrinol. 2007, 264, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Albanese, C.; Anderson, C.M.; Hilty, K.; Webb, P.; Uht, R.M.; Price, R.H.; Pestell, R.G.; Kushner, P.J. Opposing Action of Estrogen Receptors α and β on Cyclin D1 Gene Expression. J. Biol. Chem. 2002, 277, 24353–24360. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef]
- Planas-Silva, M.D.; Weinberg, R.A. Estrogen-Dependent Cyclin E-cdk2 Activation through p21 Redistribution. Mol. Cell Biol. 1997, 17, 4059–4069. [Google Scholar] [CrossRef]
- Worsley, S.D.; Ponder, B.A.; Davies, B.R. Overexpression of Cyclin D1 in Epithelial Ovarian Cancers. Gynecol. Oncol. 1997, 64, 189–195. [Google Scholar] [CrossRef]
- Banerjee, A.; Cai, S.; Xie, G.; Li, N.; Bai, X.; Lavudi, K.; Wang, K.; Zhang, X.; Zhang, J.; Patnaik, S.; et al. A Novel Estrogen Receptor β Agonist Diminishes Ovarian Cancer Stem Cells via Suppressing the Epithelial-to-Mesenchymal Transition. Cancers 2022, 14, 2311. [Google Scholar] [CrossRef]
- Prasad, S.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Srivastava, S.K. Cancer cells stemness: A doorstep to targeted therapy. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165424. [Google Scholar] [CrossRef]
- He, Y.; Alejo, S.; Venkata, P.P.; Johnson, J.D.; Loeffel, I.; Pratap, U.P.; Zou, Y.; Lai, Z.; Tekmal, R.R.; Kost, E.R.; et al. Therapeutic Targeting of Ovarian Cancer Stem Cells Using Estrogen Receptor Beta Agonist. Int. J. Mol. Sci. 2022, 23, 7159. [Google Scholar] [CrossRef]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 2010, 102, 1276–1283. [Google Scholar] [CrossRef]
- Zong, X.; Nephew, K.P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting. Cancers 2019, 11, 934. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.-M.; Nephew, K.P. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Keyvani, V.; Farshchian, M.; Esmaeili, S.-A.; Yari, H.; Moghbeli, M.; Nezhad, S.-R.K.; Abbaszadegan, M.R. Ovarian cancer stem cells and targeted therapy. J. Ovarian Res. 2019, 12, 120. [Google Scholar] [CrossRef]
- Schüler, S.; Lattrich, C.; Skrzypczak, M.; Fehm, T.; Ortmann, O.; Treeck, O. Polymorphisms in the promoter region of ESR2 gene and susceptibility to ovarian cancer. Gene 2014, 546, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kuokkanen, S.; Pal, L. Steroid Hormone Receptor Profile of Premenopausal Endometrial Polyps. Reprod. Sci. 2010, 17, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Okayasu, I. Changes in Expression of Estrogen Receptors α and β in Relation to Progesterone Receptor and pS2 Status in Normal and Malignant Endometrium. Jpn. J. Cancer Res. 2000, 91, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hojnik, M.; Sinreih, M.; Anko, M.; Hevir-Kene, N.; Knific, T.; Pirš, B.; Grazio, S.F.; Rižner, T.L. The Co-Expression of Estrogen Receptors ERα, ERβ, and GPER in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, J.; Zhou, X.; Liu, J.; Wang, Q.; Zhang, B. Roles of estrogen receptor α and β in the regulation of proliferation in endometrial carcinoma. Pathol. Res. Pract. 2020, 216, 153149. [Google Scholar] [CrossRef]
- Obata, T.; Nakamura, M.; Mizumoto, Y.; Iizuka, T.; Ono, M.; Terakawa, J.; Daikoku, T.; Fujiwara, H. Dual expression of immunoreactive estrogen receptor β and p53 is a potential predictor of regional lymph node metastasis and postoperative recurrence in endometrial endometrioid carcinoma. PLoS ONE 2017, 12, e0188641. [Google Scholar] [CrossRef]
