Therapeutic Potential of a Small-Molecule STAT3 Inhibitor in a Mouse Model of Colitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DSS-Induced Colitis Models
2.2. Immunohistochemical and TUNEL Analysis
2.3. Tunel Assay
2.4. Luminex Assay and IHC for Measurement of Y705-Phosphorylated (p)-STAT3 and Total-STAT3
2.5. RNA Isolation and mRNA Expression Analysis
2.6. Statistical Analyses
3. Results
3.1. Effect of STAT3β Deletion on DSS-Induced Colitis in Mice
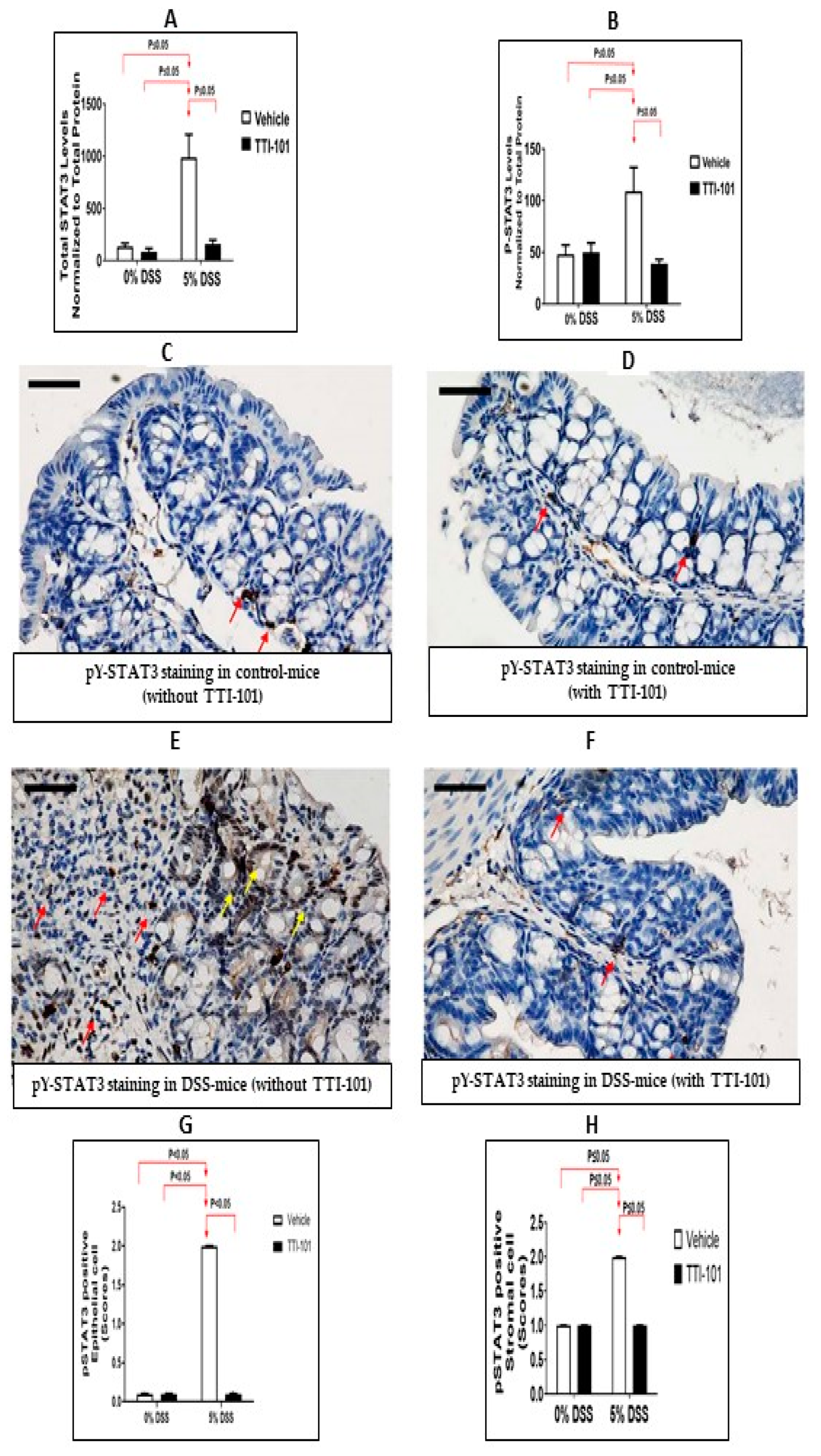
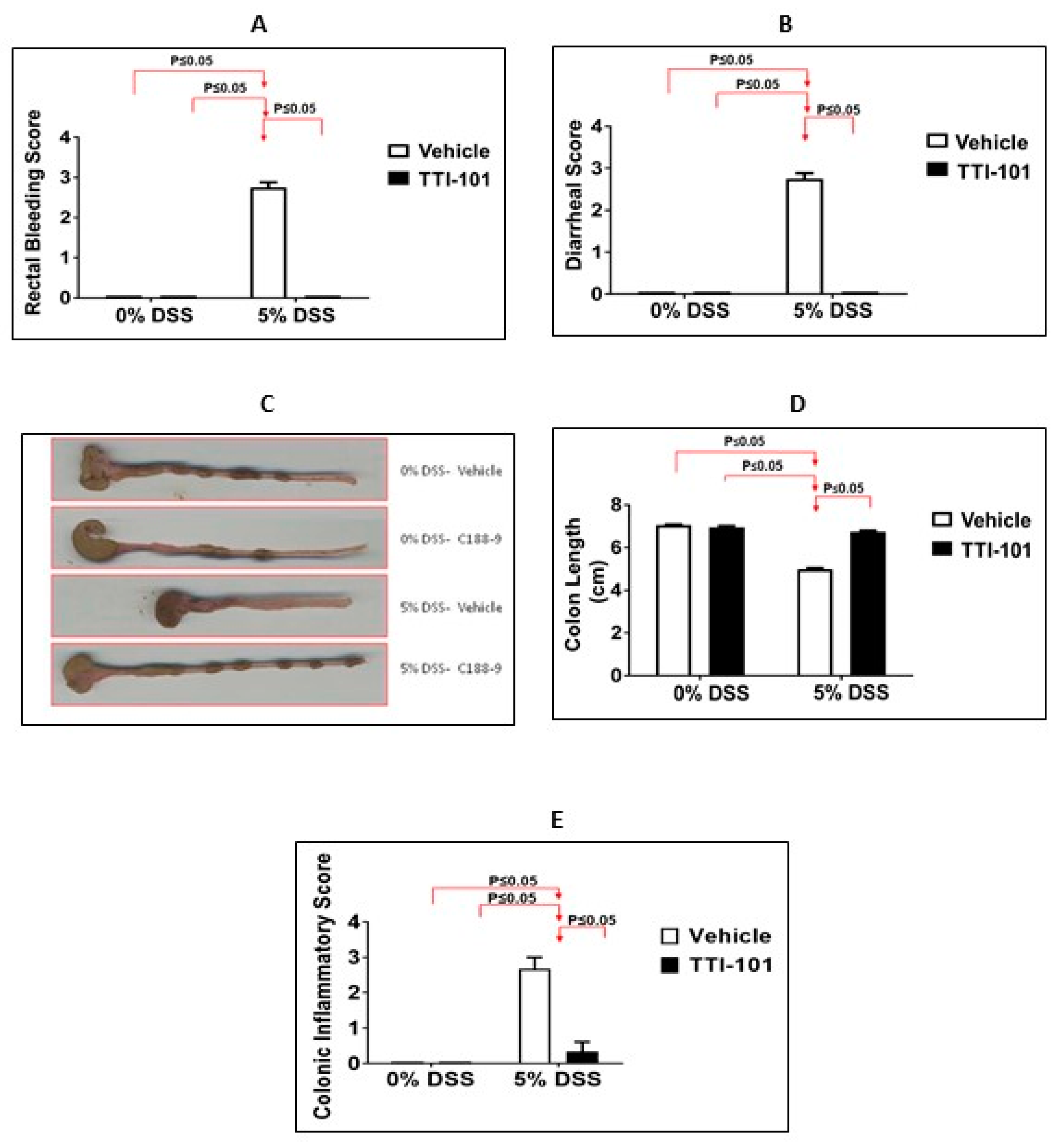
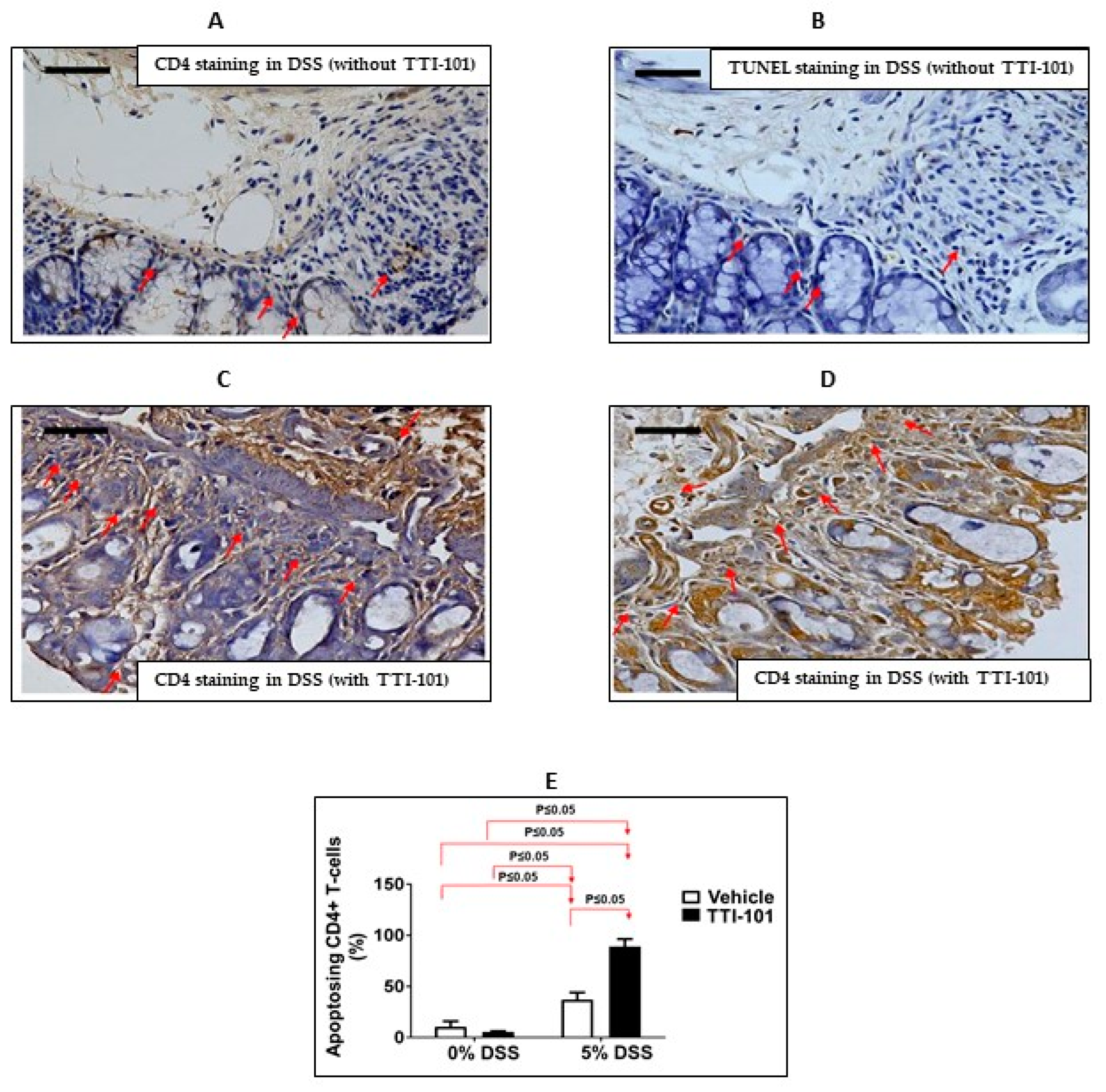
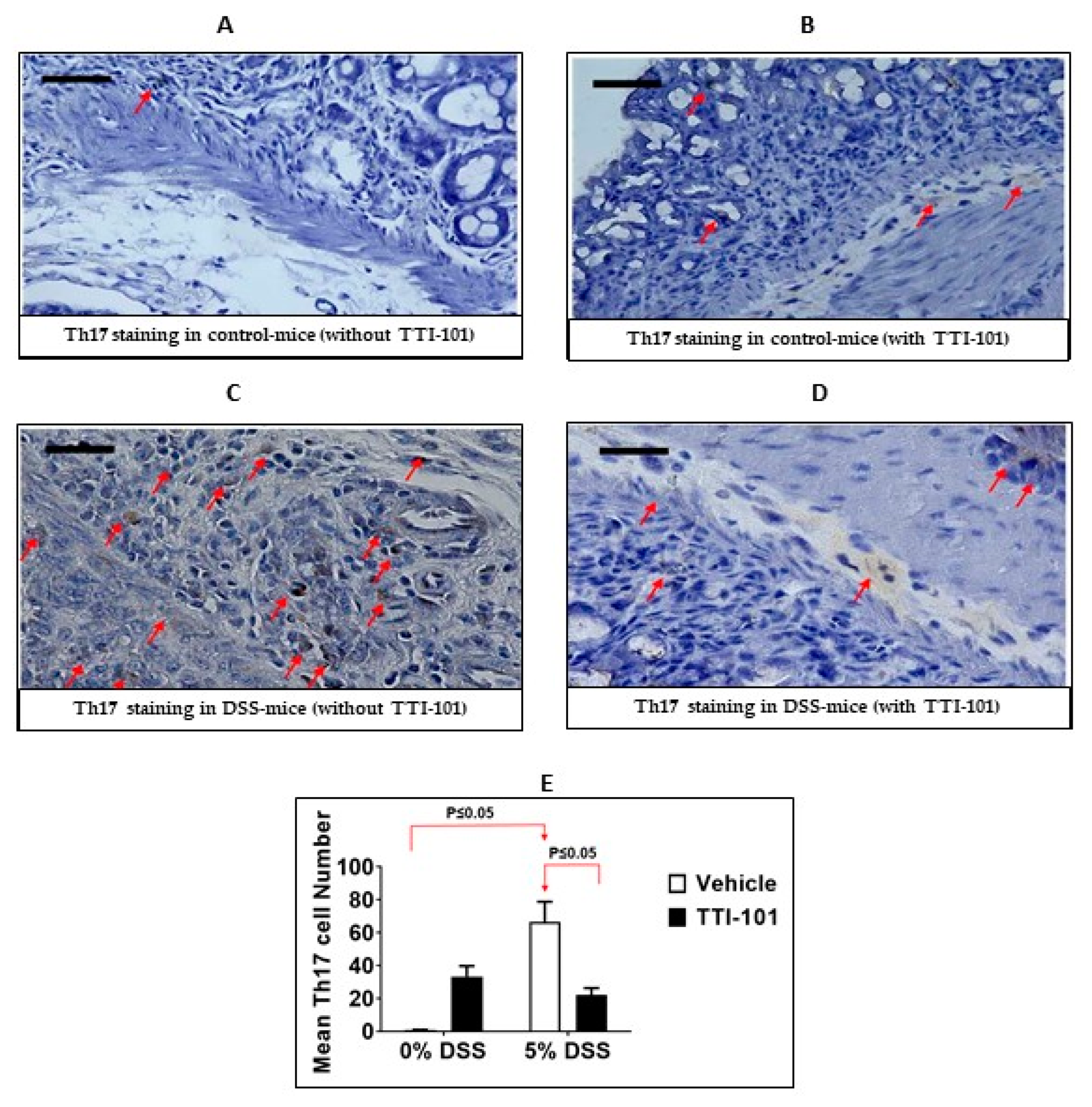
3.2. Effects of TTI-101 Treatment on DSS-Induced Colitis
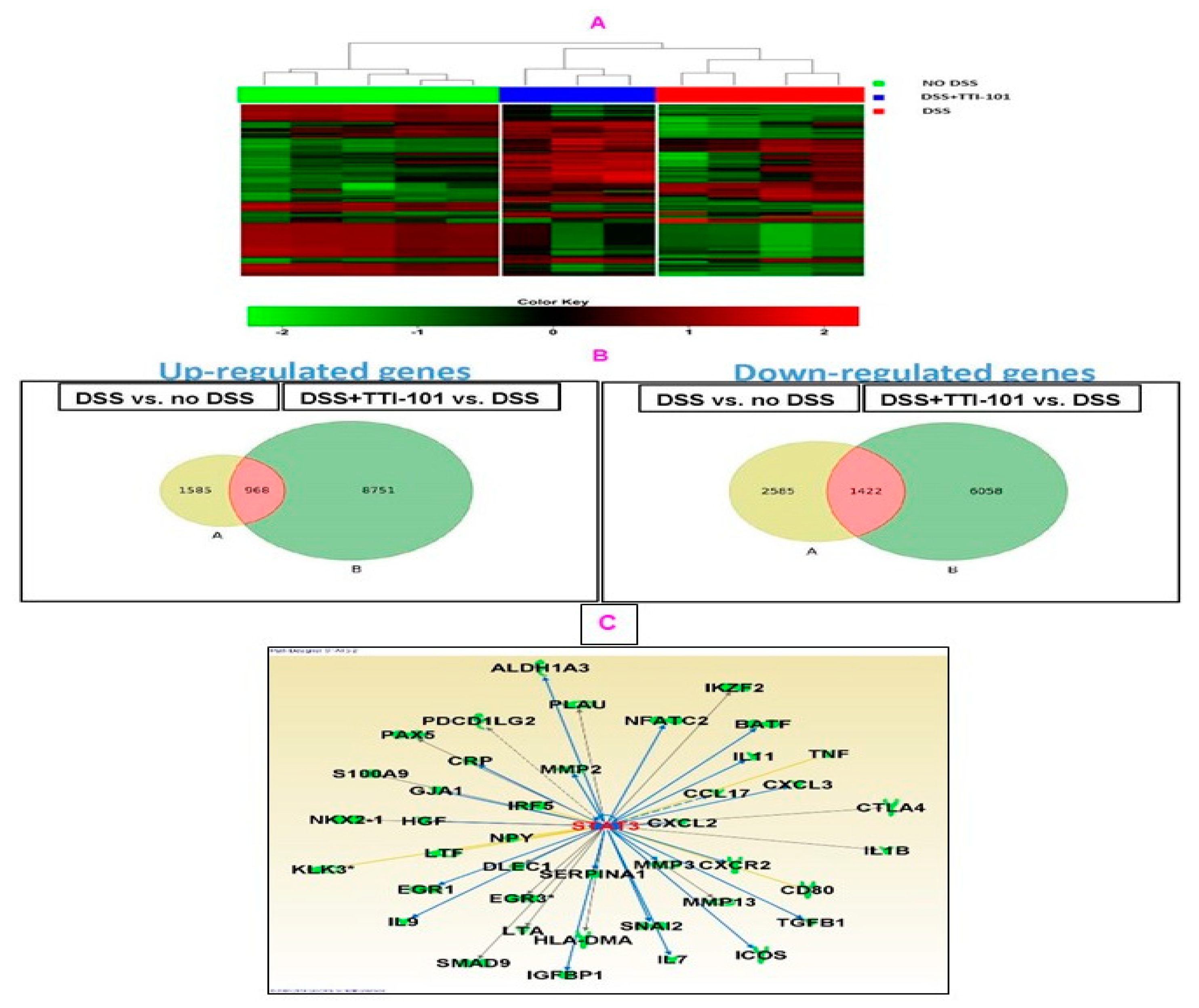
3.3. Effects of TTI-101 Treatment on Colon mRNA Levels
4. Discussion
5. General Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Castano-Milla, C.; Chaparro, M.; Gisbert, J.P. Systematic review with meta-analysis: The declining risk of colorectal cancer in ulcerative colitis. Aliment. Pharm. Ther. 2014, 39, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Konishi, T.; Kishimoto, J.; Kotake, K.; Muto, T.; Sugihara, K.; Japanese Society for Cancer of the Colon and Rectum. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: A nationwide Japanese study. Inflamm. Bowel Dis. 2011, 17, 802–808. [Google Scholar] [CrossRef]
- Lees, C.W.; Barrett, J.C.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef]
- Chapman, R.S.; Lourenco, P.C.; Tonner, E.; Flint, D.J.; Selbert, S.; Takeda, K.; Akira, S.; Clarke, A.R.; Watson, C.J. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999, 13, 2604–2616. [Google Scholar] [CrossRef]
- Atreya, R.; Mudter, J.; Finotto, S.; Mullberg, J.; Jostock, T.; Wirtz, S.; Schutz, M.; Bartsch, B.; Holtmann, M.; Becker, C.; et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000, 6, 583–588. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshizaki, K.; Kishimoto, T.; Ito, H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 2000, 164, 4878–4882. [Google Scholar] [CrossRef]
- Suzuki, A.; Hanada, T.; Mitsuyama, K.; Yoshida, T.; Kamizono, S.; Hoshino, T.; Kubo, M.; Yamashita, A.; Okabe, M.; Takeda, K.; et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 2001, 193, 471–481. [Google Scholar] [CrossRef]
