Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Preparation of Peripheral Blood-Derived Macrophages
2.3. Direct and Indirect Co-Culture Systems
2.4. cDNA Microarray
2.5. Cytokine Array
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Western Blot
2.8. STAT3 mRNA Knockdown Using Small Interfering (si) RNA
2.9. Transwell Migration and Invasion Assays
2.10. Enzyme Linked Immunosorbent Assay (ELISA)
2.11. Gelatin Gel Zymography
2.12. ESCC Cell Treatment by Recombinant Human Interleukin-8 (rhIL-8) or S100-Calcium Binding Protein A8/A9 (rhS100A8/A9)
2.13. Phosphoinositide 3-Kinase (PI3K) or p38 Mitogen-Activated Protein Kinase (MAPK) Inhibition Assays
2.14. Tissue Samples and Immunohistochemistry
2.15. Statistical Analysis
3. Results
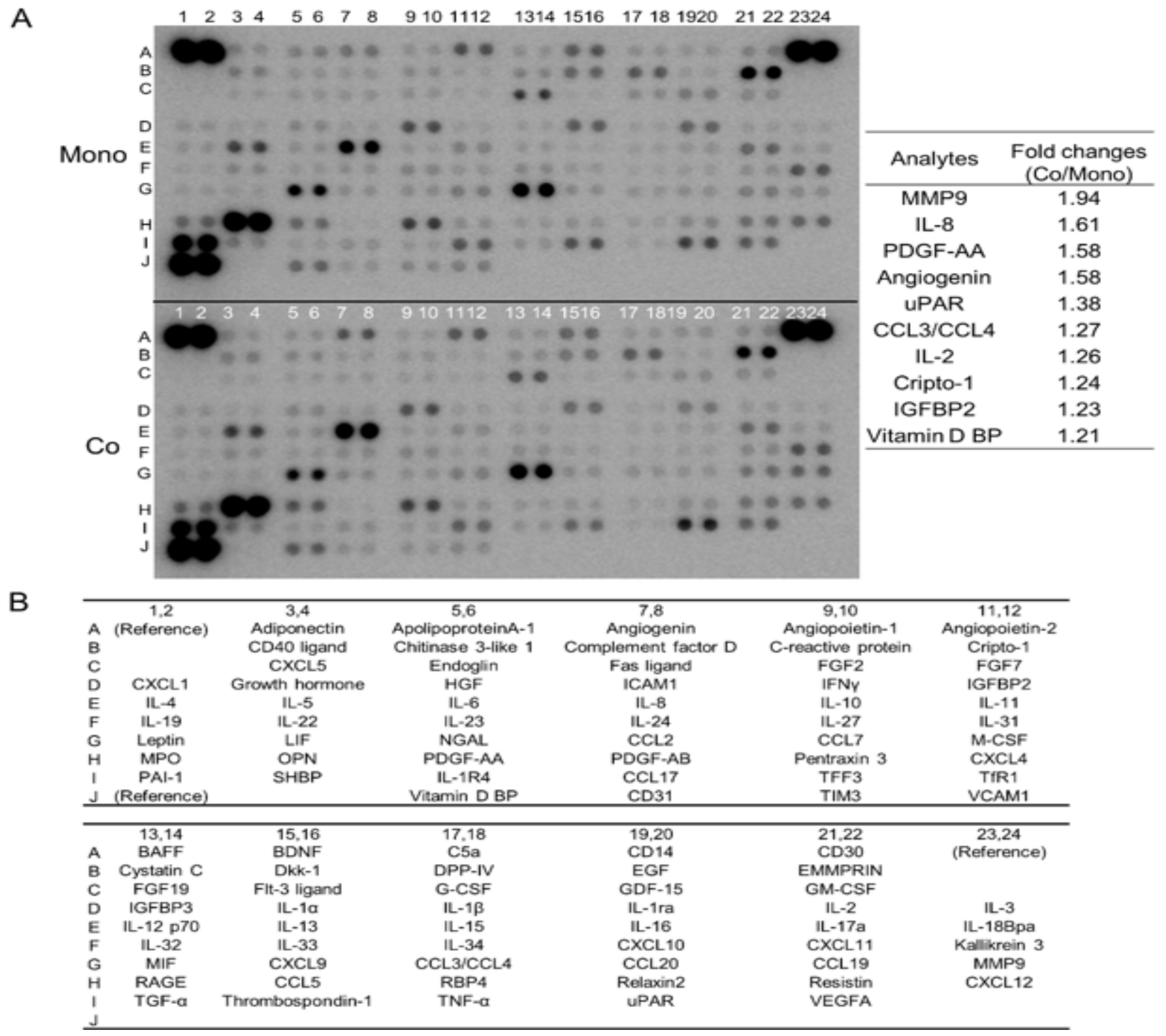
3.1. Direct Co-Culture with Macrophages Induces the Secretion of Multiple Humoral Factors from TE-11 ESCC Cells, including MMP9 and IL-8
3.2. Direct Co-Culture with Macrophages Upregulates MMP9 Gene Transcription and Protein Secretion in ESCC Cells
3.3. Direct Co-Culture with Macrophages Activates the Stat3 Signal Pathway in ESCC Cells, Resulting in MMP9 Upregulation
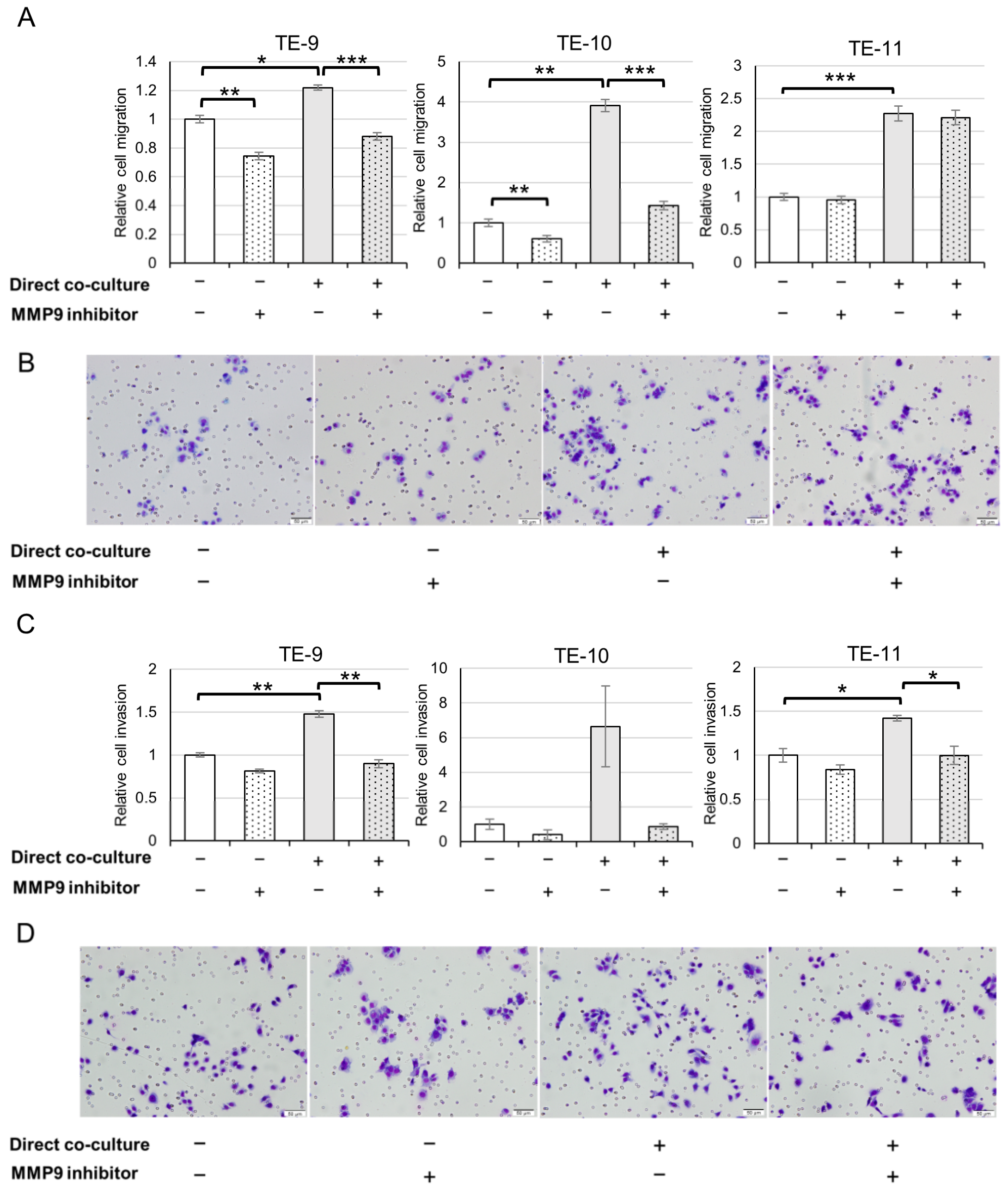
3.4. ESCC Cells Exhibit an Augmented Migration and Invasion following Direct Co-Culture with Macrophages through MMP9 Function
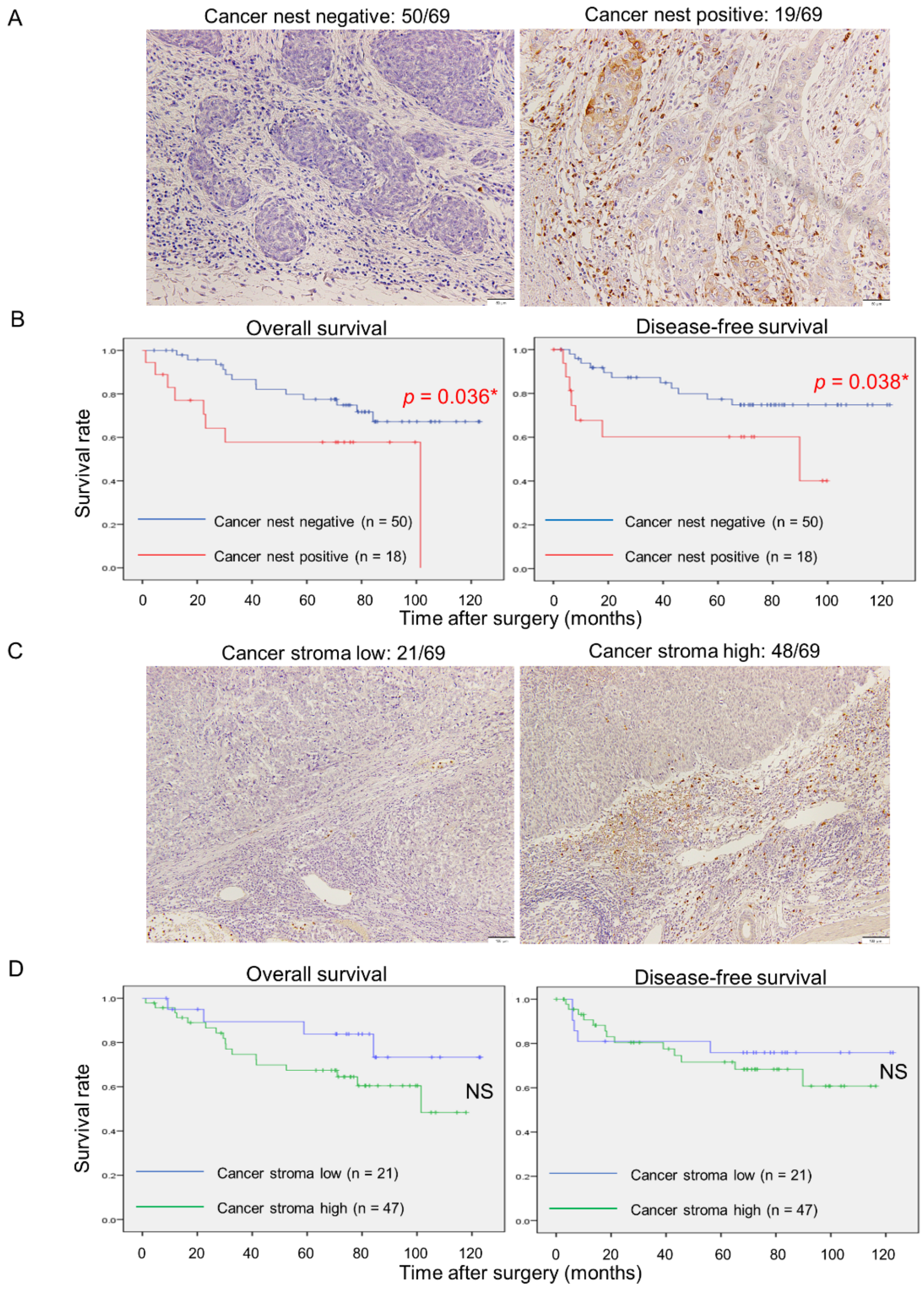
3.5. MMP9 Expression by Cancer Nests in ESCC Tissues Significantly Correlates with Aggressive Clinicopathological Factors and Poor Prognoses
3.6. Direct Co-Culture with Macrophages Also Promotes IL-8 Secretion from ESCC Cells and IL-8 Partially Induces MMP9 Production in ESCC Cells
3.7. S100A8/A9 Also Partially Induces MMP9 Production in ESCC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikh, M.; Roshandel, G.; McCormack, V.; Malekzadeh, R. Current Status and Future Prospects for Esophageal Cancer. Cancers 2023, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#a14 (accessed on 24 February 2023).