- Chakravarty, D.; Srinivasan, R.; Ghosh, S.; Gopalan, S.; Rajwanshi, A.; Majumdar, S. Estrogen receptor beta1 and the beta2/betacx isoforms in nonneoplastic endometrium and in endometrioid carcinoma. Int. J. Gynecol. Cancer 2007, 17, 905–913. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Bieche, I.; Szymczak, S.; Tozlu, S.; Lewandowski, S.A.; Girault, I.; Radwanska, K.; Szczylik, C.; Jakowicki, J.A.; Lidereau, R.; et al. Evaluation of mRNA expression of estrogen receptor beta and its isoforms in human normal and neoplastic endometrium. Int. J. Cancer 2004, 110, 783–787. [Google Scholar] [CrossRef]
- Leygue, E.; Dotzlaw, H.; Watson, P.H.; Murphy, L.C. Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res. 1999, 59, 1175. [Google Scholar] [PubMed]
- Collins, F.; Itani, N.; Esnal-Zufiaurre, A.; Gibson, D.A.; Fitzgerald, C.; Saunders, P.T.K. The ERβ5 splice variant increases oestrogen responsiveness of ERαpos Ishikawa cells. Endocr. Relat. Cancer 2020, 27, 55–66. [Google Scholar] [CrossRef]
- Mylonas, I.; Jeschke, U.; Shabani, N.; Kuhn, C.; Kriegel, S.; Kupka, M.S.; Friese, K. Normal and malignant human endometrium express immunohistochemically estrogen receptor alpha (ER-alpha), estrogen receptor beta (ER-beta) and progesterone receptor (PR). Anticancer. Res. 2005, 25, 1679. [Google Scholar] [PubMed]
- Hu, K.; Zhong, G.; He, F. Expression of estrogen receptors ERalpha and ERbeta in endometrial hyperplasia and adenocarcinoma. Int. J. Gynecol. Cancer 2005, 15, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, I. Prognostic significance and clinical importance of estrogen receptor α and β in human endometrioid adenocarcinomas. Oncol. Rep. 2010, 24, 385–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jongen, V.; Briët, J.; de Jong, R.; Hoor, K.T.; Boezen, M.; van der Zee, A.; Nijman, H.; Hollema, H. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol. Oncol. 2009, 112, 537–542. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Monterossi, G.; De Stefano, I.; Gargini, A.; Salerno, M.G.; Farulla, I.; Travaglia, D.; Vellone, V.G.; Scambia, G.; Gallo, D. The expression ratios of estrogen receptor α (ERα) to estrogen receptor β1 (ERβ1) and ERα to ERβ2 identify poor clinical outcome in endometrioid endometrial cancer. Hum. Pathol. 2013, 44, 1047–1054. [Google Scholar] [CrossRef]
- Takama, F.; Kanuma, T.; Wang, D.; Kagami, I.; Mizunuma, H. Oestrogen receptor β expression and depth of myometrial invasion in human endometrial cancer. Br. J. Cancer 2001, 84, 545–549. [Google Scholar] [CrossRef][Green Version]
- Fujimoto, J.; Sakaguchi, H.; Aoki, I.; Toyoki, H.; Tamaya, T. Clinical Implications of the Expression of Estrogen Receptor-α and -β in Primary and Metastatic Lesions of Uterine Endometrial Cancers. Oncology 2002, 62, 269–277. [Google Scholar] [CrossRef]
- Shabani, N.; Kuhn, C.; Kunze, S.; Schulze, S.; Mayr, D.; Dian, D.; Gingelmaier, A.; Schindlbeck, C.; Willgeroth, F.; Sommer, H.; et al. Prognostic significance of oestrogen receptor alpha (ERα) and beta (ERβ), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur. J. Cancer 2007, 43, 2434–2444. [Google Scholar] [CrossRef]
- Treeck, O.; Juhasz-Boess, I.; Lattrich, C.; Horn, F.; Goerse, R.; Ortmann, O. Effects of exon-deleted estrogen receptor β transcript variants on growth, apoptosis and gene expression of human breast cancer cell lines. Breast Cancer Res. Treat. 2008, 110, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Lattrich, C.; Springwald, A.; Ortmann, O. Estrogen receptor beta exerts growth-inhibitory effects on human mammary epithelial cells. Breast Cancer Res. Treat. 2010, 120, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Häring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and knockdown of estrogen receptor β differentially affect invasion of triple-negative breast cancer cells in vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, L.A.; Zhou, C.; Mendivil, A.; Malloy, K.M.; Gehrig, P.A.; Bae-Jump, V.L. Metformin is a potent inhibitor of endometrial cancer cell proliferation—Implications for a novel treatment strategy. Gynecol. Oncol. 