- Lovato, P.; Brender, C.; Agnholt, J.; Kelsen, J.; Kaltoft, K.; Svejgaard, A.; Eriksen, K.W.; Woetmann, A.; Odum, N. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J. Biol. Chem. 2003, 278, 16777–16781. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Weigmann, B.; Bartsch, B.; Kiesslich, R.; Strand, D.; Galle, P.R.; Lehr, H.A.; Schmidt, J.; Neurath, M.F. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005, 100, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Musso, A.; Dentelli, P.; Carlino, A.; Chiusa, L.; Repici, A.; Sturm, A.; Fiocchi, C.; Rizzetto, M.; Pegoraro, L.; Sategna-Guidetti, C.; et al. Signal transducers and activators of transcription 3 signaling pathway: An essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm. Bowel Dis. 2005, 11, 91–98. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. Signaling molecules: The pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr. Drug Targets 2008, 9, 369–374. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Yoshida, N.; Takeda, J.; Kishimoto, T.; Akira, S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: Generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 1998, 161, 4652–4660. [Google Scholar] [CrossRef]
- Chakraborty, A.; White, S.M.; Schaefer, T.S.; Ball, E.D.; Dyer, K.F.; Tweardy, D.J. Granulocyte colony-stimulating factor activation of Stat3 alpha and Stat3 beta in immature normal and leukemic human myeloid cells. Blood 1996, 88, 2442–2449. [Google Scholar] [CrossRef]
- Caldenhoven, E.; van Dijk, T.B.; Solari, R.; Armstrong, J.; Raaijmakers, J.A.; Lammers, J.W.; Koenderman, L.; de Groot, R.P. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J. Biol. Chem. 1996, 271, 13221–13227. [Google Scholar] [CrossRef]
- Takeda, K.; Noguchi, K.; Shi, W.; Tanaka, T.; Matsumoto, M.; Yoshida, N.; Kishimoto, T.; Akira, S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 1997, 94, 3801–3804. [Google Scholar] [CrossRef] [PubMed]
- Maritano, D.; Sugrue, M.L.; Tininini, S.; Dewilde, S.; Strobl, B.; Fu, X.; Murray-Tait, V.; Chiarle, R.; Poli, V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat. Immunol. 2004, 5, 401–409. [Google Scholar] [CrossRef]
- Moran, A.; Tsimelzon, A.I.; Mastrangelo, M.A.; Wu, Y.; Yu, B.; Hilsenbeck, S.G.; Poli, V.; Tweardy, D.J. Prevention of trauma/hemorrhagic shock-induced lung apoptosis by IL-6-mediated activation of Stat3. Clin. Transl. Sci. 2009, 2, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Orecchia, V.; Regis, G.; Musteanu, M.; Tassone, B.; Jon, C.; Forni, M.; Calautti, E.; Chiarle, R.; Eferl, R.; et al. STAT3beta controls inflammatory responses and early tumor onset in skin and colon experimental cancer models. Am. J. Cancer Res. 2014, 4, 484–494. [Google Scholar]
- Smith, P.; Mangan, N.E.; Walsh, C.M.; Fallon, R.E.; McKenzie, A.N.; van Rooijen, N.; Fallon, P.G. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 2007, 178, 4557–4566. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Eckols, T.K.; Xu, X.; Kasembeli, M.M.; Chen, Y.; Adachi, M.; Song, Y.; Mo, Q.; Lai, S.Y.; Tweardy, D.J. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget 2016, 7, 26307–26330. [Google Scholar] [CrossRef] [PubMed]
