Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients—A Systematic Review from an Ophthalmic Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (1)
- (“ocular ma*” OR “intraocular ma*” “Uveal melanoma” OR “Choroidal melanoma” OR “iris melanoma”) AND (“magnetic resonance imaging [MeSH] OR “MRI”)
3. Uveal Melanoma on MRI
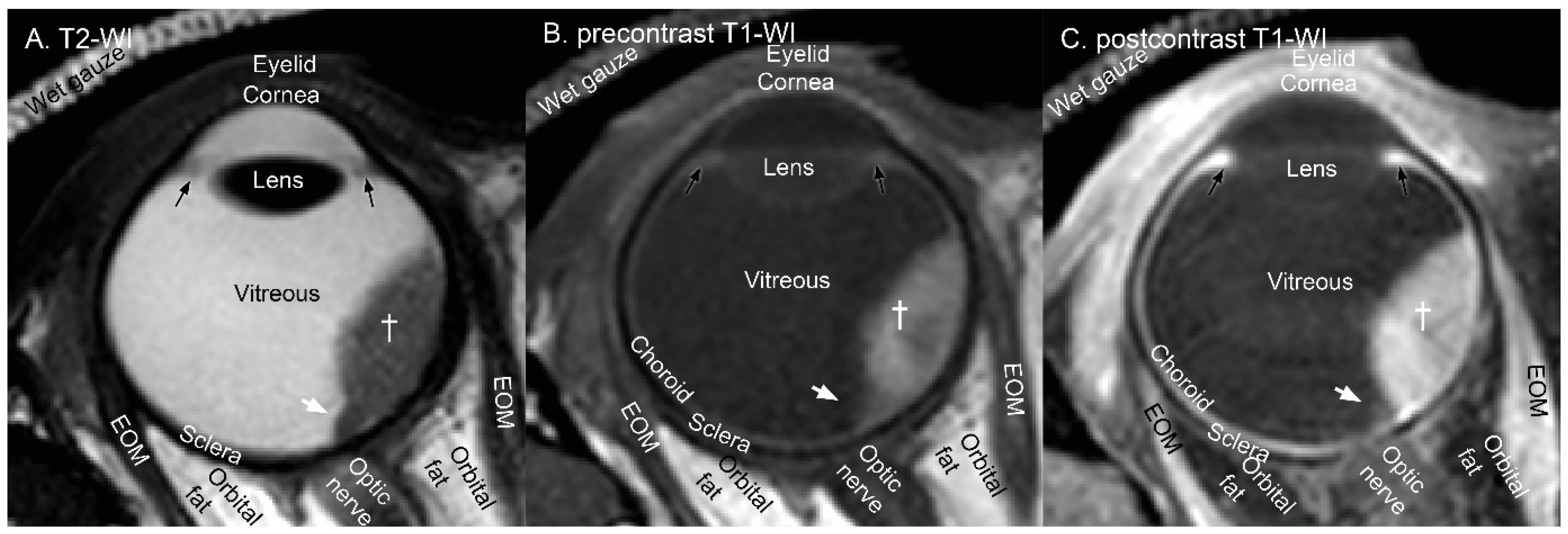
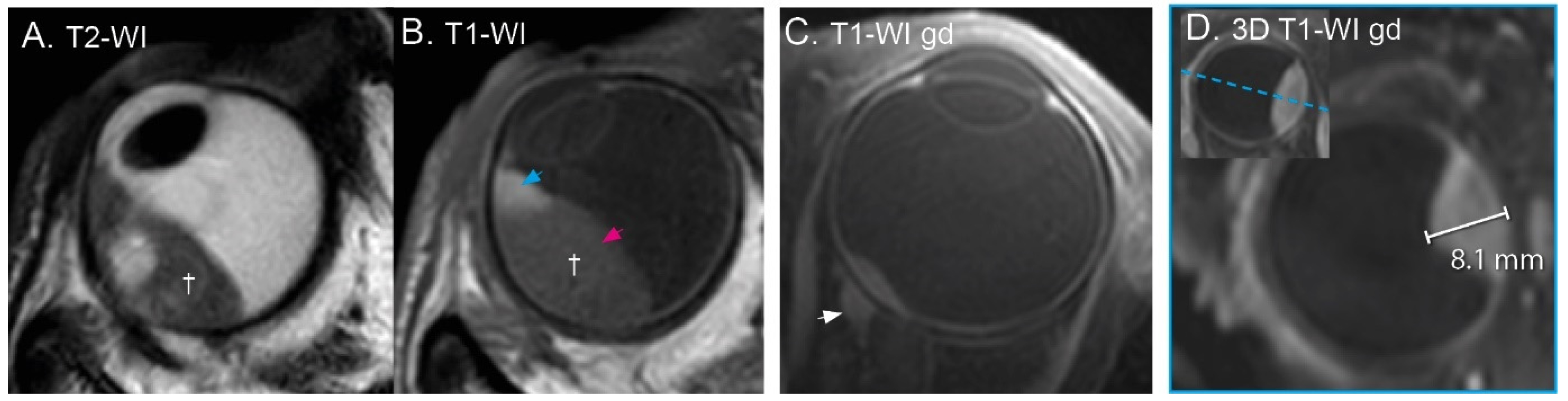
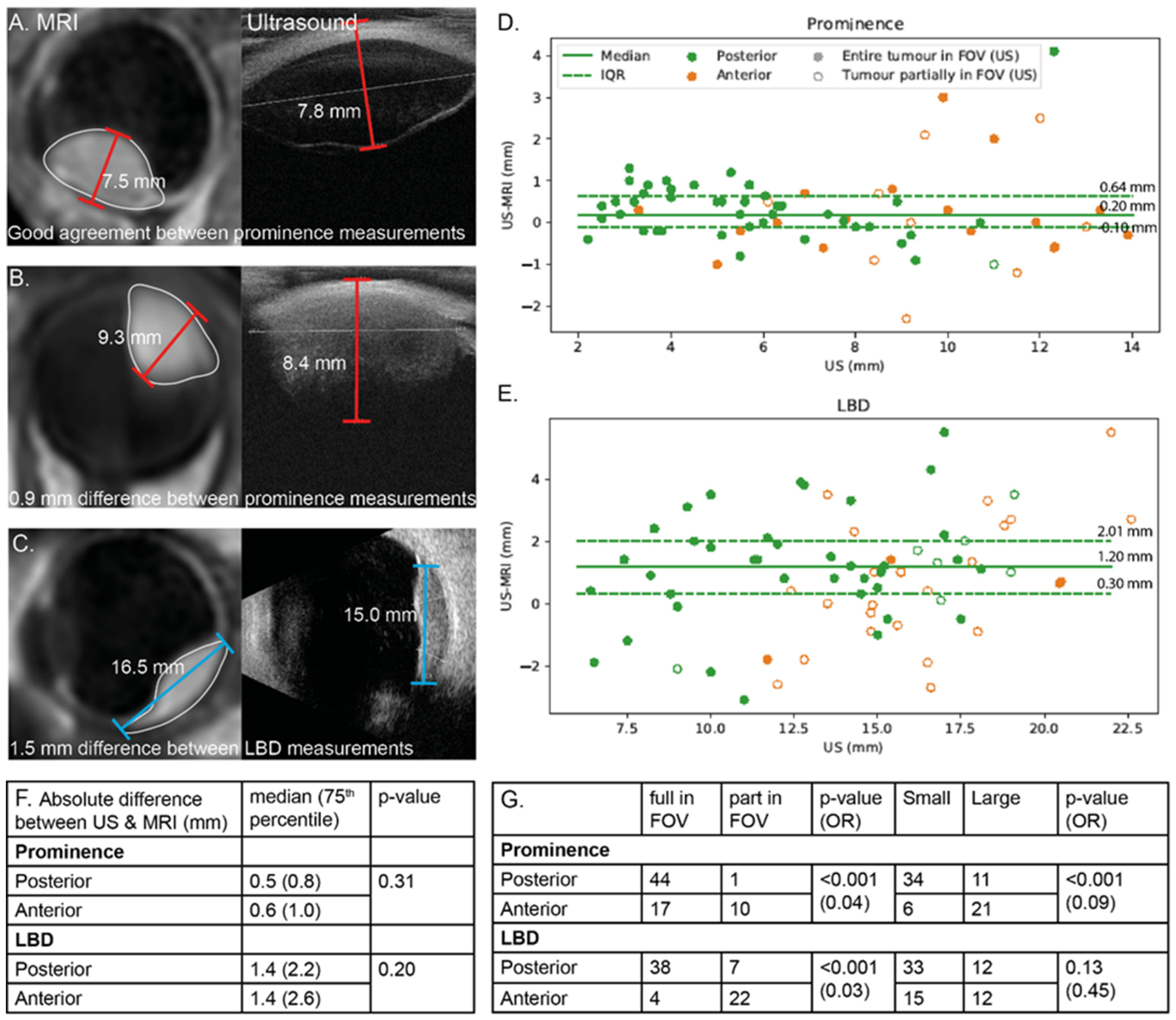
3.1. Anatomical Evaluation
3.2. Functional Scans
4. Differential Diagnosis
5. Therapy Planning
6. Follow-Up
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
- Rahman, A.M.; Selva, D.; Davis, G. Choroidal melanoma with extrascleral extension in an Australian Aboriginal man. Clin. Exp. Ophthalmol. 2007, 35, 187–189.
- Wagle, A.M.; Wu, B.C.; Gopal, L.; Sundar, G. Necrosis of uveal melanoma post-COVID-19 vaccination. Indian J. Ophthalmol. 2022, 70, 1837–1840.