2010, 116, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, H.; Zhou, X.; Li, Y.; Liu, T.; Yin, X.; Zhang, B. Role of metformin in inhibiting estrogen-induced proliferation and regulating ERα and ERβ expression in human endometrial cancer cells. Oncol. Lett. 2017, 14, 4949–4956. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Kamal, A.M.; Bulmer, J.N. Estrogen receptor β: The guardian of the endometrium. Hum. Reprod. Update 2015, 21, 174–193. [Google Scholar] [CrossRef]
- Jarzabek, K.; Koda, M.; Walentowicz-Sadlecka, M.; Grabiec, M.; Laudanski, P.; Wolczynski, S. Altered expression of ERs, aromatase, and COX2 connected to estrogen action in type 1 endometrial cancer biology. Tumor Biol. 2013, 34, 4007–4016. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Błachnio-Zabielska, A.; Walentowicz-Sadłecka, M.; Grabiec, M.; Knapp, P.A. Expression of Estrogen Receptors (α, β), Cyclooxygenase-2 and Aromatase in normal endometrium and endometrioid cancer of uterus. Adv. Med. Sci. 2013, 58, 96–103. [Google Scholar] [CrossRef][Green Version]
- Kolkova, Z.; Casslén, V.; Henic, E.; Ahmadi, S.; Ehinger, A.; Jirström, K.; Casslén, B. The G protein-coupled estrogen receptor 1 (GPER/GPR30) does not predict survival in patients with ovarian cancer. J. Ovarian Res. 2012, 5, 9. [Google Scholar] [CrossRef]
- Fujiwara, S.; Terai, Y.; Kawaguchi, H.; Takai, M.; Yoo, S.; Tanaka, Y.; Tanaka, T.; Tsunetoh, S.; Sasaki, H.; Kanemura, M.; et al. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J. Ovarian Res. 2012, 5, 35. [Google Scholar] [CrossRef]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.-J.; Verschraegen, C.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, H.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. The novel estrogen receptor GPER regulates the migration and invasion of ovarian cancer cells. Mol. Cell Biochem. 2013, 378, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Modl, S.; Thulig, M.; Weißenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Skrzypczak, M.; Ignatov, T.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor 1 (GPER-1) and agonist G-1 inhibit growth of ovarian cancer cells by activation of anti-tumoral transcriptome responses: Impact of GPER-1 mRNA on survival. J. Cancer Res. Clin. Oncol. 2020, 146, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Fraungruber, P.; Kaltofen, T.; Heublein, S.; Kuhn, C.; Mayr, D.; Burges, A.; Mahner, S.; Rathert, P.; Jeschke, U.; Trillsch, F. G Protein-Coupled Estrogen Receptor Correlates with Dkk2 Expression and Has Prognostic Impact in Ovarian Cancer Patients. Front. Endocrinol. 2021, 12, 564002. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Sandén, C.; Broselid, S.; Cornmark, L.; Andersson, K.; Daszkiewicz-Nilsson, J.; Mårtensson, U.E.A.; Olde, B.; Leeb-Lundberg, L.M.F. G Protein-Coupled Estrogen Receptor 1/G Protein-Coupled Receptor 30 Localizes in the Plasma Membrane and Traffics Intracellularly on Cytokeratin Intermediate Filaments. Mol. Pharmacol. 2011, 79, 400–410. [Google Scholar] [CrossRef]
- Kolkova, Z.; Noskova, V.; Ehinger, A.; Hansson, S.; Casslén, B. G protein-coupled estrogen receptor 1 (GPER, GPR 30) in normal human endometrium and early pregnancy decidua. Mol. Hum. Reprod. 2010, 16, 743–751. [Google Scholar] [CrossRef]