- Kasembeli, M.M.; Singhmar, P.; Ma, J.; Edralin, J.; Tang, Y.; Adams, C.; 3rd Heijnen, C.J.; Kavelaars, A.; Tweardy, D.J. TTI-101: A competitive inhibitor of STAT3 that spares oxidative phosphorylation and reverses mechanical allodynia in mouse models of neuropathic pain. Biochem. Pharmacol. 2021, 192, 114688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Dong, Y.; Chen, Z.; Eckols, T.K.; Kasembeli, M.M.; Tweardy, D.J.; Mitch, W.E. Pharmacokinetics and pharmacodynamics of TTI-101, a STAT3 inhibitor that blocks muscle proteolysis in rats with chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2020, 319, F84–F92. [Google Scholar] [CrossRef]
- Kettner, N.M.; Vijayaraghavan, S.; Durak, M.G.; Bui, T.; Kohansal, M.; Ha, M.J.; Liu, B.; Rao, X.; Wang, J.; Yi, M.; et al. Combined Inhibition of STAT3 and DNA Repair in Palbociclib-Resistant ER-Positive Breast Cancer. Clin. Cancer Res. 2019, 25, 3996–4013. [Google Scholar] [CrossRef]
- Robinson, P.; Magness, E.; Montoya, K.; Engineer, N.; Eckols, T.K.; Rodriguez, E.; Tweardy, D.J. Genetic and Small-Molecule Modulation of Stat3 in a Mouse Model of Crohn’s Disease. J. Clin. Med. 2022, 11, 7020. [Google Scholar] [CrossRef]
- Hoffman, K.A.; Villar, M.J.; Poveda, C.; Bottazzi, M.E.; Hotez, P.J.; Tweardy, D.J.; Jones, K.M. Signal Transducer and Activator of Transcription-3 Modulation of Cardiac Pathology in Chronic Chagasic Cardiomyopathy. Front. Cell Infect. Microbiol. 2021, 11, 708325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Z.; Wang, Y.; Tweardy, D.J.; Mitch, W.E. Stat3 activation induces insulin resistance via a muscle-specific E3 ubiquitin ligase Fbxo40. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E625–E635. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Yoo, W.; Stevenson, H.L.; Deshpande, D.; Shen, H.; Gagea, M.; Yoo, S.Y.; Wang, J.; Eckols, T.K.; Bharadwaj, U.; et al. Multifunctional Effects of a Small-Molecule STAT3 Inhibitor on NASH and Hepatocellular Carcinoma in Mice. Clin. Cancer Res. 2017, 23, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Bharadwaj, U.; Eckols, T.K.; Kolosov, M.; Kasembeli, M.M.; Fridley, C.; Siller, R.; Tweardy, D.J. Small-molecule targeting of signal transducer and activator of transcription (STAT) 3 to treat non-small cell lung cancer. Lung Cancer 2015, 90, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Gavino, A.C.; Nahmod, K.; Bharadwaj, U.; Makedonas, G.; Tweardy, D.J. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy 2016, 71, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Redell, M.S.; Ruiz, M.J.; Alonzo, T.A.; Gerbing, R.B.; Tweardy, D.J. Stat3 signaling in acute myeloid leukemia: Ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood 2011, 117, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kasembeli, M.M.; Jiang, X.; Tweardy, B.J.; Tweardy, D.J. Chemical probes that competitively and selectively inhibit Stat3 activation. PLoS ONE 2009, 4, e4783. [Google Scholar] [CrossRef]
- Steward-Tharp, S.M.; Laurence, A.; Kanno, Y.; Kotlyar, A.; Villarino, A.V.; Sciume, G.; Kuchen, S.; Resch, W.; Wohlfert, E.A.; Jiang, K.; et al. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood 2014, 123, 2978–2987. [Google Scholar] [CrossRef]
- Durant, L.; Watford, W.T.; Ramos, H.L.; Laurence, A.; Vahedi, G.; Wei, L.; Takahashi, H.; Sun, H.W.; Kanno, Y.; Powrie, F.; et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 2010, 32, 605–615. [Google Scholar] [CrossRef]
- Riedl, J.M.; Posch, F.; Moik, F.; Bezan, A.; Szkandera, J.; Smolle, M.A.; Kasparek, A.K.; Pichler, M.; Stoger, H.; Stotz, M.; et al. Inflammatory biomarkers in metastatic colorectal cancer: Prognostic and predictive role beyond the first line setting. Oncotarget 2017, 8, 96048–96061. [Google Scholar] [CrossRef]
- Doll, D.; Keller, L.; Maak, M.; Boulesteix, A.L.; Siewert, J.R.; Holzmann, B.; Janssen, K.P. Differential expression of the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and their impact on metastatic disease and survival. Int. J. Colorectal. Dis. 2010, 25, 573–581. [Google Scholar] [CrossRef]
- Li, A.; Varney, M.L.; Singh, R.K. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin. Exp. Metastasis 2004, 21, 571–579. [Google Scholar] [CrossRef]
- Zuo, X.; Xu, W.; Xu, M.; Tian, R.; Moussalli, M.J.; Mao, F.; Zheng, X.; Wang, J.; Morris, J.S.; Gagea, M.; et al. Metastasis regulation by PPARD expression in cancer cells. JCI Insight 2017, 2, e91419. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zhou, Q.Y.; Hu, Y.; Wen, Y.; Qiu, Z.W.; Liang, M.G.; Mo, J.L.; Xu, J.H.; Sun, C.; Liu, F.B.; et al. Hepatocyte growth factor is a prognostic marker in patients with colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 23459–23469. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Cespedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xiao, N.; Wang, J.; Wang, Z.; Zheng, S.; Shan, S.; Wang, J.; Du, J.; Wang, J. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett. 2017, 385, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, J.; Li, P.; Wang, L.; Yang, H.; Jiang, G. C/EBPbeta Promotion of MMP3-Dependent Tumor Cell Invasion and Association with Metastasis in Colorectal Cancer. Genet. Test. Mol. Biomark. 2018, 22, 5–10. [Google Scholar] [CrossRef]
