Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis
Abstract
:Simple Summary
Abstract
1. Introduction
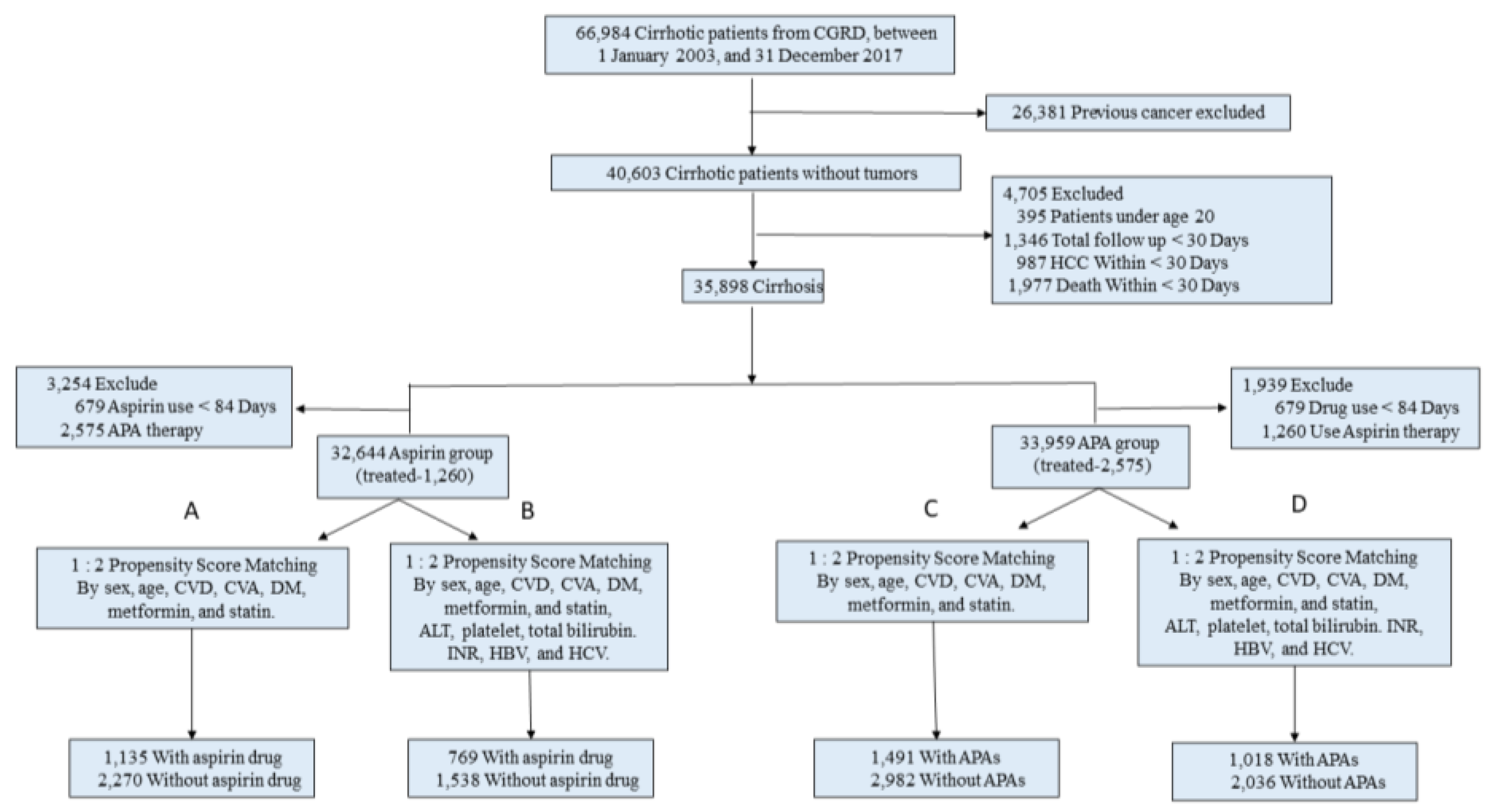
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Main Outcome
2.4. Statistical Analysis
3. Results
3.1. Patients and Study Design
3.2. Daily Aspirin and Non-Aspirin Antiplatelet Agents Reduce HCC Incidence
3.3. Daily Aspirin Is an Independent Protective Factor of HCC in Cirrhotic Patients
3.4. Daily Aspirin Is Associated with a Higher Overall Survival Rate
3.5. Daily Aspirin Reduces HCC Incidences by Multivariable Stratified Analysis
3.6. Daily Aspirin Duration Is Inversely Correlated with HCC Incidence
3.7. Gastrointestinal Bleeding Risk Was Not Increased in Daily Aspirin Users
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The Global, Regional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular Carcinoma in Cirrhosis: Incidence and Risk Factors. Gastroenterology 2004, 127 (Suppl. S1), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e2. [Google Scholar] [CrossRef]
- Singh, J.; Wozniak, A.; Cotler, S.J.; Dhanarajan, A.; Aldrich, D.; Park, D.; Kasia, C.; Schmidt, B.; Scaglione, S. Combined Use of Aspirin and Statin is Associated with a Decreased Incidence of Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2021, 56, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Roberts, L.; Sanchez, W. Chemopreventive Strategies in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2013, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, Y.-J.; Lin, C.-C.; Yen, C.-L.; Shen, C.-H.; Chang, C.-J.; Hsieh, S.-Y. Pretreatment Platelet Count Early Predicts Extrahepatic Metastasis of Human Hepatoma. Liver Int. 2015, 35, 2327–2336. [Google Scholar] [CrossRef]
- Pavlovic, N.; Rani, B.; Gerwins, P.; Heindryckx, F. Platelets as Key Factors in Hepatocellular Carcinoma. Cancers 2019, 11, 1022. [Google Scholar] [CrossRef]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating with the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef]
- Tan, R.Z.H.; Lockart, I.; Shaheed, C.A.; Danta, M. Systematic Review with Meta-Analysis: The Effects of Non-Steroidal Anti-Inflammatory Drugs and Anti-Platelet Therapy on the Incidence and Recurrence of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2021, 54, 356–367. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic Thrombocytosis in Ovarian Cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Cole, B.F.; Logan, R.F.; Halabi, S.; Benamouzig, R.; Sandler, R.S.; Grainge, M.J.; Chaussade, S.; Baron, J.A. Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials. Gynecol. Oncol. 2009, 101, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Sitia, G.; Aiolfi, R.; Di Lucia, P.; Mainetti, M.; Fiocchi, A.; Mingozzi, F.; Esposito, A.; Ruggeri, Z.M.; Chisari, F.V.; Iannacone, M.; et al. Antiplatelet Therapy Prevents Hepatocellular Carcinoma and Improves Survival in a Mouse Model of Chronic Hepatitis B. Proc. Natl. Acad. Sci. USA 2012, 109, E2165–E2172. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Daily Aspirin Use Reduces Risk of Fibrosis Progression in Patients With Nonalcoholic Fatty Liver Disease, Providing New Uses for an Old Drug. Clin. Gastroenterol. Hepatol. 2019, 17, 2651–2653. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Wangensteen, K.J.; FitzGerald, G.A. Aspirin in Hepatocellular Carcinoma. Cancer Res. 2021, 81, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Antiplatelet Therapy Improves the Prognosis of Patients with Hepatocellular Carcinoma. Cancers 2020, 12, 3215. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Hsu, Y.-C.; Tseng, H.-C.; Yu, S.-H.; Lin, J.-T.; Wu, M.-S.; Wu, C.-Y. Association of Daily Aspirin Therapy With Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. JAMA Intern. Med. 2019, 179, 633–640. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Hsu, Y.-C.; Tseng, H.-C.; Lin, J.-T.; Wu, M.-S.; Wu, C.-Y. Association of Daily Aspirin Therapy With Hepatocellular Carcinoma Risk in Patients With Chronic Hepatitis C Virus Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2784–2792.e7. [Google Scholar] [CrossRef]
- Simon, T.G.; Ma, Y.; Ludvigsson, J.F.; Chong, D.Q.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; Chung, R.T.; Zhang, X.; et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018, 4, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Jeng, W.-J.; Pan, M.-H.; Hsieh, Y.-C.; Lu, S.-N.; Chen, C.-J.; Yang, H.-I. Incidence of Hepatocellular Carcinoma in a Community-Based Taiwanese Population without Chronic HBV/HCV Infection. JHEP Rep. 2021, 4, 100410. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, G.; Luo, S.; Yin, W.; Song, H. The Value of Platelet Count in Evaluating the Degree of Liver Fibrosis in Patients with Chronic Hepatitis B. J. Clin. Lab. Anal. 2020, 34, e23270. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Memel, Z.N.; Arvind, A.; Moninuola, O.; Philpotts, L.; Chung, R.T.; Corey, K.E.; Simon, T.G. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol. Commun. 2020, 5, 133–143. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef]
- Tripodi, A.; Salerno, F.; Chantarangkul, V.; Clerici, M.; Cazzaniga, M.; Primignani, M.; Mannucci, P.M. Evidence of Normal Thrombin Generation in Cirrhosis Despite Abnormal Conventional Coagulation Tests. Hepatology 2005, 41, 553–558. [Google Scholar] [CrossRef]
- Gatt, A.; Riddell, A.; Calvaruso, V.; Tuddenham, E.G.; Makris, M.; Burroughs, A.K. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J. Thromb. Haemost. 2010, 8, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Basili, S.; Iuliano, L.; Camastra, C.; Giammarresi, C.; Santarone, S.; Rocca, B.; Landolfi, R.; Ciabattoni, G.; Cordova, C.; et al. Increased Thromboxane Metabolites Excretion in Liver Cirrhosis. Thromb. Haemost. 1998, 79, 747–751. [Google Scholar] [CrossRef]
- Panasiuk, A.; Prokopowicz, D.; Zak, J.; Matowicka-Karna, J.; Osada, J.; Wysocka, J. Activation of blood platelets in chronic hepatitis and liver cirrhosis P-selectin expression on blood platelets and secretory activity of beta-thromboglobulin and platelet factor-4. Hepato Gastroenterol. 2001, 48, 818–822. [Google Scholar] [PubMed]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.; de Maat, M.P.; de Groot, P.G.; Leebeek, F.W. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Raparelli, V.; Nocella, C.; Bartimoccia, S.; Novo, M.; Severino, A.; De Falco, E.; Cammisotto, V.; Pasquale, C.; Crescioli, C.; et al. Gut-Derived Endotoxin Stimulates Factor VIII Secretion from Endothelial Cells. Implications for Hypercoagulability in Cirrhosis. J. Hepatol. 2017, 67, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qu, G.; Sun, C.; Liu, H.; Jiang, Y.; Li, N.; Wu, B.; Gao, J.; Feng, L.; Xie, P.; et al. Does Aspirin Reduce the Incidence, Recurrence, and Mortality of Hepatocellular Carcinoma? A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis. Eur. J. Clin. Pharmacol. 2022, 79, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chung, G.E.; Lee, J.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef] [PubMed]