- Watanabe, M.; Toh, Y.; Ishihara, R.; Kono, K.; Matsubara, H.; Miyazaki, T.; Morita, M.; Murakami, K.; Muro, K.; Numasaki, H.; et al. Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus Off. J. Jpn. Esophageal Soc. 2023, 20, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nabeya, Y.; Okazumi, S.-I.; Matsubara, H.; Shiratori, T.; Gunji, Y.; Kobayashi, S.; Hayashi, H.; Ochiai, T. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery 2003, 133, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Shigeoka, M.; Urakawa, N.; Nakamura, T.; Nishio, M.; Watajima, T.; Kuroda, D.; Komori, T.; Kakeji, Y.; Semba, S.; Yokozaki, H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1112–1119. [Google Scholar] [CrossRef]
- Urakawa, N.; Utsunomiya, S.; Nishio, M.; Shigeoka, M.; Takase, N.; Arai, N.; Kakeji, Y.; Koma, Y.-I.; Yokozaki, H. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab. Investig. 2015, 95, 491–503. [Google Scholar] [CrossRef]
- Hosono, M.; Koma, Y.-I.; Takase, N.; Urakawa, N.; Higashino, N.; Suemune, K.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget 2017, 8, 106071–106088. [Google Scholar] [CrossRef]
- Fujikawa, M.; Koma, Y.-I.; Hosono, M.; Urakawa, N.; Tanigawa, K.; Shimizu, M.; Kodama, T.; Sakamoto, H.; Nishio, M.; Shigeoka, M.; et al. Chemokine (C-C Motif) Ligand 1 Derived from Tumor-Associated Macrophages Contributes to Esophageal Squamous Cell Carcinoma Progression via CCR8-Mediated Akt/Proline-Rich Akt Substrate of 40 kDa/Mammalian Target of Rapamycin Pathway. Am. J. Pathol. 2021, 191, 686–703. [Google Scholar] [CrossRef]
- Kodama, T.; Koma, Y.-I.; Arai, N.; Kido, A.; Urakawa, N.; Nishio, M.; Shigeoka, M.; Yokozaki, H. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. 2020, 100, 1140–1157. [Google Scholar] [CrossRef]
- Tanigawa, K.; Tsukamoto, S.; Koma, Y.-I.; Kitamura, Y.; Urakami, S.; Shimizu, M.; Fujikawa, M.; Kodama, T.; Nishio, M.; Shigeoka, M.; et al. S100A8/A9 Induced by Interaction with Macrophages in Esophageal Squamous Cell Carcinoma Promotes the Migration and Invasion of Cancer Cells via Akt and p38 MAPK Pathways. Am. J. Pathol. 2022, 192, 536–552. [Google Scholar] [CrossRef]