- Das, D.; Deka, P.; Verma, G.; Kuri, G.C.; Bhattacharjee, H.; Bharali, G.; Pandey, D.; Koul, A.; Das, B.; Deka, A. IgG4-related intraocular inflammation masquerading as ciliary body melanoma in a young girl. Indian J. Ophthalmol. 2016, 64, 601–603.
- Kimura, D.; Kida, T.; Sato, T.; Fukumoto, M.; Kohmoto, R.; Kojima, S.; Mizuno, H.; Sakaguchi, H.; Sugasawa, J.; Ikeda, T. A Case of Retinal Detachment with Unique Optical Coherence Tomography Findings after Gamma Knife® Radiosurgery Treatment for Choroidal Melanoma. Case Rep. Ophthalmol. 2018, 9, 17–23.
- Shields, J.A.; Shields, C.L.; Kimmel, A.S.; Eagle, R.C., Jr. Contralateral blindness from chiasmal extension of unsuspected choroidal melanoma. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 384–387.
- Arevalo, J.F.; Shields, C.L.; Shields, J.A. Giant nodular posterior scleritis simulating choroidal melanoma and birdshot retinochoroidopathy. Ophthalmic Surg. Lasers Imaging Retin. 2003, 34, 403–405.
- Cotliar, J.M.; Shields, C.L.; Meyer, D.R. Chronic orbital inflammation and fibrosis after retrobulbar alcohol and chlorpromazine injections in a patient with choroidal melanoma. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 410–411.
- Sheck, L.H.; Ng, Y.S.; Watson, M.; Vincent, A.L. Clinical findings and molecular diagnosis of retinoblastoma in older children. Ophthalmic Genet. 2013, 34, 238–242.
- Tsukikawa, M.; Akinpelu, B.; Wangaryattawanich, P.; Scherpelz, K.; Stacey, A.W. Uveal melanoma incidentally diagnosed with neuroimaging, a case series of 3 patients. Radiol. Case Rep. 2022, 17, 54–59.
- Richter, M.N.; Bechrakis, N.E.; Stoltenburg-Didinger, G.; Foerster, M.H. Transscleral resection of a ciliary body leiomyoma in a child: Case report and review of the literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 953–957.
- Lindner, T.; Langner, S.; Falke, K.; Walter, U.; Krüger, P.C.; Pohlmann, A.; Zimpfer, A.; Stahnke, T.; Hadlich, S.; Guthoff, R.; et al. Anatomic and pathological characterization of choroidal melanoma using multimodal imaging: what is practical, what is needed? Melanoma Res. 2015, 25, 252–258.
- Wei, W.; Mo, J.; Jie, Y.; Li, B. Adenoma of the retinal pigment epithelium: A report of 3 cases. Can. J. Ophthalmol. 2010, 45, 166–170.
References
- Hawkes, R.C.; Holland, G.N.; Moore, W.S.; Rizk, S.; Worthington, B.S.; Kean, D.M. NMR imaging in the evaluation of orbital tumors. AJNR Am. J. Neuroradiol. 1983, 4, 254–256. [Google Scholar] [CrossRef]
- De Keizer, R.; Vielvoye, G.; de Wolff-Rouendaal, D. Nuclear Magnetic Resonance Imaging of Intraocular Tumors. Am. J. Ophthalmol. 1986, 102, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, J.-W.M.; Ferreira, T.A.; Soemarwoto, K.P.; Genders, S.W.; Teeuwisse, W.M.; Webb, A.G.; Luyten, G.P.M. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; McDonald, C.; Ito, Y.; Tofts, P.; Latif, Z.; Gross, J. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: Eliminating blinking artifacts and demonstrating proof of concept. Magn. Reson. Med. 2001, 46, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Langner, S.; Graessl, A.; Rieger, J.; Schwerter, M.; Muhle, M.; Lysiak, D.; Kraus, O.; Wuerfel, J.; Guthoff, R.F.; et al. High spatial resolution in vivo magnetic resonance imaging of the human eye, orbit, nervus opticus and optic nerve sheath at 7.0 Tesla. Exp. Eye Res. 2014, 125, 89–94. [Google Scholar] [CrossRef]
- Lemke, A.J.; Alai-Omid, M.; Hengst, S.A.; Kazi, I.; Felix, R. Eye imaging with a 3.0-T MRI using a surface coil—A study on volunteers and initial patients with uveal melanoma. Eur. Radiol. 2006, 16, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Richdale, K.; Wassenaar, P.; Teal Bluestein, K.; Abduljalil, A.; Christoforidis, J.A.; Lanz, T.; Knopp, M.V.; Schmalbrock, P. 7 Tesla MR imaging of the human eye in vivo. J. Magn. Reason. Imaging JMRI 2009, 30, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Graessl, A.; Muhle, M.; Schwerter, M.; Rieger, J.; Oezerdem, C.; Santoro, D.; Lysiak, D.; Winter, L.; Hezel, F.; Waiczies, S.; et al. Ophthalmic Magnetic Resonance Imaging at 7 T Using a 6-Channel Transceiver Radiofrequency Coil Array in Healthy Subjects and Patients with Intraocular Masses. Investig. Radiol. 