| Clinical Outcome and Treatment Group | Number with Event/ Total No. | Incidence Rate (Per 100 Person-Year) | Univariate Cox Model | Multivariable Cox Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude HR | (95% CI) | p-Value | Adjusted HR | (95% CI) | p-Value | |||||
| 3-year outcomes | ||||||||||
| Incident HCC | ||||||||||
| Aspirin | 47/1135 | 1.49 | 0.46 | 0.31 | 0.69 | 0.0002 | 0.57 | 0.37 | 0.87 | 0.009 |
| APA c | 93/1491 | 2.49 | 0.62 | 0.46 | 0.83 | 0.0015 | 0.82 | 0.59 | 1.14 | 0.243 |
| 5-year outcomes | ||||||||||
| Incident HCC | ||||||||||
| Aspirin | 82/1135 | 2.14 | 0.55 | 0.40 | 0.76 | 0.0003 | 0.630 | 0.45 | 0.88 | 0.007 |
| APA c | 138/1491 | 2.90 | 0.63 | 0.49 | 0.82 | 0.0005 | 0.81 | 0.61 | 1.08 | 0.151 |
| Event and Duration of Low-Dose Aspirin | Univariate Cox Model | Multivariable Cox Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR | (95% CI) | p-Value | Adjusted HR | (95% CI) | p-Value | |||
| Aspirin a | ||||||||
| Nonuser | 1.00 (reference) | 1.00 (reference) | ||||||
| 3 m to <1 y | 0.73 | 0.52 | 1.03 | 0.071 | 0.88 | 0.58 | 1.34 | 0.553 |
| 1 to <3 y | 0.73 | 0.50 | 1.07 | 0.103 | 0.56 | 0.31 | 0.99 | 0.048 |
| ≥3 y | 0.35 | 0.21 | 0.60 | 0.0001 | 0.37 | 0.18 | 0.76 | 0.007 |
| Aspirin b | ||||||||
| Nonuser | 1.00 (reference) | 1.00 (reference) | ||||||
| 3 m to <1 y | 0.93 | 0.62 | 1.39 | 0.723 | 0.85 | 0.55 | 1.31 | 0.455 |
| 1 to <3 y | 0.50 | 0.28 | 0.90 | 0.020 | 0.54 | 0·30 | 0.98 | 0.044 |
| ≥3 y | 0.34 | 0.17 | 0.68 | 0.002 | 0.39 | 0.19 | 0.79 | 0.009 |
| Clinical Outcome and Treatment Group | Multivariable Cox Model | |||
|---|---|---|---|---|
| Adjusted HR | (95% CI) | p-Value | ||
| Patients without previous GI bleeding | ||||
| 3-year outcomes | 0.66 | 0.49 | 0.90 | 0.009 |
| 5-year outcomes | 0.65 | 0.50 | 0.86 | 0.002 |
| Patients with previous GI bleeding | ||||
| 3-year outcomes | 0.51 | 0.36 | 0.71 | <0.0001 |
| 5-year outcome | 0.51 | 0.36 | 0.72 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Hsu, C.-Y.; Yen, T.-H.; Wu, T.-H.; Yu, M.-C.; Hsieh, S.-Y. Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis. Cancers 2023, 15, 2946. https://doi.org/10.3390/cancers15112946
Lee C-H, Hsu C-Y, Yen T-H, Wu T-H, Yu M-C, Hsieh S-Y. Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis. Cancers. 2023; 15(11):2946. https://doi.org/10.3390/cancers15112946
Chicago/Turabian StyleLee, Chern-Horng, Chiu-Yi Hsu, Tzung-Hai Yen, Tsung-Han Wu, Ming-Chin Yu, and Sen-Yung Hsieh. 2023. "Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis" Cancers 15, no. 11: 2946. https://doi.org/10.3390/cancers15112946
APA StyleLee, C.-H., Hsu, C.-Y., Yen, T.-H., Wu, T.-H., Yu, M.-C., & Hsieh, S.-Y. (2023). Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis. Cancers, 15(11), 2946. https://doi.org/10.3390/cancers15112946