- Kodaira, H.; Koma, Y.; Hosono, M.; Higashino, N.; Suemune, K.; Nishio, M.; Shigeoka, M.; Yokozaki, H. ANXA10 induction by interaction with tumor-associated macrophages promotes the growth of esophageal squamous cell carcinoma. Pathol. Int. 2019, 69, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Koma, Y.-I.; Tanigawa, K.; Tsukamoto, S.; Azumi, Y.; Miyako, S.; Urakami, S.; Kodama, T.; Nishio, M.; Shigeoka, M.; et al. Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma. Cancers 2023, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Nishihira, T.; Hashimoto, Y.; Katayama, M.; Mori, S.; Kuroki, T. Molecular and cellular features of esophageal cancer cells. J. Cancer Res. Clin. Oncol. 1993, 119, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; Miller, R.A.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. WikiPathways: Connecting communities. Nucleic Acids Res. 2020, 49, D613–D621. [Google Scholar] [CrossRef] [PubMed]
- Japanese Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part I. Esophagus 2016, 14, 1–36. [Google Scholar] [CrossRef]
- Japanese Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part II and III. Esophagus 2017, 14, 37–65. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Lin, S.-C.; Zhang, M.-Q.; Guo, X.-T.; Ma, K.; Wang, L.-X.; Huang, W.-T.; Wang, Z.; Yu, X.; Wang, C.-G.; et al. Association and prognostic significance of alpha-L-fucosidase-1 and matrix metalloproteinase 9 expression in esophageal squamous cell carcinoma. World J. Gastrointest. Oncol. 2022, 14, 498–510. [Google Scholar] [CrossRef]
- Ohashi, K.; Nemoto, T.; Nakamura, K.; Nemori, R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer 2000, 88, 2201–2209. [Google Scholar] [CrossRef]