2014, 49, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, J.W.M.; van Rijn, G.A.; Luyten, G.P.M.; Webb, A.G. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 2013, 26, 1864–1869. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Fonk, L.G.; Jaarsma-Coes, M.G.; van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.-W.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part I: MR imaging with pathologic correlation and technical considerations. Insights Into Imaging 2021, 12, 1–27. [Google Scholar] [CrossRef]
- Van Vught, L.; Dekker, C.E.; Stoel, B.C.; Luyten, G.P.M.; Beenakker, J.M. Evaluation of intraocular lens position and retinal shape in negative dysphotopsia using high-resolution magnetic resonance imaging. J. Cataract. Refract. Surg. 2021, 47, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wan, C. Application of advanced magnetic resonance imaging in glaucoma: A narrative review. Quant. Imaging Med. Surg. 2022, 12, 2106–2128. [Google Scholar] [CrossRef]
- Niendorf, T.; Beenakker, J.W.M.; Langner, S.; Erb-Eigner, K.; Bach Cuadra, M.; Beller, E.; Millward, J.M.; Niendorf, T.M.; Stachs, O. Ophthalmic Magnetic Resonance Imaging: Where Are We (Heading to)? Curr. Eye Res. 2021, 46, 1251–1270. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Saraiva, P.; Genders, S.W.; Buchem, M.V.; Luyten, G.P.M.; Beenakker, J.-W. CT and MR imaging of orbital inflammation. Neuroradiology 2018, 60, 1253–1266. [Google Scholar] [CrossRef]
- Diogo, M.; Jager, M.; Ferreira, T. CT and MR Imaging in the Diagnosis of Scleritis. AJNR Am. J. Neuroradiol. 2016, 37, 2334–2339. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Pinheiro, C.F.; Saraiva, P.; Jaarsma-Coes, M.G.; Van Duinen, S.G.; Genders, S.W.; Marinkovic, M.; Beenakker, J.-W.M. MR and CT Imaging of the Normal Eyelid and its Application in Eyelid Tumors. Cancers 2020, 12, 658. [Google Scholar] [CrossRef]
- Sims, J.R.; Chen, A.M.; Sun, Z.; Deng, W.; Colwell, N.A.; Colbert, M.K.; Zhu, J.; Sainulabdeen, A.; Faiq, M.A.; Chan, K.C.; et al. Role of Structural, Metabolic, and Functional MRI in Monitoring Visual System Impairment and Recovery. J. Magn. Reson. Imaging 2021, 54, 1706–1729. [Google Scholar] [CrossRef]
- Gracitelli, C.P.; Gerente, V.M.; Furlanetto, R.L.; Amaro, E.; Paranhos, A. Magnetic Resonance Imaging for Glaucoma Evaluation. Eur. J. Gastroenterol. Hepatol. 2020, 29, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.J.; Kazi, I.; Felix, R. Magnetic resonance imaging of orbital tumors. Eur. Radiol. 2006, 16, 2207–2219. [Google Scholar] [CrossRef]
- De Graaf, P.; on behalf of the European Retinoblastoma Imaging Collaboration (ERIC); Göricke, S.; Rodjan, F.; Galluzzi, P.; Maeder, P.; Castelijns, J.A.; Brisse, H.J. Guidelines for imaging retinoblastoma: Imaging principles and MRI standardization. Pediatr. Radiol. 2012, 42, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Caltabiano, R.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part II: Treatment indications and complications. Insights Into Imaging 2021, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Gaudana, R.; Boddu, S.H. Imaging Techniques in the Diagnosis and Management of Ocular Tumors: Prospects and Challenges. AAPS J. 2018, 20, 97. [Google Scholar] [CrossRef]
- Solnik, M.; Paduszyńska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Chorągiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of Uveal Melanoma—Current Standard and Methods in Development. Cancers 2022, 14, 3147. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Durairaj, P.; Yeung, J. Uveal Melanoma: A Review of the Literature. Oncol. Ther. 2018, 6, 87–104. [Google Scholar] [CrossRef]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S.; et al. Uveal melanoma: Quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef]
