Surgery of Colorectal Liver Metastases Involving the Inferior Vena Cava: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
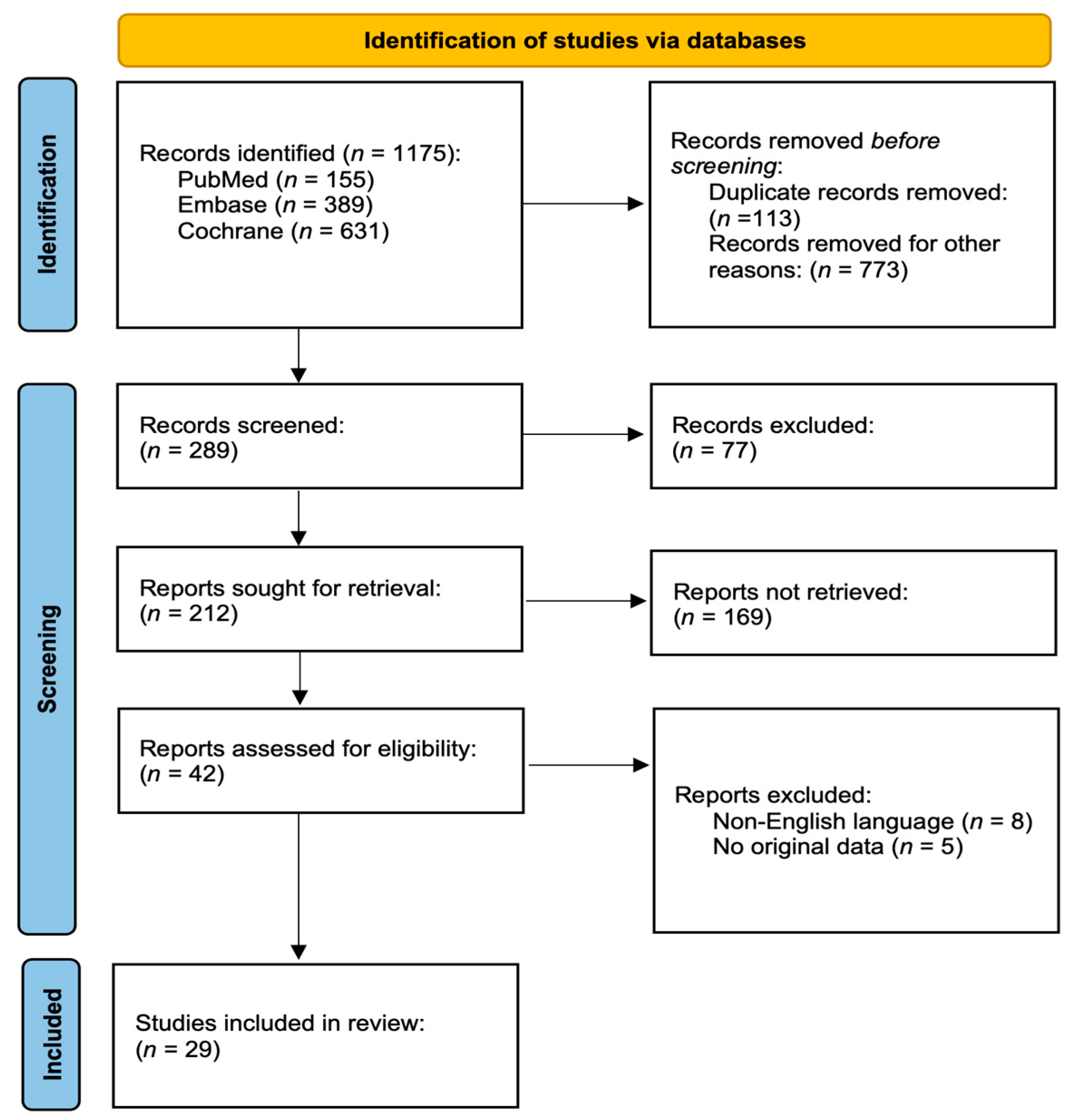
2. Materials and Methods
2.1. Literature Review
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Variables Studied
3. Results
3.1. Patients and Surgical Characteristics
3.2. Morbidity and Mortality Outcomes
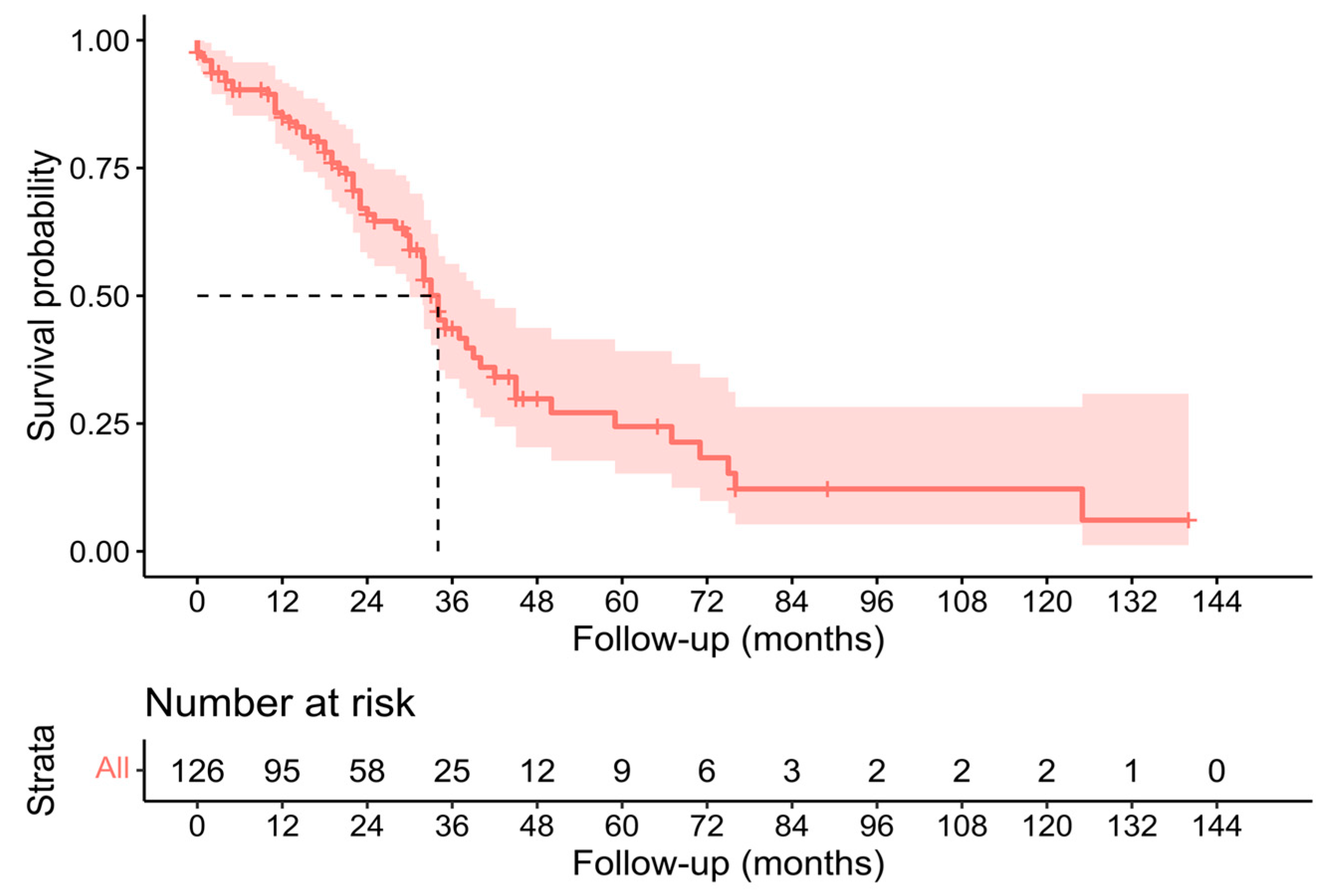
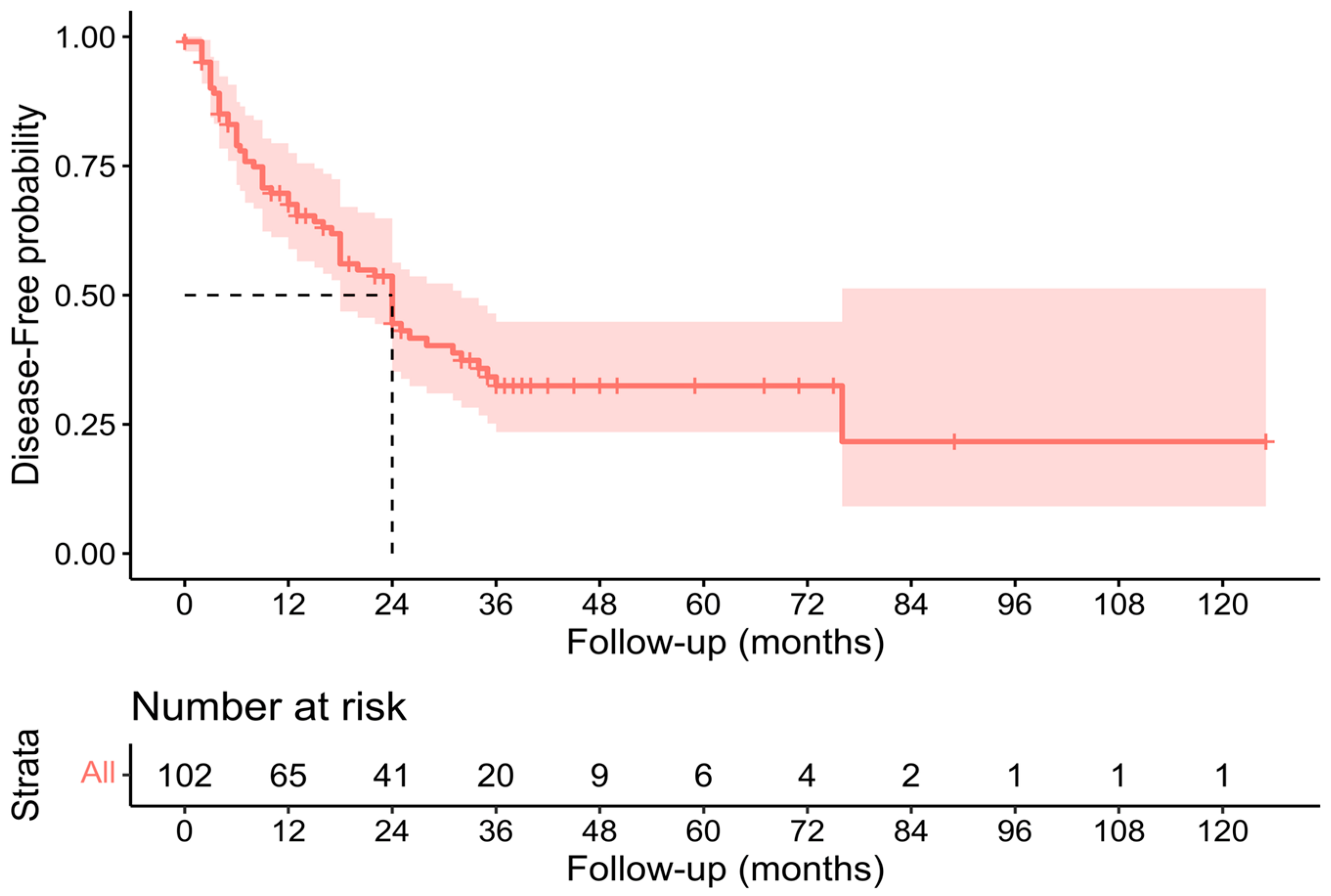
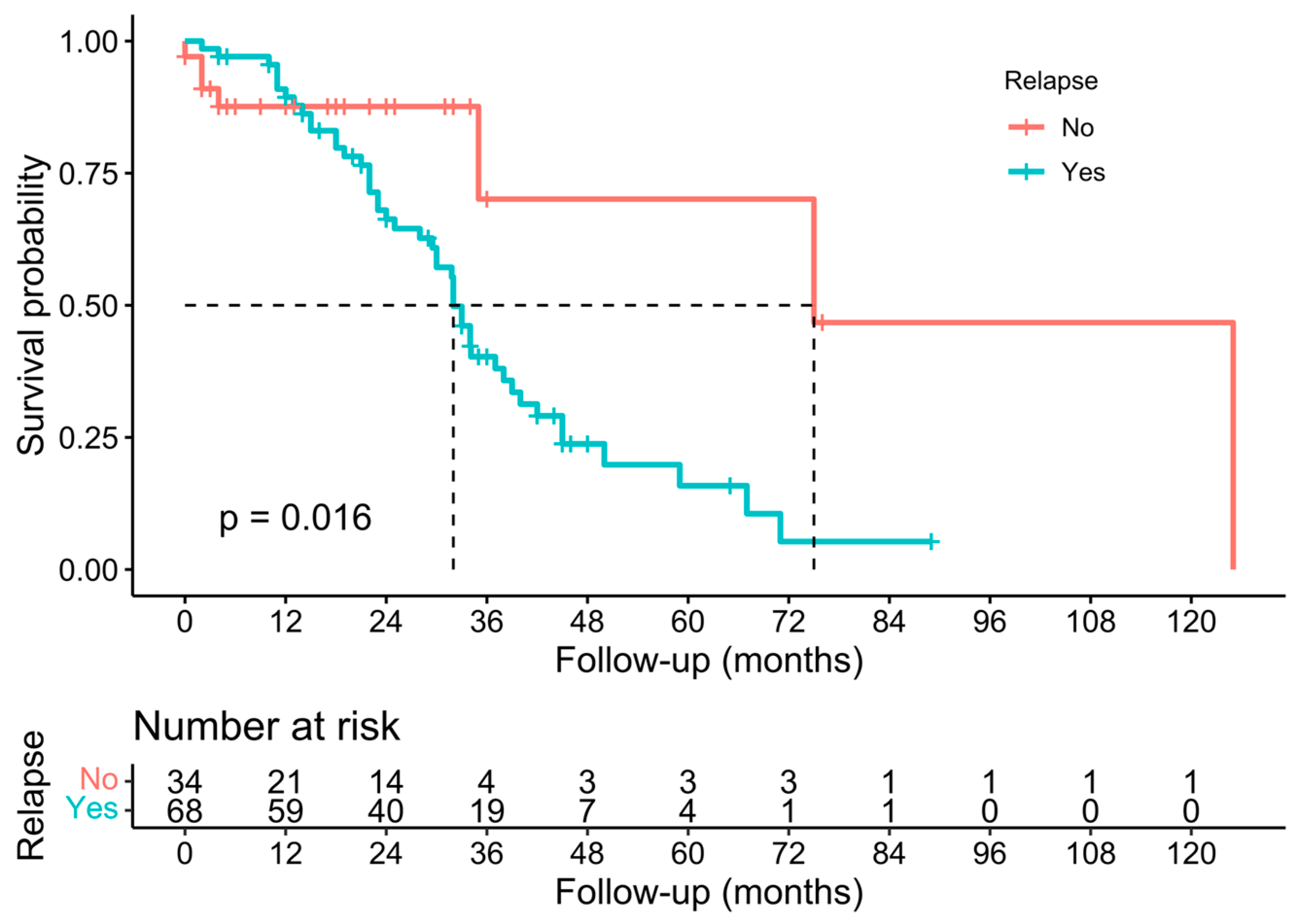
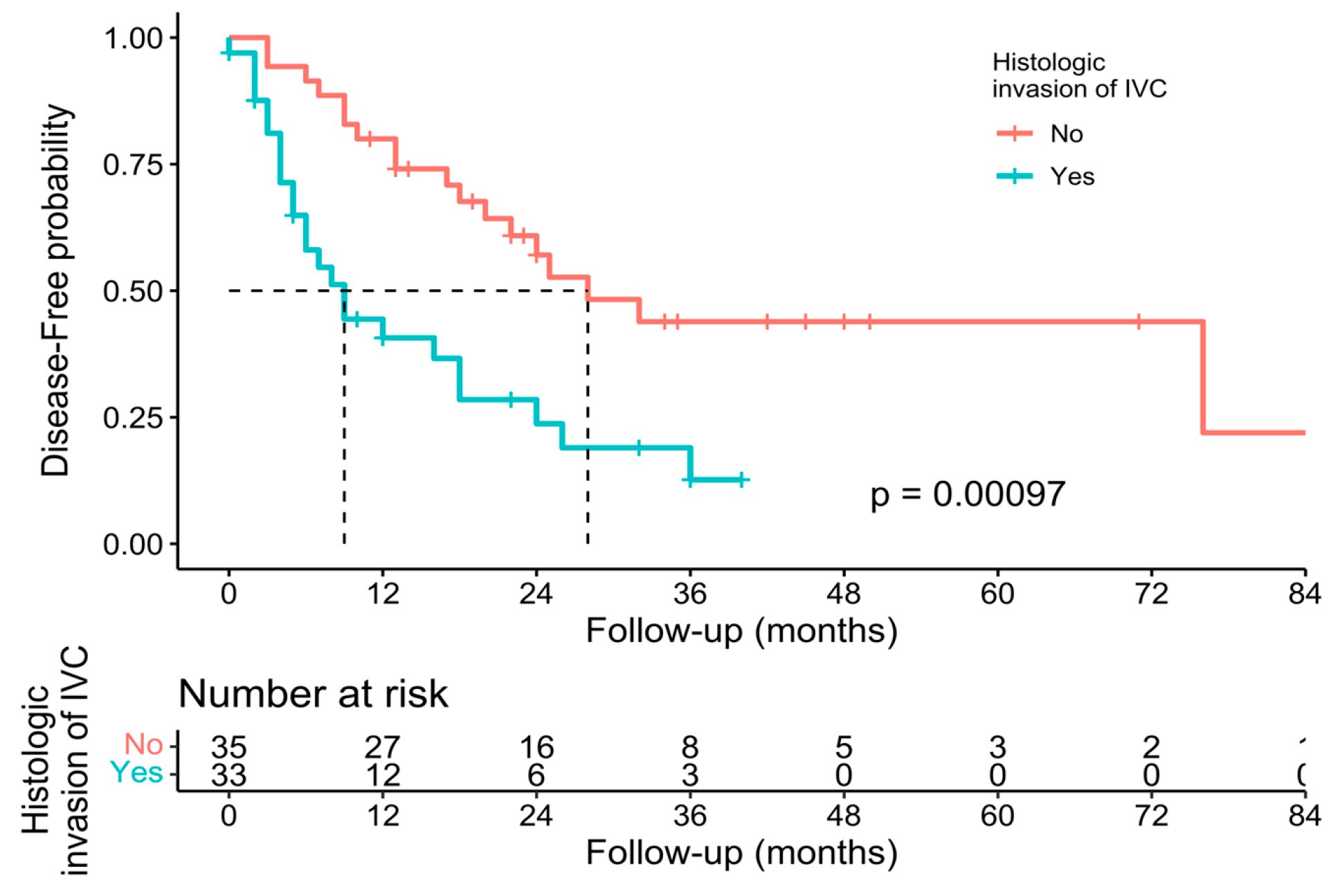
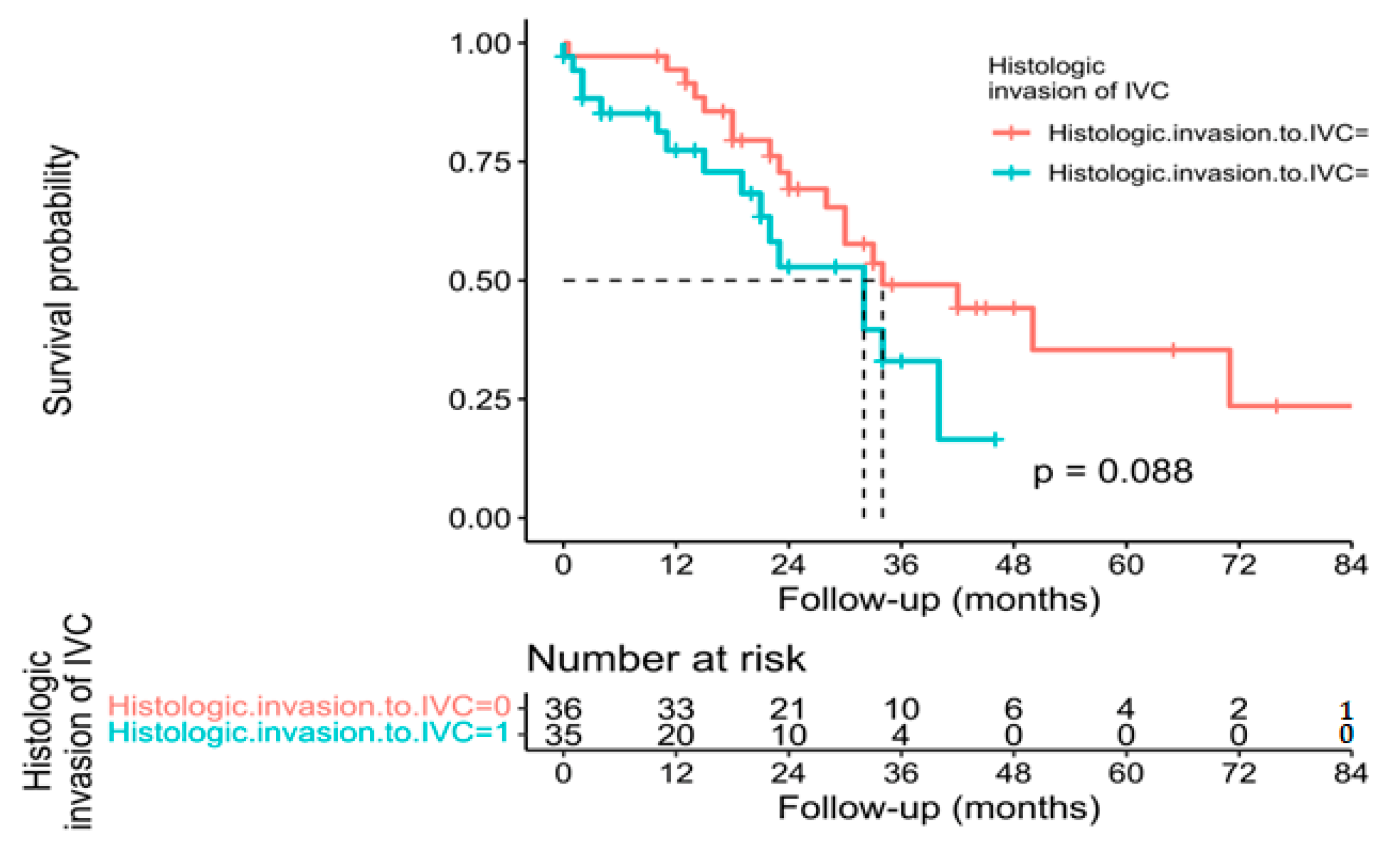
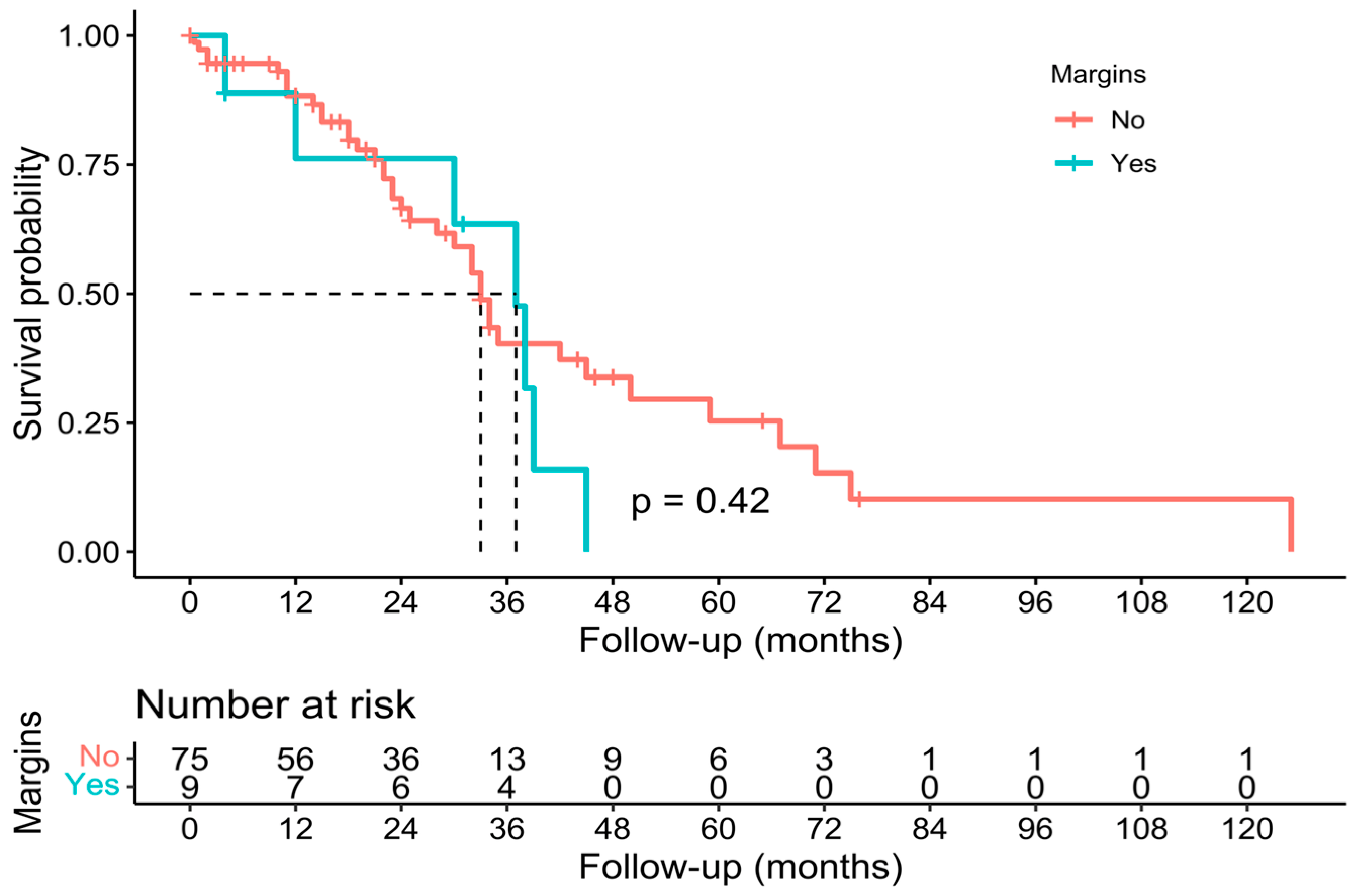