- Mukherjee, S.; Roth, M.J.; Dawsey, S.M.; Yan, W.; Rodriguez-Canales, J.; Erickson, H.S.; Hu, N.; Goldstein, A.M.; Taylor, P.R.; Richardson, A.M.; et al. Increased matrix metalloproteinase activation in esophageal squamous cell carcinoma. J. Transl. Med. 2010, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, Y.; Yoshida, T.; Yagawa, T.; Saiki, Y.; Takeo, S.; Harada, T.; Okazawa, T.; Yanai, H.; Okita, K. Matrix metalloproteinase-7 and matrix metalloproteinase-9 are associated with unfavourable prognosis in superficial oesophageal cancer. Br. J. Cancer 2003, 89, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hu, Y.; Zhang, M.-F.; Luo, K.-J.; Xie, X.-Y.; Wen, J.; Fu, J.-H.; Yang, H. MMP1 promotes tumor growth and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2016, 377, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fogg, K.C.; Olson, W.R.; Miller, J.N.; Khan, A.; Renner, C.; Hale, I.; Weisman, P.S.; Kreeger, P.K. Alternatively activated macrophage-derived secretome stimulates ovarian cancer spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett. 2019, 458, 92–101. [Google Scholar] [CrossRef]
- Carroll, M.J.; Kapur, A.; Felder, M.; Patankar, M.S.; Kreeger, P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget 2016, 7, 86608–86620. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, H.; Chang, H.; Feng, Z.; Zhang, C.; Yang, X. The interaction of interleukin-8 and PTEN inactivation promotes the malignant progression of head and neck squamous cell carcinoma via the STAT3 pathway. Cell Death Dis. 2020, 11, 405. [Google Scholar] [CrossRef]
- Yuan, X.; Li, Y.; Zhang, A.Z.; Jiang, C.H.; Li, F.P.; Xie, Y.F.; Li, J.F.; Liang, W.H.; Zhang, H.J.; Liu, C.X.; et al. Tumor-associated macrophage polarization promotes the progression of esophageal carcinoma. Aging 2020, 13, 2049–2072. [Google Scholar] [CrossRef]
- Kamoshida, G.; Matsuda, A.; Miura, R.; Takashima, Y.; Katsura, A.; Tsuji, T. Potentiation of tumor cell invasion by co-culture with monocytes accompanying enhanced production of matrix metalloproteinase and fibronectin. Clin. Exp. Metastasis 2013, 30, 289–297. [Google Scholar] [CrossRef]
- Komohara, Y.; Horlad, H.; Ohnishi, K.; Fujiwara, Y.; Bai, B.; Nakagawa, T.; Suzu, S.; Nakamura, H.; Kuratsu, J.-I.; Takeya, M. Importance of direct macrophage—Tumor cell interaction on progression of human glioma. Cancer Sci. 2012, 103, 2165–2172. [Google Scholar] [CrossRef]