- Tartaglione, T.; Pagliara, M.M.; Sciandra, M.; Caputo, C.G.; Calandrelli, R.; Fabrizi, G.; Gaudino, S.; Blasi, M.A.; Colosimo, C. Uveal melanoma: Evaluation of extrascleral extension using thin-section MR of the eye with surface coils. La Radiol. Med. 2014, 119, 775–783. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Jaarsma-Coes, M.G.; Marinkovic, M.; Verbist, B.; Verdijk, R.M.; Jager, M.J.; Luyten, G.P.M.; Beenakker, J.-W.M. MR imaging characteristics of uveal melanoma with histopathological validation. Neuroradiology 2021, 64, 171–184. [Google Scholar] [CrossRef]
- Récsán, Z.; Karlinger, K.; Fodor, M.; Zalatnai, A.; Papp, M.; Salacz, G. MRI for the Evaluation of Scleral Invasion and Extrascleral Extension of Uveal Melanomas. Clin. Radiol. 2002, 57, 371–376. [Google Scholar] [CrossRef]
- Hosten, N.; Bornfeld, N.; Wassmuth, R.; Lemke, A.J.; Sander, B.; E Bechrakis, N.; Félix, R. Uveal melanoma: Detection of extraocular growth with MR imaging and US. Radiology 1997, 202, 61–67. [Google Scholar] [CrossRef]
- Coleman, D.J.; Silverman, R.H.; Rondeau, M.J.; O Lloyd, H.; Daly, S. Explaining the current role of high-frequency ultrasound in ophthalmic diagnosis. Expert. Rev. Ophthalmol. 2006, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, L.; Jaarsma-Coes, M.G.; Verbist, B.M.; Vu, T.K.; Marinkovic, M.; Rasch, C.R.; Luyten, G.P.; Beenakker, J.-W.M. Automatic Three-Dimensional Magnetic Resonance-based measurements of tumour prominence and basal diameter for treatment planning of uveal melanoma. Phys. Imaging Radiat. Oncol. 2022, 24, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma-Coes, M.G.; Ferreira, T.A.; Marinkovic, M.; Vu, T.K.; van Vught, L.; van Haren, G.R.; Rodrigues, M.F.; Klaver, Y.L.; Verbist, B.M.; Luyten, G.P.; et al. Comparison of MRI-based and conventional measurements for proton beam therapy of uveal melanoma. Ophthalmol. Retina 2022, 7, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kuai, X.-P.; Chen, X.-S.; Tao, X.-F. Assessment of dynamic contrast-enhanced magnetic resonance imaging in the differentiation of malignant from benign orbital masses. Eur. J. Radiol. 2013, 82, 1506–1511. [Google Scholar] [CrossRef]
- De Graaf, P.; Pouwels, P.; Rodjan, F.; Moll, A.; Imhof, S.; Knol, D.; Sanchez, E.; van der Valk, P.; Castelijns, J. Single-Shot Turbo Spin-Echo Diffusion-Weighted Imaging for Retinoblastoma: Initial Experience. AJNR Am. J. Neuroradiol. 2012, 33, 110–118. [Google Scholar] [CrossRef]
- Erb-Eigner, K.; Willerding, G.; Taupitz, M.; Hamm, B.; Asbach, P. Diffusion-Weighted Imaging of Ocular Melanoma. Investig. Radiol. 2013, 48, 702–707. [Google Scholar] [CrossRef]
- Kamrava, M.; Sepahdari, A.R.; Leu, K.; Wang, P.-C.; Roberts, K.; Demanes, D.J.; McCannel, T.; Ellingson, B.M. Quantitative multiparametric MRI in uveal melanoma: Increased tumor permeability may predict monosomy 3. Neuroradiology 2015, 57, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Sepahdari, A.R.; Politi, L.S.; Aakalu, V.K.; Kim, H.J.; Razek, A.A. Diffusion-weighted imaging of orbital masses: Multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am. J. Neuroradiol. 2014, 35, 170–175. [Google Scholar] [CrossRef]
- Sepahdari, A.R.; Aakalu, V.K.; Setabutr, P.; Shiehmorteza, M.; Naheedy, J.H.; Mafee, M.F. Indeterminate Orbital Masses: Restricted Diffusion at MR Imaging with Echo-planar Diffusion-weighted Imaging Predicts Malignancy. Radiology 2010, 256, 554–564. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Chou, R.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Yang, W.L.; Wang, Z.Y.; Chen, W.; Zhao, Q.; Li, Y.F.; Cui, R.; Shen, L.; Wei, W.B. [Comparative analysis on the significances of contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging in uveal melanoma diagnosis]. Zhonghua Yan Ke Za Zhi 2018, 54, 194–198. [Google Scholar]
- Buerk, B.M.; Pulido, J.S.; Chiong, I.; Folberg, R.; Edward, D.P.; Duffy, M.T.; Thulborn, K.R. Vascular perfusion of choroidal melanoma by 3.0 tesla magnetic resonance imaging. Trans. Am. Ophthalmol. Soc. 2004, 102, 209–218. [Google Scholar] [PubMed]