- Gao, F.; Ma, X.; Ostmann, A.B.; Das, S.K. GPR30 Activation Opposes Estrogen-Dependent Uterine Growth via Inhibition of Stromal ERK1/2 and Estrogen Receptor Alpha (ERα) Phosphorylation Signals. Endocrinology 2011, 152, 1434–1447. [Google Scholar] [CrossRef]
- Smith, H.O.; Leslie, K.; Singh, M.; Qualls, C.R.; Revankar, C.M.; Joste, N.E.; Prossnitz, E. GPR30: A novel indicator of poor survival for endometrial carcinoma. Am. J. Obstet. Gynecol. 2007, 196, 386.e1–386.e11. [Google Scholar] [CrossRef]
- Osaku, D.; Oishi, T.; Kawamura, N.; Iida, Y.; Komatsu, H.; Kudoh, A.; Chikumi, J.; Sato, S.; Harada, T. Differential expression of estrogen receptor subtypes in ovarian high-grade serous carcinoma and clear cell carcinoma. Reprod. Med. Biol. 2021, 20, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-X.; Xiong, W.; Wang, M.-L.; Yang, J.; Shi, H.-J.; Chen, H.-Q.; Niu, G. Nuclear G protein-coupled oestrogen receptor (GPR30) predicts poor survival in patients with ovarian cancer. J. Int. Med. Res. 2018, 46, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, X.; He, C.; Hua, G.; Tsai, M.-Y.; Davis, J.S. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013, 4, e869. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, Y.; Wen, H.; Jiang, X.; Cao, X.; Zhang, G.; Liu, G. A novel estrogen receptor GPER mediates proliferation induced by 17β-estradiol and selective GPER agonist G-1 in estrogen receptor α (ERα)-negative ovarian cancer cells. Cell Biol. Int. 2014, 38, 631–638. [Google Scholar] [CrossRef]
- Albanito, L.; Madeo, A.; Lappano, R.; Vivacqua, A.; Rago, V.; Carpino, A.; Oprea, T.I.; Prossnitz, E.R.; Musti, A.M.; Andò, S.; et al. G Protein–Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17β-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res. 2007, 67, 1859–1866. [Google Scholar] [CrossRef]
- Scaling, A.L.; Prossnitz, E.R.; Hathaway, H.J. GPER Mediates Estrogen-Induced Signaling and Proliferation in Human Breast Epithelial Cells and Normal and Malignant Breast. Horm. Cancer 2014, 5, 146–160. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.-F.; Yang, M.-L.; Wu, T.-Y.; Xu, C.-J.; Wang, J.-M.; Li, C.-J.; Li, X. G Protein–Coupled Receptor 30 Mediates the Anticancer Effects Induced by Eicosapentaenoic Acid in Ovarian Cancer Cells. Cancer Res. Treat. 2020, 52, 815–829. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, T.; Liu, M.; Ren, S.; Yang, H.; Zeng, S.; Zhao, H.; Chen, L.; Ming, T.; Meng, X.; et al. Shikonin, a naphthalene ingredient: Therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine 2022, 94, 153805. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Tang, X.; Guo, L.; Tang, X.; Zhu, T.; Zhao, T.; Zhang, W.; Zhang, P. Shikonin Mediates Apoptosis through G Protein-Coupled Estrogen Receptor of Ovarian Cancer Cells. Evid.-Based Complement. Altern. Med. 2022, 2022, 6517732. [Google Scholar] [CrossRef]
- Girgert, R.; Emons, G.; Gründker, C. Estrogen Signaling in ERα-Negative Breast Cancer: ERβ and GPER. Front. Endocrinol. 2018, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Heublein, S.; Jeschke, U.; Kuhn, C.; Hester, A.; Czogalla, B.; Mahner, S.; Rottmann, M.; Mayr, D.; Schmoeckel, E.; et al. The G-Protein-Coupled Estrogen Receptor (GPER) Regulates Trimethylation of Histone H3 at Lysine 4 and Represses Migration and Proliferation of Ovarian Cancer Cells In Vitro. Cells 2021, 10, 619. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Schüler, S.; Lattrich, C.