- Umakoshi, M.; Takahashi, S.; Itoh, G.; Kuriyama, S.; Sasaki, Y.; Yanagihara, K.; Yashiro, M.; Maeda, D.; Goto, A.; Tanaka, M. Macrophage-mediated transfer of cancer-derived components to stromal cells contributes to establishment of a pro-tumor microenvironment. Oncogene 2019, 38, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Levenson, A.S.; Satcher, R.L. Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. Int. J. Mol. Med. 2006, 17, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Waltera, A.; Schulz, D.; Schaefer, N.; Stoeckl, S.; Pion, E.; Haerteis, S.; Reichert, T.E.; Ettl, T.; Bauer, R.J. Opposing MMP-9 Expression in Mesenchymal Stromal Cells and Head and Neck Tumor Cells after Direct 2D and 3D Co-Culture. Int. J. Mol. Sci. 2023, 24, 1293. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Komohara, Y.; Tashiro, H.; Ohtake, H.; Nakagawa, T.; Katabuchi, H.; Takeya, M. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010, 101, 2128–2136. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, Y.; Li, X.; Cao, X.; Chen, A.; Xu, C.; Cao, J.; Luo, X. Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. Biomed. Pharmacother. 2018, 103, 262–271. [Google Scholar] [CrossRef]
- Jia, Z.-H.; Jia, Y.; Guo, F.-J.; Chen, J.; Zhang, X.-W.; Cui, M.-H. Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer. PLoS ONE 2017, 12, e0183622. [Google Scholar] [CrossRef]
- Suzuki, T.; Kuwabara, Y.; Iwata, H.; Mitani, M.; Shinoda, N.; Sato, A.; Mitsui, A.; Sugiura, M.; Kato, J.; Fujii, Y. Role of matrix metalloproteinase-9 in in vitro invasion of esophageal carcinoma cells. J. Surg. Oncol. 2002, 81, 80–86. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Sun, P.; Paller, A.S. Ganglioside GM3 Inhibits Matrix Metalloproteinase-9 Activation and Disrupts Its Association with Integrin. J. Biol. Chem. 2003, 278, 25591–25599. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999, 13, 35–48. [Google Scholar] [CrossRef]