- Russo, A.; Mariotti, C.; Longo, A.; Foti, P.V.; Avitabile, T.; Uva, M.G.; Franco, L.M.; Bonfiglio, V.; Milone, P.; Ettorre, G.C.; et al. Diffusion-weighted magnetic resonance imaging and ultrasound evaluation of choroidal melanomas after proton-beam therapy. La Radiol. Med. 2015, 120, 634–640. [Google Scholar] [CrossRef]
- Sepahdari, A.; Kapur, R.; Aakalu, V.; Villablanca, J.; Mafee, M. Diffusion-Weighted Imaging of Malignant Ocular Masses: Initial Results and Directions for Further Study. AJNR Am. J. Neuroradiol. 2012, 33, 314–319. [Google Scholar] [CrossRef]
- Tang, M.; Ferreira, T.; Jaarsma-Coes, M.; Klaassen, L.; Marinkovic, M.; Vu, K.; Rasch, C.; Creutzberg, C.; Horeweg, N.; Klaver, Y.; et al. MR-based follow-up after brachytherapy and proton beam therapy in uveal melanoma. Acta Ophthalmol. 2022, 100. [Google Scholar] [CrossRef]
- Schueller, P.; Dogan, A.; Panke, J.E.; Micke, O.; Willich, N. Does the imaging method have an influence on the measured tumor height in ruthenium plaque therapy of uveal melanoma? Strahlenther. Onkol. 2005, 181, 320–325. [Google Scholar] [CrossRef]
- Beenakker, J.-W.M.; Rasch, C.R. Letter to the Editor of Radiotherapy and Oncology regarding the paper titled “MRI and FUNDUS image fusion for improved ocular biometry in Ocular Proton Therapy” by Via et al. Radiother. Oncol. 2022, 176, 251. [Google Scholar] [CrossRef] [PubMed]
- Oberacker, E.; Paul, K.; Huelnhagen, T.; Oezerdem, C.; Winter, L.; Pohlmann, A.; Boehmert, L.; Stachs, O.; Heufelder, J.; Weber, A.; et al. Magnetic resonance safety and compatibility of tantalum markers used in proton beam therapy for intraocular tumors: A 7.0 Tesla study. Magn. Reson. Med. 2017, 78, 1533–1546. [Google Scholar] [CrossRef]
- Bustin, A. Editorial for “A Comparison of 3T and 7T MRI for the Clinical Evaluation of Uveal Melanoma”. J. Magn. Reson. Imaging 2022, 55, 1516–1517. [Google Scholar] [CrossRef]
- Tang, M.C.; Jaarsma-Coes, M.G.; Ferreira, T.A.; Fonk, L.Z.G.; Marinkovic, M.; Luyten, G.P.; Beenakker, J.M. A Comparison of 3 T and 7 T MRI for the Clinical Evaluation of Uveal Melanoma. J. Magn. Reson. Imaging 2022, 55, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Gach, H.M.; Mackey, S.L.; Rehman, S.; Kadbi, M.; Zoberi, J.E.; Garcia-Ramirez, J.; Grigsby, P.W. Magnetic resonance imaging metal artifact reduction for eye plaque patient with dental braces. J. Contemp. Brachyther. 2017, 9, 490–495. [Google Scholar] [CrossRef]
- Jaarsma-Coes, M.G.; Ferreira, T.A.G.; van Haren, G.R.; Marinkovic, M.; Beenakker, J.-W.M. MRI enables accurate diagnosis and follow-up in uveal melanoma patients after vitrectomy. Melanoma Res. 2019, 29, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, G.A.; Mourik, J.E.M.; Teeuwisse, W.M.; Luyten, G.P.M.; Webb, A.G. Magnetic Resonance Compatibility of Intraocular Lenses Measured at 7 Tesla. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Islamaj, E.; Van Vught, L.; Jordaan-Kuip, C.P.; Vermeer, K.A.; Ferreira, T.A.; De Waard, P.W.T.; Lemij, H.G.; Beenakker, J.-W.M. Magnetic resonance imaging reveals possible cause of diplopia after Baerveldt glaucoma implantation. PLoS ONE 2022, 17, e0276527. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Inì, C.; Broggi, G.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results. Cancers 2022, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, T.W.; Kim, S.; Choung, H.; Lee, M.J.; Kim, N.; Khwarg, S.I.; Yu, Y.S. Effects on Periocular Tissues after Proton Beam Radiation Therapy for Intraocular Tumors. J. Korean Med. Sci. 2018, 33, e120. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ji, X.; Liu, M.; Xia, Z.; Zheng, H.; Yin, Q.; Wang, H.; Li, Y. The value of MRI in evaluating the efficacy and complications with the treatment of intra-arterial chemotherapy for retinoblastoma. Oncotarget 2017, 8, 38413–38425. [Google Scholar] [CrossRef]