- Mendonsa, A.M.; VanSaun, M.N.; Ustione, A.; Piston, D.W.; Fingleton, B.M.; Gorden, D.L. Host and tumor derived MMP13 regulate extravasation and establishment of colorectal metastases in the liver. Mol. Cancer 2015, 14, 49. [Google Scholar] [CrossRef]
- Ji, H.; Greening, D.W.; Barnes, T.W.; Lim, J.W.; Tauro, B.J.; Rai, A.; Xu, R.; Adda, C.; Mathivanan, S.; Zhao, W.; et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 2013, 13, 1672–1686. [Google Scholar] [CrossRef]
- Yang, P.; Chen, T.; Xu, Z.; Zhu, H.; Wang, J.; He, Z. Long noncoding RNA GAPLINC promotes invasion in colorectal cancer by targeting SNAI2 through binding with PSF and NONO. Oncotarget 2016, 7, 42183–42194. [Google Scholar] [CrossRef]
- Feng, J.; Song, D.; Jiang, S.; Yang, X.; Ding, T.; Zhang, H.; Luo, J.; Liao, J.; Yin, Q. Quercetin restrains TGF-beta1-induced epithelial-mesenchymal transition by inhibiting Twist1 and regulating E-cadherin expression. Biochem. Biophys. Res. Commun. 2018, 498, 132–138. [Google Scholar] [CrossRef]
- Jiao, S.F.; Sun, K.; Chen, X.J.; Zhao, X.; Cai, N.; Liu, Y.J.; Xu, L.M.; Kong, X.M.; Wei, L.X. Inhibition of tumor necrosis factor alpha reduces the outgrowth of hepatic micrometastasis of colorectal tumors in a mouse model of liver ischemia-reperfusion injury. J. Biomed. Sci. 2014, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.E.; Colon-Gonzalez, F.; Blomain, E.; Kim, G.W.; Aing, A.; Stoecker, B.; Rock, J.; Snook, A.E.; Zhan, T.; Hyslop, T.M.; et al. Obesity-Induced Colorectal Cancer Is Driven by Caloric Silencing of the Guanylin-GUCY2C Paracrine Signaling Axis. Cancer Res. 2016, 76, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Heijink, D.M.; Fehrmann, R.S.; de Vries, E.G.; Koornstra, J.J.; Oosterhuis, D.; van der Zee, A.G.; Kleibeuker, J.H.; de Jong, S. A bioinformatical and functional approach to identify novel strategies for chemoprevention of colorectal cancer. Oncogene 2011, 30, 2026–2036. [Google Scholar] [CrossRef]
- Hinkel, I.; Duluc, I.; Martin, E.; Guenot, D.; Freund, J.N.; Gross, I. Cdx2 controls expression of the protocadherin Mucdhl, an inhibitor of growth and beta-catenin activity in colon cancer cells. Gastroenterology 2012, 142, 875–885.e3. [Google Scholar] [CrossRef]
- Dougherty, U.; Mustafi, R.; Sadiq, F.; Almoghrabi, A.; Mustafi, D.; Kreisheh, M.; Sundaramurthy, S.; Liu, W.; Konda, V.J.; Pekow, J.; et al. The renin-angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer. Clin. Cancer Res. 2014, 20, 5848–5859. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Nagata, N.; Kuroda, Y.; Itoh, H.; Kawahara, A.; Kuroki, N.; Ookuma, R.; Bell, D.A. Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genetic polymorphism and susceptibility to gastric and colorectal adenocarcinoma. Carcinogenesis 1996, 17, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.J.; Rerko, R.M.; Yan, M.; Platzer, P.; Guda, K.; Dotson, A.; Lawrence, E.; Dannenberg, A.J.; Lovgren, A.K.; Luo, G.; et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12098–12102. [Google Scholar] [CrossRef]