- Bannikov, G.A.; Karelina, T.V.; Collier, I.E.; Marmer, B.L.; Goldberg, G.I. Substrate Binding of Gelatinase B Induces Its Enzymatic Activity in the Presence of Intact Propeptide. J. Biol. Chem. 2002, 277, 16022–16027. [Google Scholar] [CrossRef] [PubMed]
- Tadbir, A.A.; Mardani, M.; Pourshahidi, S.; Nezarati, K.; Bahadori, P. Prognostic value of matrix metalloproteinase-9 expression in oral squamous cell carcinoma and its association with angiogenesis. J. Clin. Exp. Dent. 2016, 8, e130–e135. [Google Scholar] [CrossRef]
- Kabashima, A.; Maehara, Y.; Kakeji, Y.; Baba, H.; Koga, T.; Sugimachi, K. Clinicopathological features and overexpression of matrix metalloproteinases in intramucosal gastric carcinoma with lymph node metastasis. Clin. Cancer Res. 2000, 6, 3581–3584. [Google Scholar]
- Joseph, C.; Alsaleem, M.; Orah, N.; Narasimha, P.L.; Miligy, I.M.; Kurozumi, S.; Ellis, I.O.; Mongan, N.; Green, A.R.; Rakha, E.A. Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast Cancer Res. Treat. 2020, 182, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Leite, K.R.M.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Martins, J.M.A.; Rabelo-Santos, S.H.; Westin, M.C.D.A.; Zeferino, L.C. Tumoral and stromal expression of MMP-2, MMP-9, MMP-14, TIMP-1, TIMP-2, and VEGF-A in cervical cancer patient survival: A competing risk analysis. BMC Cancer 2020, 20, 660. [Google Scholar] [CrossRef]
- Sillanpää, S.; Anttila, M.; Voutilainen, K.; Ropponen, K.; Turpeenniemi-Hujanen, T.; Puistola, U.; Tammi, R.; Tammi, M.; Sironen, R.; Saarikoski, S.; et al. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 296–303. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patel, K.D. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J. Leukoc. Biol. 2005, 78, 279–288. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Proost, P.; Wuyts, A.; Van Damme, J.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000, 96, 2673–2681. [Google Scholar] [CrossRef]