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor (GPER) expression in endometrial adenocarcinoma and effect of agonist G-1 on growth of endometrial adenocarcinoma cell lines. Steroids 2013, 78, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Krakstad, C.; Trovik, J.; Wik, E.; Engelsen, I.B.; Werner, H.M.J.; Birkeland, E.; Raeder, M.B.; Øyan, A.M.; Stefansson, I.M.; Kalland, K.H.; et al. Loss of GPER identifies new targets for therapy among a subgroup of ERα-positive endometrial cancer patients with poor outcome. Br. J. Cancer 2012, 106, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Lan, L.; Liu, R.; Wu, Y.; Qu, Q.; Wen, K. Tamoxifen has a proliferative effect in endometrial carcinoma mediated via the GPER/EGFR/ERK/cyclin D1 pathway: A retrospective study and an in vitro study. Mol. Cell Endocrinol. 2016, 437, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.-Y.; Xie, B.-Y.; Yang, B.-Y.; Ning, C.-C.; Shan, W.-W.; Gu, C.; Luo, X.-Z.; Chen, X.-J.; Zhang, Z.-B.; Feng, Y.-J. Increased TET1 Expression in Inflammatory Microenvironment of Hyperinsulinemia Enhances the Response of Endometrial Cancer to Estrogen by Epigenetic Modulation of GPER. J. Cancer 2017, 8, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-Q.; Zhou, L.; Chen, X.-Y.; Wan, X.-P.; He, Y.-Y. The G protein-coupled receptor GPR30 mediates the proliferative and invasive effects induced by hydroxytamoxifen in endometrial cancer cells. Biochem. Biophys. Res. Commun. 2012, 420, 343–349. [Google Scholar] [CrossRef]
- De Marco, P.; Bartella, V.; Vivacqua, A.; Lappano, R.; Santolla, M.F.; Morcavallo, A.; Pezzi, V.; Belfiore, A.; Maggiolini, M. Insulin-like growth factor-I regulates GPER expression and function in cancer cells. Oncogene 2013, 32, 678–688. [Google Scholar] [CrossRef]
- Wan, J.; Yin, Y.; Zhao, M.; Shen, F.; Chen, M.; Chen, Q. The positivity of G-protein-coupled receptor-30 (GPR 30), an alternative estrogen receptor is not different between type 1 and type 2 endometrial cancer. Oncotarget 2017, 8, 90897–90904. [Google Scholar] [CrossRef]
- He, Y.-Y.; Du, G.-Q.; Cai, B.; Yan, Q.; Zhou, L.; Chen, X.-Y.; Lu, W.; Yang, Y.-X.; Wan, X.-P. Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2. J. Cancer Res. Clin. Oncol. 2012, 138, 775–783. [Google Scholar] [CrossRef]
- Ge, X.; Guo, R.; Qiao, Y.; Zhang, Y.; Lei, J.; Wang, X.; Li, L.; Hu, D. The G Protein–Coupled Receptor GPR30 Mediates the Nontranscriptional Effect of Estrogen on the Activation of PI3K/Akt Pathway in Endometrial Cancer Cells. Int. J. Gynecol. Cancer 2013, 23, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Sehouli, J.; Denkert, C.; Mustea, A.; Könsgen, D.; Koch, I.; Wei, L.; Lichtenegger, W. Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J. Mol. Med. 2005, 83, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Alam, S.M.; Jahan, I.; Sato, E.; Sakaguchi, H.; Tamaya, T. Clinical implication of estrogen-related receptor (ERR) expression in ovarian cancers. J. Steroid Biochem. Mol. Biol. 2007, 104, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Expression of estrogen-related receptors in ovarian cancer and impact on survival. J. Cancer Res. Clin. Oncol. 2021, 147, 2555–2567. [Google Scholar] [CrossRef]
- Huang, W.; Chen, L.; Sun, P. ERRα expression in ovarian cancer and promotes ovarian cancer cells migration in vitro. Arch. Gynecol. Obstet. 2022, 305, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, C.; Moreira-Barbosa, C.; Brunelli, L.; Ostano, P.; Panini, N.; Lupi, M.; Anastasia, A.; Fiordaliso, F.; Salio, M.; Formenti, L.; et al. PGC1α/β Expression Predicts Therapeutic Response to Oxidative Phosphorylation Inhibition in Ovarian Cancer. Cancer Res. 2022, 82, 1423–1434. [Google Scholar] [CrossRef]