- Girnun, G.D.; Smith, W.M.; Drori, S.; Sarraf, P.; Mueller, E.; Eng, C.; Nambiar, P.; Rosenberg, D.W.; Bronson, R.T.; Edelmann, W.; et al. APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc. Natl. Acad. Sci. USA 2002, 99, 13771–13776. [Google Scholar] [CrossRef]
- Ziegler, P.K.; Bollrath, J.; Pallangyo, C.K.; Matsutani, T.; Canli, O.; De Oliveira, T.; Diamanti, M.A.; Muller, N.; Gamrekelashvili, J.; Putoczki, T.; et al. Mitophagy in Intestinal Epithelial Cells Triggers Adaptive Immunity during Tumorigenesis. Cell 2018, 174, 88–101.e16. [Google Scholar] [CrossRef]
- Poffenberger, M.C.; Metcalfe-Roach, A.; Aguilar, E.; Chen, J.; Hsu, B.E.; Wong, A.H.; Johnson, R.M.; Flynn, B.; Samborska, B.; Ma, E.H.; et al. LKB1 deficiency in T cells promotes the development of gastrointestinal polyposis. Science 2018, 361, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Heddergott, R.; Stark, N.; Edmunds, S.J.; Li, J.; Conradi, L.C.; Bohnenberger, H.; Ceteci, F.; Greten, F.R.; Dobbelstein, M.; Moll, U.M. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell 2018, 34, 298–314.e7. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Entrez Gene Name | DSS vs. No DSS | DSS + TTI-101 vs. DSS | Inflammation | T-Cell Apoptosis | Metastases |
|---|---|---|---|---|---|---|
| ALDH1A3 | aldehyde dehydrogenase 1 family member A3 | 1.64 | −2.12 | PRO-INF | No Role | No Role |
| BATF | basic leucine zipper ATF-like transcription factor | 1.39 | −1.63 | PRO-INF | No Role | No Role |
| CCL17 | C-C motif chemokine ligand 17 | 1.59 | −1.55 | PRO-INF | No Role | No Role |
| CRP | C-reactive protein | 1.22 | −1.25 | PRO-INF | ANTI-APOP | PRO-MET |
| CTLA4 | cytotoxic T-lymphocyte associated protein 4 | 1.18 | −1.37 | ANTI-INF | PRO-APOP | PRO-MET |
| CXCL2 | C-X-C motif chemokine ligand 2 | 1.91 | −1.82 | PRO-INF | ANTI-APOP | PRO-MET |
| CXCL3 | C-X-C motif chemokine ligand 3 | 1.3 | −1.47 | PRO-INF | No Role | PRO-MET |
| CXCR2 | C-X-C motif chemokine receptor 2 | 1.52 | −1.19 | PRO-INF | No Role | PRO-MET |
| DLEC1 | deleted in lung and esophageal cancer 1 | 1.26 | −1.45 | No Role | No Role | No Role |
| EGR3 | early growth response 3 | 1.4 | −2.12 | ANTI-INF | No Role | Anti-MET |
| EGR1 | early growth response 1 | 1.57 | −1.65 | PRO-INF | ANTI-APOP | Anti-MET |