- Blanchette-Farra, N.; Kita, D.; Konstorum, A.; Tesfay, L.; Lemler, D.; Hegde, P.; Claffey, K.P.; Torti, F.M.; Torti, S.V. Contribution of three-dimensional architecture and tumor-associated fibroblasts to hepcidin regulation in breast cancer. Oncogene 2018, 37, 4013–4032. [Google Scholar] [CrossRef] [PubMed]




| Number of Cases | Cancer Nest Expression of MMP9 a | p-Value | Stromal Expression of MMP9 b | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Negative (n = 50) | Positive (n = 19) | Low (n = 21) | High (n = 48) | |||||
| Age | ||||||||
| <65 | 32 | 24 | 8 | 0.661 | 10 | 22 | 0.891 | |
| ≥65 | 37 | 26 | 11 | 11 | 26 | |||
| Histological grade c | ||||||||
| CIS + WDSCC | 15 | 12 | 3 | 0.460 | 5 | 10 | 0.783 | |
| MDSCC + PDSCC | 54 | 38 | 16 | 16 | 38 | |||
| Depth of invasion c | ||||||||
| ≤T1 | 49 | 39 | 10 | 0.038 * | 15 | 34 | 0.960 | |
| ≥T2 | 20 | 11 | 9 | 6 | 14 | |||
| Lymphatic vessel invasion c | ||||||||
| Negative | 37 | 29 | 8 | 0.237 | 13 | 24 | 0.362 | |
| Positive | 32 | 21 | 11 | 8 | 24 | |||
| Blood vessel invasion c | ||||||||
| Negative | 43 | 33 | 10 | 0.306 | 15 | 28 | 0.302 | |
| Positive | 26 | 17 | 9 | 6 | 20 | |||
| Lymph node metastasis c | ||||||||
| Negative | 41 | 32 | 9 | 0.209 | 14 | 27 | 0.417 | |
| Positive | 28 | 18 | 10 | 7 | 21 | |||
| Stage d | ||||||||
| ≤I | 38 | 30 | 8 | 0.182 | 13 | 25 | 0.450 | |
| ≥II | 31 | 20 | 11 | 8 | 23 | |||
| CD68 positive cells e | ||||||||
| Low | 35 | 30 | 5 | 0.012 * | 11 | 24 | 0.856 | |
| High | 34 | 20 | 14 | 10 | 24 | |||
| CD163 positive cells e | ||||||||
| Low | 34 | 30 | 4 | 0.004 ** | 11 | 23 | 0.733 | |
| High | 35 | 20 | 15 | 10 | 25 | |||
| CD204 positive cells e | ||||||||
| Low | 34 | 33 | 1 | <0.001 *** | 13 | 21 | 0.165 | |
| High | 35 | 17 | 18 | 8 | 27 | |||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Number of Cases | p-Value | HR | 95% CI | p-Value | ||
| Age | ||||||
| <65 | 32 | 0.377 | ||||
| ≥65 | 36 | |||||
| Histological grade a | ||||||
| CIS + WDSCC | 15 | 0.481 | ||||
| MDSCC + PDSCC | 53 | |||||
| Depth of tumor invasion a | ||||||