- Luo, C.; Widlund, H.R.; Puigserver, P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer 2016, 2, 619–631. [Google Scholar] [CrossRef]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.G.; Wikman, H.; Pantel, K.; Haigis, M.C.; De Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Huang, X.; Ruan, G.; Liu, G.; Gao, Y.; Sun, P. Immunohistochemical Analysis of PGC-1α and ERRα Expression Reveals Their Clinical Significance in Human Ovarian Cancer. OncoTargets Ther. 2020, 13, 13055–13062. [Google Scholar] [CrossRef]
- Huang, X.; Ruan, G.; Sun, P. Estrogen-related receptor alpha copy number variation is associated with ovarian cancer histological grade. J. Obstet. Gynaecol. Res. 2021, 47, 1878–1883. [Google Scholar] [CrossRef]
- Lam, S.S.; Mak, A.S.; Yam, J.W.; Cheung, A.N.; Ngan, H.Y.; Wong, A.S. Targeting Estrogen-Related Receptor Alpha Inhibits Epithelial-to-Mesenchymal Transition and Stem Cell Properties of Ovarian Cancer Cells. Mol. Ther. 2014, 22, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.M.; Chaudhary, S.; Mishra, D.R.; Naik, S.K.; Suklabaidya, S.; Adhya, A.K.; Mishra, S.K. Estrogen receptor α dependent regulation of estrogen related receptor β and its role in cell cycle in breast cancer. BMC Cancer 2018, 18, 607. [Google Scholar] [CrossRef]
- Yu, S.; Wong, Y.C.; Wang, X.H.; Ling, M.T.; Ng, C.F.; Chen, S.; Chan, F.L. Orphan nuclear receptor estrogen-related receptor-β suppresses in vitro and in vivo growth of prostate cancer cells via p21WAF1/CIP1 induction and as a potential therapeutic target in prostate cancer. Oncogene 2008, 27, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kinoshita, Y.; Hosokawa, K.; Mori, T.; Yamaguchi, T.; Honjo, H. Function of Estrogen-Related Receptor α in Human Endometrial Cancer. J. Clin. Endocrinol. Metab. 2006, 91, 1573–1577. [Google Scholar] [CrossRef][Green Version]
- Sun, P.; Mao, X.; Gao, M.; Huang, M.; Chen, L.; Ruan, G.; Huang, W.; Braicu, E.I.; Sehouli, J. Novel endocrine therapeutic strategy in endometrial carcinoma targeting estrogen-related receptor α by XCT790 and siRNA. Cancer Manag. Res. 2018, 10, 2521–2535. [Google Scholar] [CrossRef]
- Matsushima, H.; Mori, T.; Ito, F.; Yamamoto, T.; Akiyama, M.; Kokabu, T.; Yoriki, K.; Umemura, S.; Akashi, K.; Kitawaki, J. Anti-tumor effect of estrogen-related receptor alpha knockdown on uterine endometrial cancer. Oncotarget 2016, 7, 34131–34148. [Google Scholar] [CrossRef]
- Fujimoto, J.; Sato, E. Clinical implication of estrogen-related receptor (ERR) expression in uterine endometrial cancers. J. Steroid Biochem. Mol. Biol. 2009, 116, 71–75. [Google Scholar] [CrossRef]
- Huang, M.; Chen, L.; Mao, X.; Liu, G.; Gao, Y.; You, X.; Gao, M.; Sehouli, J.; Sun, P. ERRα inhibitor acts as a potential agonist of PPARγ to induce cell apoptosis and inhibit cell proliferation in endometrial cancer. Aging 2020, 12, 23029–23046. [Google Scholar] [CrossRef]
- Mao, X.; Lei, H.; Yi, T.; Su, P.; Tang, S.; Tong, Y.; Dong, B.; Ruan, G.; Mustea, A.; Sehouli, J.; et al. Lipid reprogramming induced by the TFEB-ERRα axis enhanced membrane fluidity to promote EC progression. J. Exp. Clin. Cancer Res. 2022, 41, 28. [Google Scholar] [CrossRef]
- Deblois, G.; St-Pierre, J.; Giguère, V. The PGC-1/ERR signaling axis in cancer. Oncogene 2013, 32, 3483–3490. [Google Scholar] [CrossRef]
- De Vitto, H.; Ryu, J.; Calderon-Aparicio, A.; Monts, J.; Dey, R.; Chakraborty, A.; Lee, M.-H.; Bode, A.M.; Dong, Z. Estrogen-related receptor alpha directly binds to p53 and cooperatively controls colon cancer growth through the regulation of mitochondrial biogenesis and function. Cancer Metab. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mao, X.; Huang, M.; Lei, H.; Xue, L.; Sun, P. PGC-1α and ERRα in patients with endometrial cancer: A translational study for predicting myometrial invasion. Aging 2020, 12, 16963–16980. [Google Scholar] [CrossRef] [PubMed]