- Jeong, H.; Sa, H.-S. Uveal Melanoma with Massive Extraocular Extension through the Sclerocorneal Limbus. Korean J. Ophthalmol. 2015, 29, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Houle, V.; Bélair, M.; Allaire, G.S. AIRP Best Cases in Radiologic-Pathologic Correlation: Choroidal Melanoma. Radiographics 2011, 31, 1231–1236. [Google Scholar] [CrossRef]
- Khetan, V.; Gupta, K.; Mohan, E.R.; Gopal, L. Uveal melanoma presenting as cataract and staphyloma. Indian J. Ophthalmol. 2009, 57, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Rebolleda, G.; Suárez Figueroa, M.; Muñoz-Negrete, F.J.; Rocamora, A. Magnetic resonance imaging in cavitary choroidal melanoma. Eur. J. Ophthalmol. 2000, 10, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Raghavendra, R.; Ratra, V.; Krishnakumar, S.; Gopal, L.; Shanmugam, M.P. Diffuse malignant melanoma of the choroid simulating metastatic tumour in the choroid. Indian J. Ophthalmol. 2000, 48, 137–140. [Google Scholar] [PubMed]
- Nguyen, T.-N.; Edelstein, C.; Mansour, M.; Burnier, M.N. Primary choroidal melanoma masquerading as a hemorrhagic lesion in a patient with ocular trauma. Can. J. Ophthalmol. 2003, 38, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Minija, C.; Shanmugam, M.P. Subretinal lipid exudation associated with untreated choroidal melanoma. Indian J. Ophthalmol. 2011, 59, 233–235. [Google Scholar] [CrossRef]
- Becerra, E.M.; Saornil, M.A.; Blanco, G.; Méndez, M.C.; Muiños, Y.; Esteban, M.R. Cavitary choroidal melanoma. Can. J. Ophthalmol. 2005, 40, 619–622. [Google Scholar] [CrossRef]
- Jiblawi, A.; Chanbour, H.; Tayba, A.; Khayat, H.; Jiblawi, K. Magnetic Resonance Imaging Diagnosis of Choroidal Melanoma. Cureus 2021, 13, e16628. [Google Scholar] [CrossRef]
- Koolstra, K.; Beenakker, J.M.; Koken, P.; Webb, A.; Börnert, P. Cartesian MR fingerprinting in the eye at 7T using compressed sensing and matrix completion-based reconstructions. Magn. Reson. Med. 2019, 81, 2551–2565. [Google Scholar] [CrossRef]
- Damento, G.M.; Koeller, K.K.; Salomão, D.R.; Pulido, J.S. T2 Fluid-Attenuated Inversion Recovery Imaging of Uveal Melanomas and Other Ocular Pathology. Ocul. Oncol. Pathol. 2016, 2, 251–261. [Google Scholar] [CrossRef]
- Hirunpat, P.; Sanghan, N.; Hirunpat, S. White matter: A good reference for the signal intensity evaluation in magnetic resonance imaging for the diagnosis of uveal melanoma. Neuroradiol. J. 2021, 34, 113–119. [Google Scholar] [CrossRef]
- Wei, W.; Jia, G.; von Tengg-Kobligk, H.; Heverhagen, J.T.; Abdel-Rahman, M.; Wei, L.; Christoforidis, J.B.; Davidorf, F.; Knopp, M.V. Dynamic Contrast-Enhanced Magnetic Resonance Imaging of Ocular Melanoma as a Tool to Predict Metastatic Potential. J. Comput. Assist. Tomogr. 2017, 41, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma-Coes, M.G.; Ferreira, T.A.; van Houdt, P.J.; van der Heide, U.A.; Luyten, G.P.M.; Beenakker, J.-W.M. Eye-specific quantitative dynamic contrast-enhanced MRI analysis for patients with intraocular masses. Magn. Reson. Mater. Phys. Biol. Med. 2022, 35, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, X.; Wei, W.; Xian, J. Using a novel MR imaging sign to differentiate retinal pigment epithelium from uveal melanoma. Neuroradiology 2020, 62, 347–352. [Google Scholar] [CrossRef]
- Stroszczynski, C.; Hosten, N.; Bornfeld, N.; Wiegel, T.; Schueler, A.; Foerster, P.; Lemke, A.J.; Hoffmann, K.T.; Felix, R. Choroidal hemangioma: MR findings and differentiation from uveal melanoma. AJNR Am. J. Neuroradiol. 1998, 19, 1441–1447. [Google Scholar] [PubMed]
- Lemke, A.-J.; Hosten, N.; Wiegel, T.; Prinz, R.D.; Richter, M.; Bechrakis, N.E.; Foerster, P.I.; Felix, R. Intraocular metastases: Differential diagnosis from uveal melanomas with high-resolution MRI using a surface coil. Eur. Radiol. 2001, 11, 2593–2601. [Google Scholar] [CrossRef]
- Nagesh, C.; Rao, R.; Hiremath, S.; Honavar, S. Magnetic resonance imaging of the orbit, Part 1: Basic principles and radiological approach. Indian J. Ophthalmol. 2021, 69, 2574. [Google Scholar] [CrossRef]