3.3. Pathological, Oncological, and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zidani, R.; Brienza, S.; Dogliotti, L.; Perpoint, B.; Rotarski, M.; Letourneau, Y.; Chollet, P.; Le Rol, A.; Focan, C. International Organization for Cancer Chronotherapy A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma. Int. Organ. Cancer Chronother. Cancer 1999, 85, 2532–2540. [Google Scholar]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and Fluorouracil with or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Fong, Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J. Clin. 1999, 49, 514–523. [Google Scholar] [CrossRef]
- Bismuth, H.; Adam, R.; Lévi, F.; Farabos, C.; Waechter, F.; Castaing, D.; Majno, P.; Engerran, L. Resection of Nonresectable Liver Metastases from Colorectal Cancer After Neoadjuvant Chemotherapy. Ann. Surg. 1996, 224, 509–522. [Google Scholar] [CrossRef]
- Giacchetti, S.; Perpoint, B.; Zidani, R.; Le Bail, N.; Faggiuolo, R.; Focan, C.; Chollet, P.; Llory, J.; Letourneau, Y.; Coudert, B.; et al. Phase III Multicenter Randomized Trial of Oxaliplatin Added to Chronomodulated Fluorouracil–Leucovorin as First-Line Treatment of Metastatic Colorectal Cancer. J. Clin. Oncol. 2000, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Noun, R.; Zante, E.; Ballet, T.; Sauvanet, A. Portal Triad Clamping or Hepatic Vascular Exclusion for Major Liver Resection. A controlled study. Ann. Surg. 1996, 224, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Abu Hilal, M.; Lodge, J. Pushing back the frontiers of resectability in liver cancer surgery. Eur. J. Surg. Oncol. 2008, 34, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Grazi, G.L.; Mazziotti, A.; Jovine, E.; Pierangeli, F.; Ercolani, G.; Gallucci, A.; Cavallari, A. Total Vascular Exclusion of the Liver During Hepatic Surgery. Arch. Surg. 1997, 132, 1104–1109. [Google Scholar] [CrossRef]
- Malassagne, B.; Cherqui, D.; Alon, R.; Brunetti, F.; Humeres, R.; Fagniez, P.-L. Safety of selective vascular clamping for major hepatectomies. J. Am. Coll. Surg. 1998, 187, 482–486. [Google Scholar] [CrossRef]
- Torzilli, G.; Makuuchi, M.; Midorikawa, Y.; Sano, K.; Inoue, K.; Takayama, T.; Kubota, K. Liver Resection Without Total Vascular Exclusion: Hazardous or Beneficial? An analysis of our experience. Ann. Surg. 2001, 233, 167–175. [Google Scholar] [CrossRef]
- Smyrniotis, V.E.; Kostopanagiotou, G.G.; Gamaletsos, E.L.; Vassiliou, J.G.; Voros, D.C.; Fotopoulos, A.C.; Contis, J.C. Total versus selective hepatic vascular exclusion in major liver resections. Am. J. Surg. 2002, 183, 173–178. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- CEBM, University of Oxford. Oxford Centre for Evidence-Based Medicine: Levels of Evidence. March 2009. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 22 February 2023).