| GJA1 | gap junction protein alpha 1 | 1.19 | −1.19 | PRO-INF | No Role | PRO-MET |
| HGF | hepatocyte growth factor | 1.39 | −1.35 | PRO-INF | No Role | PRO-MET |
| HLA-DMA | major histocompatibility complex, class II, DM alpha | 1.26 | −1.45 | No Role | No Role | No Role |
| ICOS | inducible T-cell costimulator | 1.16 | −1.28 | PRO-INF | ANTI-APOP | No Role |
| IGFBP1 | insulin-like growth factor binding protein 1 | 1.39 | −1.41 | PRO-INF | No Role | No Role |
| IKZF2 | IKAROS family zinc finger 2 | 1.21 | −1.27 | PRO-INF | ANTI-APOP | No Role |
| IL7 | interleukin 7 | 1.42 | −1.22 | PRO-INF | ANTI-APOP | No Role |
| IL9 | interleukin 9 | 1.48 | −1.41 | PRO-INF | ANTI-APOP | No Role |
| IL11 | interleukin 11 | 1.18 | −1.42 | PRO-INF | ANTI-APOP | PRO-MET |
| IL1B | interleukin 1 beta | 1.59 | −1.34 | PRO-INF | ANTI-APOP | No Role |
| LTA | lymphotoxin alpha | 1.43 | −1.71 | PRO-INF | No Role | Anti-MET |
| MMP2 | matrix metallopeptidase 2 | 1.32 | −1.24 | PRO-INF | No Role | PRO-MET |
| MMP3 | matrix metallopeptidase 3 | 2.48 | −2.17 | PRO-INF | No Role | PRO-MET |
| MMP13 | matrix metallopeptidase 13 | 1.58 | −1.73 | PRO-INF | No Role | PRO-MET |
| NFATC2 | nuclear factor of activated T-cells 2 | 1.18 | −1.42 | PRO-INF | ANTI-APOP | No Role |
| NKX2-1 | NK2 homeobox 1 | 1.31 | −1.99 | ANTI-INF | No Role | No Role |
| PDCD1LG2 | programmed cell death 1 ligand 2 | 1.29 | −1.46 | ANTI-INF | ANTI-APOP | No Role |
| PLAU | plasminogen activator, urokinase | 1.38 | −1.58 | PRO-INF | No Role | No Role |
| PAX5 | paired box 5 | 1.34 | −2.34 | No Role | No Role | No Role |
| S100A9 | S100 calcium binding protein A9 | 1.58 | −1.71 | PRO-INF | No Role | PRO-MET |
| SERPINA1 | serpin family A member 1 | 1.3 | −1.47 | PRO-INF | No Role | No Role |
| SMAD9 | SMAD family member 9 | 1.23 | −1.38 | PRO/ANTI-INF | No Role | No Role |
| SNAI2 | snail family transcriptional repressor 2 | 1.34 | −1.12 | PRO-INF | No Role | PRO-MET |
| TGFB1 | transforming growth factor beta 1 | 1.22 | −1.33 | PRO-INF/ANTI-INF | ANTI-APOP | PRO-MET |
| Symbol | Entrez Gene Name | DSS vs. No DSS | DSS + TTI-101 vs. DSS |
|---|---|---|---|
| ALB | albumin | 1.21 | −1.31 |
| ATP4B | ATPase H+/K+ transporting beta subunit | 1.48 | −2.2 |
| BPI | bactericidal/permeability-increasing protein | 1.15 | −1.4 |
| C19orf57 | chromosome 19 open reading frame 57 | 1.38 | −1.65 |
| CCL17 | C-C motif chemokine ligand 17 | 1.59 | −1.55 |
| CD80 | CD80 molecule | 1.21 | −1.34 |
| CRP | C-reactive protein | 1.22 | −1.25 |
| CSF2RB | colony stimulating factor 2 receptor beta common subunit | 1.85 | −1.48 |
| CXCL2 | C-X-C motif chemokine ligand 2 | 1.91 | −1.82 |
| CXCL3 | C-X-C motif chemokine ligand 3 | 1.3 | −1.47 |