| ≤T1 | 48 | <0.001 *** | 4.547 | 0.752–27.502 | 0.010 * | |
| ≥T2 | 20 | |||||
| Lymphatic vessel invasion a | ||||||
| Negative | 37 | 0.001 ** | 1.659 | 0.332–8.298 | 0.711 | |
| Positive | 31 | |||||
| Blood vessel invasion a | ||||||
| Negative | 43 | 0.030 * | 0.865 | 0.260–2.883 | 0.983 | |
| Positive | 25 | |||||
| Lymph node metastasis a | ||||||
| Negative | 43 | <0.001 *** | 0.886 | 0.089–8.813 | 0.061 | |
| Positive | 25 | |||||
| Stage b | ||||||
| ≤I | 38 | <0.001 *** | ||||
| ≥II | 30 | |||||
| CD68 positive cells c | ||||||
| Low | 33 | 0.009 ** | ||||
| High | 35 | |||||
| CD163 positive cells c | ||||||
| Low | 34 | 0.049 * | ||||
| High | 34 | |||||
| CD204 positive cells c | ||||||
| Low | 34 | 0.002 ** | 0.252 | 0.037–1.703 | 0.502 | |
| High | 34 | |||||
| Cancer nest MMP9 d | ||||||
| Negative | 50 | 0.038 * | 4.845 | 1.399–16.681 | 0.026 * | |
| Positive | 18 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukamoto, S.; Koma, Y.-i.; Kitamura, Y.; Tanigawa, K.; Azumi, Y.; Miyako, S.; Urakami, S.; Hosono, M.; Kodama, T.; Nishio, M.; et al. Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis. Cancers 2023, 15, 2987. https://doi.org/10.3390/cancers15112987
Tsukamoto S, Koma Y-i, Kitamura Y, Tanigawa K, Azumi Y, Miyako S, Urakami S, Hosono M, Kodama T, Nishio M, et al. Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis. Cancers. 2023; 15(11):2987. https://doi.org/10.3390/cancers15112987
Chicago/Turabian StyleTsukamoto, Shuichi, Yu-ichiro Koma, Yu Kitamura, Kohei Tanigawa, Yuki Azumi, Shoji Miyako, Satoshi Urakami, Masayoshi Hosono, Takayuki Kodama, Mari Nishio, and et al. 2023. "Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis" Cancers 15, no. 11: 2987. https://doi.org/10.3390/cancers15112987
APA StyleTsukamoto, S., Koma, Y.-i., Kitamura, Y., Tanigawa, K., Azumi, Y., Miyako, S., Urakami, S., Hosono, M., Kodama, T., Nishio, M., Shigeoka, M., & Yokozaki, H. (2023). Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis. Cancers, 15(11), 2987. https://doi.org/10.3390/cancers15112987