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Gardner, B.; Bender, S.; Praeger, P.I. En bloc caudate lobe and partial vena cava resection using a gott shunt for retrohepatic caval bypass. J. Surg. Oncol. 1992, 50, 267–269. [Google Scholar] [CrossRef]
- Del Campo, C.; Konok, G.P. Use of a pericardial xenograft patch in repair of resected retrohepatic vena cava. Can. J. Surg. 1994, 37, 59–61. [Google Scholar] [PubMed]
- Kubota, K.; Makuuchi, M.; Kobayashi, T.; Sakamoto, Y.; Inoue, K.; Torzilli, G.; Takayama, T. Reconstruction of the inferior vena cava using a hepatic venous patch obtained from resected liver. Hepato-Gastroenterol. 1997, 44, 378–379. [Google Scholar]
- Miyazaki, M.; Ito, H.; Nakagawa, K.; Ambiru, S.; Shimizu, H.; Okuno, A.; Nukui, Y.; Yoshitomi, H.; Kusashio, K.; Furuya, S.; et al. Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am. J. Surg. 1999, 177, 294–298. [Google Scholar] [CrossRef]
- Tono, T.; Ohzato, H.; Fukunaga, M.; Hasuike, Y.; Nakagawa, H.; Monden, T.; Okamura, J.; Kikkawa, N.; Takatsuka, Y. Surgical treatment of hepatic caudate lobe metastases originating from colorectal primaries. Int. Surg. 2001, 85, 237–242. [Google Scholar]
- Lodge, J.P.A.; Ammori, B.J.; Prasad, K.R.; Bellamy, M.C. Ex Vivo and In Situ Resection of Inferior Vena Cava with Hepatectomy for Colorectal Metastases. Ann. Surg. 2001, 231, 471–479. [Google Scholar] [CrossRef]
- Togo, S.; Tanaka, K.; Endo, I.; Morioka, D.; Miura, Y.; Masunari, H.; Kubota, T.; Nagano, Y.; Masui, H.; Sekido, H.; et al. Caudate Lobectomy Combined with Resection of the Inferior Vena cava and Its Reconstruction by a Pericardial Autograft Patch. Dig. Surg. 2002, 19, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Arii, S.; Teramoto, K.; Kawamura, T.; Takamatsu, S.; Sato, E.; Nakamura, N.; Iwai, T.; Mori, A.; Tanaka, J.; Imamura, M. Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumors. J. Am. Coll. Surg. 2003, 196, 243–249. [Google Scholar] [CrossRef]
- Aoki, T.; Sugawara, Y.; Imamura, H.; Seyama, Y.; Minagawa, M.; Hasegawa, K.; Kokudo, N.; Makuuchi, M. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer1. J. Am. Coll. Surg. 2004, 198, 366–372. [Google Scholar] [CrossRef]
- Hemming, A.W.; Langham, M.R.; Reed, A.I.; Van Der Werf, W.J.; Howard, R.J. Resection of the inferior vena cava for hepatic malignancy. Am. Surg. 2001, 67, 1081–1087, discussion 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Hemming, A.W.; Reed, A.I.; Langham, M.R.; Fujita, S.; Howard, R.J. Combined Resection of the Liver and Inferior Vena Cava for Hepatic Malignancy. Ann. Surg. 2004, 239, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Nardo, B.; Ercolani, G.; Montalti, R.; Bertelli, R.; Gardini, A.; Beltempo, P.; Puviani, L.; Pacilè, V.; Vivarelli, M.; Cavallari, A. Hepatic Resection for Primary or Secondary Malignancies with Involvement of the Inferior Vena Cava: Is This Operation Safe or Hazardous? J. Am. Coll. Surg. 2005, 201, 671–679. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nagino, M.; Kamiya, J.-I.; Hayakawa, N.; Nimura, Y. Surgical treatment for colorectal liver metastases involving the paracaval portion of the caudate lobe. Surgery 2005, 137, 26–32. [Google Scholar] [CrossRef]
- Johnson, S.T.; Blitz, M.; Kneteman, N.; Bigam, D. Combined Hepatic and Inferior Vena Cava Resection for Colorectal Metastases. J. Gastrointest. Surg. 2006, 10, 220–226. [Google Scholar] [CrossRef]
- Delis, S.G.; Madariaga, J.; Ciancio, G. Combined liver and inferior vena cava resection for hepatic malignancy. J. Surg. Oncol. 2007, 96, 258–264. [Google Scholar] [CrossRef]
- Varma, D.; Ogata, S.; Belghiti, J. Isolated total caval clamping with “preserved remnant liver perfusion” for combined hepatic and venacaval resection in tumors involving venacava. Surgery 2007, 141, 112–116. [Google Scholar] [CrossRef]
- Hashimoto, T.; Minagawa, M.; Aoki, T.; Hasegawa, K.; Sano, K.; Imamura, H.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Caval Invasion by Liver Tumor Is Limited. J. Am. Coll. Surg. 2008, 207, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, Z.; Jeong, G.-A.; Chung, J.-C.; Chu, C.-W.; Shin, E.-J.; Kim, H.-C. Ante-situm liver resection in recurrent liver metastasis from colorectal cancer. Hepato-Gastroenterol. 2009, 56, 508–511. [Google Scholar]
- Tanaka, K.; Matsuyama, R.; Takeda, K.; Matsuo, K.; Nagano, Y.; Endo, I. Aggressive liver resection including major-vessel resection for colorectal liver metastases. World J. Hepatol. 2009, 1, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Malde, D.J.; Khan, A.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P.A. Inferior vena cava resection with hepatectomy: Challenging but justified. HPB 2011, 13, 802–810. [Google Scholar] [CrossRef]