| GABRB2 | gamma-aminobutyric acid type A receptor beta2 subunit | 1.24 | −1.38 |
| GABRR2 | gamma-aminobutyric acid type A receptor rho2 subunit | 1.28 | −1.65 |
| GABRR3 | gamma-aminobutyric acid type A receptor rho3 subunit (gene/pseudogene) | 1.27 | −1.49 |
| GIPR | gastric inhibitory polypeptide receptor | 1.22 | −1.56 |
| GPER1 | G protein-coupled estrogen receptor 1 | 1.38 | −1.61 |
| GRIN2D | glutamate ionotropic receptor NMDA type subunit 2D | 1.35 | −1.78 |
| ICOSLG/LOC102723996 | inducible T-cell costimulator ligand | 1.26 | −1.22 |
| IL7 | interleukin 7 | 1.42 | −1.22 |
| IL25 | interleukin 25 | 1.44 | −1.68 |
| IL1B | interleukin 1 beta | 1.59 | −1.34 |
| IL1RL1 | interleukin 1 receptor like 1 | 1.24 | −1.35 |
| IL3RA | interleukin 3 receptor subunit alpha | 1.43 | −1.89 |
| IRF5 | interferon regulatory factor 5 | 1.21 | −1.38 |
| LTF | lactotransferrin | 1.45 | −1.59 |
| LY6E | lymphocyte antigen 6 family member E | 1.24 | −1.33 |
| MMP2 | matrix metallopeptidase 2 | 1.32 | −1.24 |
| ORMDL3 | ORMDL sphingolipid biosynthesis regulator 3 | 1.3 | −1.69 |
| PDCD1LG2 | programmed cell death 1 ligand 2 | 1.29 | −1.46 |
| PPP2R2B | protein phosphatase 2 regulatory subunit Bbeta | 1.17 | −1.19 |
| SLC24A4 | solute carrier family 24 members 4 | 1.39 | −1.47 |
| SLC6A3 | solute carrier family 6 members 3 | 1.12 | −1.28 |
| SLPI | secretory leukocyte peptidase inhibitor | 1.78 | −2.06 |
| SNX20 | sorting nexin 20 | 1.57 | −2 |
| TGFB1 | transforming growth factor beta 1 | 1.22 | −1.33 |
| TNF | tumor necrosis factor | 1.29 | −1.43 |
| TNFSF8 | TNF superfamily member 8 | 1.42 | −1.72 |
| TREM1 | triggering receptor expressed on myeloid cells 1 | 1.32 | −1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, P.; Montoya, K.; Magness, E.; Rodriguez, E.; Villalobos, V.; Engineer, N.; Yang, P.; Bharadwaj, U.; Eckols, T.K.; Tweardy, D.J. Therapeutic Potential of a Small-Molecule STAT3 Inhibitor in a Mouse Model of Colitis. Cancers 2023, 15, 2977. https://doi.org/10.3390/cancers15112977
Robinson P, Montoya K, Magness E, Rodriguez E, Villalobos V, Engineer N, Yang P, Bharadwaj U, Eckols TK, Tweardy DJ. Therapeutic Potential of a Small-Molecule STAT3 Inhibitor in a Mouse Model of Colitis. Cancers. 2023; 15(11):2977. https://doi.org/10.3390/cancers15112977
Chicago/Turabian StyleRobinson, Prema, Kelsey Montoya, Emily Magness, Emma Rodriguez, Viviana Villalobos, Nikita Engineer, Peng Yang, Uddalak Bharadwaj, Thomas Kris Eckols, and David John Tweardy. 2023. "Therapeutic Potential of a Small-Molecule STAT3 Inhibitor in a Mouse Model of Colitis" Cancers 15, no. 11: 2977. https://doi.org/10.3390/cancers15112977