- Yoriki, K.; Mori, T.; Kokabu, T.; Matsushima, H.; Umemura, S.; Tarumi, Y.; Kitawaki, J. Estrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 2019, 9, 6697. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Shang, J.; Zhaang, Z.; Cui, B.; Lin, Y.; Yang, Y.; Song, Y.; Yu, S.; Xia, J. Estrogen related receptor alpha triggers the migration and invasion of endometrial cancer cells via up regulation of TGFB1. Cell Adhes. Migr. 2018, 12, 538–547. [Google Scholar] [CrossRef]
- Kokabu, T.; Mori, T.; Matsushima, H.; Yoriki, K.; Kataoka, H.; Tarumi, Y.; Kitawaki, J. Antitumor effect of XCT790, an ERRα inverse agonist, on ERα-negative endometrial cancer cells. Cell Oncol. 2019, 42, 223–235. [Google Scholar] [CrossRef]
- Sun, P.-M.; Gao, M.; Wei, L.-H.; Mustea, A.; Wang, J.-L.; Könsgen, D.; Lichtenegger, W.; Sehouli, J. An estrogen receptor alpha-dependent regulation of estrogen receptor-related receptor alpha in the proliferation of endometrial carcinoma cells. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S2), 564–568. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Huang, M.; Chen, L.; Lei, H.; Lin, H.; Mao, X.; Sun, P. ERRγ, a Novel Biomarker, Associates with Pathoglycemia of Endometrial Cancer to Predict Myometrial Invasion. J. Oncol. 2022, 2022, 5283388. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mori, T.; Sawada, M.; Kuroboshi, H.; Tatsumi, H.; Yoshioka, T.; Matsushima, H.; Iwasaku, K.; Kitawaki, J. Estrogen-Related Receptor-γ Regulates Estrogen Receptor-α Responsiveness in Uterine Endometrial Cancer. Int. J. Gynecol. Cancer 2012, 22, 1509–1516. [Google Scholar] [CrossRef]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The Role of Hyperglycemia in Endometrial Cancer Pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Ko, E.M.; Walter, P.; Clark, L.; Jackson, A.; Franasiak, J.; Bolac, C.; Havrilesky, L.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; et al. The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: Prevalence and prognostic significance. Gynecol. Oncol. 2014, 133, 28–32. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Wei, Z.; Lin, C.S.; Fang, S.; Ahmadian, M.; Kida, Y.; Tseng, T.; Dai, Y.; Yu, R.T.; Liddle, C.; et al. ERRγ Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells. Cell Metab. 2016, 23, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.; Kim, D.-K.; Choi, H.-S. ERRγ: A Junior Orphan with a Senior Role in Metabolism. Trends Endocrinol. Metab. 2017, 28, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, A.; Prabu, P.; Mohan, V.; Gibert, Y.; Balasubramanyam, M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol. Cell Biochem. 2019, 458, 171–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schüler-Toprak, S.; Skrzypczak, M.; Gründker, C.; Ortmann, O.; Treeck, O. Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer. Cancers 2023, 15, 2845. https://doi.org/10.3390/cancers15102845
Schüler-Toprak S, Skrzypczak M, Gründker C, Ortmann O, Treeck O. Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer. Cancers. 2023; 15(10):2845. https://doi.org/10.3390/cancers15102845
Chicago/Turabian StyleSchüler-Toprak, Susanne, Maciej Skrzypczak, Carsten Gründker, Olaf Ortmann, and Oliver Treeck. 2023. "Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer" Cancers 15, no. 10: 2845. https://doi.org/10.3390/cancers15102845
APA StyleSchüler-Toprak, S., Skrzypczak, M., Gründker, C., Ortmann, O., & Treeck, O. (2023). Role of Estrogen Receptor β, G-Protein Coupled Estrogen Receptor and Estrogen-Related Receptors in Endometrial and Ovarian Cancer. Cancers, 15(10), 2845. https://doi.org/10.3390/cancers15102845