- Nuzzo, G.; Giordano, M.; Giuliante, F.; Lopez-Ben, S.; Albiol, M.; Figueras, J. Complex liver resection for hepatic tumours involving the inferior vena cava. Eur. J. Surg. Oncol. 2011, 37, 921–927. [Google Scholar] [CrossRef]
- Polistina, F.; Fabbri, A.; Ambrosino, G. Hepatic Colorectal Metastases Involving Infra-Hepatic Inferior Vena Cava in High Risk Patients for Extended Resection: An Alternative Method for Achieving Radical Resection in Patient with Borderline Liver Remnant. Indian J. Surg. 2013, 75, 220–225. [Google Scholar] [CrossRef]
- Anonymous. Abstracts—APASL 2013. Hepatol. Int. 2013, 7, 1–754. [Google Scholar] [CrossRef]
- Hemming, A.W.; Mekeel, K.L.; Zendejas, I.; Kim, R.D.; Sicklick, J.K.; Reed, A.I. Resection of the Liver and Inferior Vena Cava for Hepatic Malignancy. J. Am. Coll. Surg. 2013, 217, 115–124. [Google Scholar] [CrossRef]
- Guerrini, G.P.; Soliani, P. Repeated hepatic resection combined with inferior vena cava replacement: Case report and review of literature. Int. J. Surg. Case Rep. 2015, 6, 114–117. [Google Scholar] [CrossRef]
- Marangoni, G.; Hakeem, A.; Khan, A.; Rotimi, O.; Lodge, J.P. Repeat hepatectomy with inferior vena cava re-resection for colorectal liver metastases: Case report and review of the literature. Surg. Today 2015, 45, 1450–1456. [Google Scholar] [CrossRef]
- Nomi, T.; Fuks, D.; Agrawal, A.; Kawaguchi, Y.; Ogiso, S.; Gayet, B. Totally Laparoscopic Right Hepatectomy Combined with Resection of the Inferior Vena Cava by Anterior Approach. Ann. Surg. Oncol. 2015, 22, 851. [Google Scholar] [CrossRef]
- Ko, S.; Kirihataya, Y.; Matsusaka, M.; Mukogawa, T.; Ishikawa, H.; Watanabe, A. Parenchyma-Sparing Hepatectomy with Vascular Reconstruction Techniques for Resection of Colorectal Liver Metastases with Major Vascular Invasion. Ann. Surg. Oncol. 2016, 23, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tardu, A.; Kayaalp, C.; Yilmaz, S.; Tolan, K.; Ersan, V.; Karagul, S.; Ertuğrul, I.; Kirmizi, S. Reresection of Colorectal Liver Metastasis with Vena Cava Resection. Case Rep. Surg. 2016, 2016, 8173048. [Google Scholar] [CrossRef] [PubMed]
- Vladov, N.; Kostadinov, R.; Mihaylov, V.; Takorov, I.; Lukanova, T.; Yakova, M.; Trichkov, T.; Odisseeva, E.; Mutafchiyski, V. Single-Centre Experience of Supra-Renal Vena Cava Resection and Reconstruction. World J. Surg. 2021, 45, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Koep, L.J.; Weil, R.; Lilly, J.R.; Putnam, C.W.; Aldrete, J.A. Right trisegmentectomy for hepatic neoplasms. Surgery, Gynecol. Obstet. 1980, 150, 208–214. [Google Scholar]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Mohammad, W.M.; Balaa, F.K. Surgical Management of Colorectal Liver Metastases. Clin. Colon Rectal Surg. 2009, 22, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, L.; Xu, D.; Wan, T.; Si, X. A pooled analysis of combined liver and inferior vena cava resection for hepatic malignancy. HPB 2017, 19, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Nimura, Y.; Nishio, H.; Ebata, T.; Igami, T.; Matsushita, M.; Nishikimi, N.; Kamei, Y. Hepatectomy With Simultaneous Resection of the Portal Vein and Hepatic Artery for Advanced Perihilar Cholangiocarcinoma: An Audit of 50 Consecutive Cases. Ann. Surg. 2010, 252, 115–123. [Google Scholar] [CrossRef]
- Mehrabi, A.; Fonouni, H.; Golriz, M.; Hofer, S.; Hafezi, M.; Rahbari, N.N.; Weitz, J.; Büchler, M.W.; Schmidt, J. Hypothermic ante situm Resection in Tumors of the Hepatocaval Confluence. Dig. Surg. 2011, 28, 100–108. [Google Scholar] [CrossRef]
- Hannoun, L.; Delriviere, L.; Gibbs, P.; Borie, D.; Vaillant, J.C.; Delva, E. Major extended hepatic resections in diseased livers using hypothermic protection: Preliminary results from the first 12 patients treated with this new technique. J. Am. Coll. Surg. 1996, 183, 597–605. [Google Scholar] [PubMed]
- Azoulay, D.; Pascal, G.; Salloum, C.; Adam, R.; Castaing, D.; Tranecol, N. Vascular reconstruction combined with liver resection for malignant tumours. Br. J. Surg. 2013, 100, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
| Year | Author | Country | N° Pat. | Mean Age | M/F | MNM | Type of Hepatectomy | Vascular Control | IVC Repair |
|---|---|---|---|---|---|---|---|---|---|
| 1992 | Gardner [16] | USA | 1 | 77 | 0/1 | 1 | 1 S1 resection | 1 THVE | 1 PC |
| 1994 | Del Campo [17] | Canada | 1 | 64 | 1/0 | 4 | 1 RH | 1 IVC clamp | 1 PBP |
| 1997 | Kubota [18] | Japan | 1 | 59 | 0/1 | ND | 1 RH | 1 LC | 1 Aut. patch |
| 1999 | Miyazaki [19] | Japan | 14 | ND | ND | ND | ND | ND | ND |
| 2000 | Tono [20] | Japan | 3 | 62.3 | 2/1 | 1.3 | 2 extended LHs + CL 1 S1 resection | 3 LC | 3 PCs |
| 2000 | Lodge [21] | UK | 8 | 62 | 6/2 | 1.6 | 4 ex vivo hepatectomies 3 RHs 1 extended RH + CL | 2 Portosystemic venovenous bypasses 2 Portosystemic venovenous bypasses + HVE 2 Systemic venovenous bypasses + HVE 1 LC 1 IVC clamp | 3 Dacron patches 1 Dacron tube 2 PTFE tube grafts 1 PC 1 Aut. graft |
| 2002 | Togo [22] | Japan | 1 | 53 | 0/1 | 5 | 1 LH + CL | IVC clamp | 1 Aut. patch |
| 2003 | Arii [23] | Japan | 2 | 57.5 | 1/1 | 1 | 1 RT + CL 1 RH | 1 Active ventriculovenous shunt (biopump) 1 Passive ventriculovenous shunt | 2 PTFE grafts |
| 2004 | Aoki [24] | Japan | 3 | 60.6 | 1/2 | 3 | 2 extended RHs 1 wedge resection | 3 LC | 1 Aut. graft 2 PCs |
| 2004 | Hemming [25,26] | USA | 6 | ND | ND | 2.75 | 3 RTs 1 LT. 1 Ex vivo hepatectomy 1 RH + CL | ND | 4 PTFE tube grafts 1 PTFE patch 1 PC |
| 2005 | Nardo [27] | Italy | 11 | 55.3 | 8/3 | 2.2 | 4 extended LHs 4 extended RHs 1 RH 1 LH 1 segmentectomy | 6 THVEs 1 IVC clamp 2 IHPCs 2 IHPCs/LC | 7 PCs 3 PTFE grafts 1 PBP |
| 2005 | Yamamoto [28] | Japan | 4 | 61.2 | 3/1 | NA | 2 S1 resections 2 RHs + CL | 3 LC 1 IVC clamp | 3 PCs 1 Aut. graft |
| 2006 | Johnson [29] | Canada | 11 | 60.5 | 4/7 | 1.1 | 3 RTs 3 RHs 2 LHs 1 RH + COR 1 RT + COR 1 LT+ COR | 10 THVEs 1 LC | 8 PTFE patches 2 PCs 1 PTFE tube graft |
| 2007 | Delis [30] | USA | 6 | 46.2 | 3/3 | ND | 4 RHs 2 LTs | 3 THVEs 3 PHVEs | 6 PTFE tube grafts |
| 2007 | Varma [31] | France | 1 | 66 | 1/0 | ND | ND | 1 IVC clamp | 1 PC |
| 2008 | Hashimoto [32] | Japan | 15 | 62 | 9/6 | 2 extended RHs 1 right lateral sectionectomy 1 extended LH 9 wedge resections 1 S1 resection 1 segmentectomy | 14 LC 1 IVC clamp | 12 PCs 2 Aut. grafts 1 PTFE patch | |
| 2009 | Kim [33] | Korea | 1 | 56 | 1/0 | 1 | 1 segmentectomy + CL | 1 THVE | 1 Aut. graft |
| 2009 | Tanaka [34] | Japan | 20 | 63.4 | 10/10 | 3.7 | ND | 17 LC 3 THVEs | 17 PCs 2 PTFE grafts 1 Aut. patch |
| 2011 | Malde [35] | UK | 21 | 57.9 | 11/10 | ND | 5 RHs 4 RTs 4 RTs + CL 3 LHs 1 LH + COR 1 LT + CL 1 S1 + COR 1 RH + CL 1 segmentectomy | 13 THVEs 4 THVEs, IHP, ex vivo 1 THVE, IHP, ante situm | 14 PCs 3 Dacron grafts 2 PTFE grafts 2 PBP |
| 2011 | Nuzzo [36] | Italy, Spain | 11 | 55.3 | 6/5 | ND | 3 RHs 6 extended RHs 2 extended LHs | ND | 7 PCs 4 PTFE grafts |
| 2013 | Polistina [37] | Italy | 2 | 55 | 1/1 | 2.5 | 1 extended RH 1 extended LH | 2 THVEs | 2 Aut. patches |
| 2013 | Ie [38] | Japan | 9 | 55 | 4/5 | ND | 6 RHs 2 segmentectomies 1 wedge resection | 9 LC | 9 PCs |
| 2013 | Hemming [39] | USA | 13 | ND | 9/4 | ND | 5 RTs 5 RHs 2 LTs 1 LH | ND | 9 PTFE grafts 2 ND patches 2 PCs |
| 2015 | Guerrini [40] | Italy | 1 | 67 | 1/0 | 1 | 1 segmentectomy | 1 THVE | 1 PTFE graft |
| 2015 | Marangoni [41] | UK | 1 | 52 | 0/1 | 1 | 1 segmentectomy + CL | 1 THVE | 1 PTFE patch |
| 2015 | Nomi [42] | France | 1 | 58 | 0/1 | ND | 1 RH + CL | ND | 1 PC |
| 2016 | Ko [43] | Japan | 6 | ND | ND | 6.3 | 2 extended RHs 1 segmentectomy 2 RHs 1 wedge resection | 6 THVEs | ND |
| 2016 | Tardu [44] | Turkey | 1 | 66 | 0/1 | 5 | 1 RH + COR | 1 THVE | 1 Aut. graft |
| 2021 | Vladov [45] | Bulgaria | 13 | 58.8 | 5/8 | ND | 7 RHs 2 RH + CL 1 LH + wedge resection 3 segmentectomies | ND | 11 PCs 1 PTFE graft 1 Dacron patch |
| Year | Author | n | 1 Year-OS | 3 Year-OS | 5 Year-OS | MS (Months) | Median DFS (Months) |
|---|---|---|---|---|---|---|---|
| 1999 | Miyazaki [19] | 14 | 64% | 33% | 22% | ||
| 2005 | Nardo [27] | 11 | 81.8% | - | 51.9% | ||
| 2006 | Johnson [29] | 11 | 34 | 9 | |||
| 2008 | Hashimoto [32] | 15 | 52.9% | 47.3% | - | ||
| 2011 | Malde [35] | 21 | 75.9% | 19.6% | 28 | - | |
| 2011 | Nuzzo [36] | 11 | 16 | ||||
| 2013 | Ie [38] | 9 | 100% | 60% | 40% | 13 (3–114) | |
| 2021 | Vladov [45] | 13 | 46% | 23% | 0% | ||
| 2023 | Present study | 126 | 71.4% | 19.8% | 7.1% | 34 (30–40) | 24 (18–32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serradilla-Martín, M.; Oliver-Guillén, J.R.; Ruíz-Quijano, P.; Palomares-Cano, A.; de la Plaza-Llamas, R.; Ramia, J.M. Surgery of Colorectal Liver Metastases Involving the Inferior Vena Cava: A Systematic Review. Cancers 2023, 15, 2965. https://doi.org/10.3390/cancers15112965
Serradilla-Martín M, Oliver-Guillén JR, Ruíz-Quijano P, Palomares-Cano A, de la Plaza-Llamas R, Ramia JM. Surgery of Colorectal Liver Metastases Involving the Inferior Vena Cava: A Systematic Review. Cancers. 2023; 15(11):2965. https://doi.org/10.3390/cancers15112965
Chicago/Turabian StyleSerradilla-Martín, Mario, José Ramón Oliver-Guillén, Pablo Ruíz-Quijano, Ana Palomares-Cano, Roberto de la Plaza-Llamas, and José Manuel Ramia. 2023. "Surgery of Colorectal Liver Metastases Involving the Inferior Vena Cava: A Systematic Review" Cancers 15, no. 11: 2965. https://doi.org/10.3390/cancers15112965
APA StyleSerradilla-Martín, M., Oliver-Guillén, J. R., Ruíz-Quijano, P., Palomares-Cano, A., de la Plaza-Llamas, R., & Ramia, J. M. (2023). Surgery of Colorectal Liver Metastases Involving the Inferior Vena Cava: A Systematic Review. Cancers, 15(11), 2965. https://doi.org/10.3390/cancers15112965