- Orman, G.; Huisman, T. A descriptive neuroimaging study of retinoblastoma in children: Magnetic resonance imaging features. Pol. J. Radiol. 2022, 87, 363–368. [Google Scholar] [CrossRef]
- De Graaf, P.; Barkhof, F.; Moll, A.C.; Imhof, S.M.; Knol, D.L.; van der Valk, P.; Castelijns, J.A. Retinoblastoma: MR imaging parameters in detection of tumor extent. Radiology 2005, 235, 197–207. [Google Scholar] [CrossRef]
- Nagesh, C.; Rao, R.; Hiremath, S.; Honavar, S. Magnetic resonance imaging of the orbit, Part 2: Characterization of orbital pathologies. Indian J. Ophthalmol. 2021, 69, 2585. [Google Scholar] [CrossRef]
- Damianidis, C.; Konstantinou, D.; Kyriakou, V.; Arvaniti, M.; Kotziamani, N.; Rodokalakis, G.; Agriou, A.; Emmanouilidou, M.; Tsitouridis, I. Magnetic Resonance Imaging and Ultrasonographic Evaluation of Retinal Detachment in Orbital Uveal Melanomas. Neuroradiol. J. 2010, 23, 329–338. [Google Scholar] [CrossRef]
- Via, R.; Pica, A.; Antonioli, L.; Paganelli, C.; Fattori, G.; Spaccapaniccia, C.; Lomax, A.; Weber, D.C.; Schalenbourg, A.; Baroni, G.; et al. MRI and FUNDUS image fusion for improved ocular biometry in Ocular Proton Therapy. Radiother. Oncol. 2022, 174, 16–22. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Huang, Y.; Hei, Y.; Wang, L.; Xiao, L. Orbital Extension of Uveal Melanoma: Treatment and Survival Analysis. Int. Ophthalmol. Clin. 2019, 59, 37–51. [Google Scholar] [CrossRef]
- Blanco, G. Diagnosis and treatment of orbital invasion in uveal melanoma. Can. J. Ophthalmol. 2004, 39, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fusetti, S.; Parrozzani, R.; Urban, F.; Gurabardhi, M.; Ferronato, G.; Midena, E. Modified Enucleation for Choroidal Melanoma with Large Extrascleral Extension. Orbit 2010, 29, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Modarres, M.; Rezanejad, A.; Falavarjani, K.G. Recurrence and massive extraocular extension of choroidal malignant melanoma after vitrectomy and endoresection. Indian J. Ophthalmol. 2014, 62, 731–733. [Google Scholar] [CrossRef]
- Mittica, N.; Vemuganti, G.K.; Duffy, M.; Torczynski, E.; Edward, D.P. Late Orbital Recurrence of a Choroidal Melanoma Following Internal Resection: Report of a Case and Review of the Literature. Surv. Ophthalmol. 2003, 48, 181–190. [Google Scholar] [CrossRef]
- Kiratli, H.; Koç, I.; Tarlan, B. Orbital Extension of an Unsuspected Choroidal Melanoma Presumably through an Aqueous Tube Shunt. Ocul. Oncol. Pathol. 2016, 2, 144–147. [Google Scholar] [CrossRef]
- Sambuelli, R.; Luna, J.D.; Reviglio, V.E.; Aoki, A.; Juarez, C.P. Small choroidal melanoma with massive extraocular extension: Invasion through posterior scleral emissary channels. Int. Ophthalmol. 2001, 24, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.H.; Ricks, C.; Harrie, R.P. Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma. BMC Ophthalmol. 2018, 18, 320. [Google Scholar] [CrossRef]
- Jacinto, F.A.; Margo, C.E. Neglected Choroidal Melanoma Tracking Along Optic Nerve to Brain. Ophthalmology 2016, 123, 2488. [Google Scholar] [CrossRef]
- Singh, A.D.; Platt, S.M.; Lystad, L.; Lowe, M.; Oh, S.; Jones, S.E.; Alzahrani, Y.; Plesec, T. Optic Nerve Assessment Using 7-Tesla Magnetic Resonance Imaging. Ocul. Oncol. Pathol. 2016, 2, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.; Estes, A.; Ulrich, L.; Thomas, D.; Gay, D. Epibulbar Plasmacytoma Masquerading as Subconjunctival Hemorrhage in a Patient With Multiple Myeloma. Cornea 2017, 36, 249–251. [Google Scholar] [CrossRef]
- Maheshwari, A.; Finger, P.T. Cancers of the eye. Cancer Metastasis Rev. 2018, 37, 677–690. [Google Scholar] [CrossRef]
- Mahajan, A.; Crum, A.; Johnson, M.H.; Materin, M.A. Ocular Neoplastic Disease. Semin. Ultrasound CT MRI 2011, 32, 28–37. [Google Scholar] [CrossRef]
- Surukrattanaskul, S.; Keyurapan, B.; Wangtiraumnuay, N. Correlation between clinical presentations, radiological findings and high risk histopathological features of primary enucleated eyes with advanced retinoblastoma at Queen Sirikit National Institute of Child Health: 5 years result. PLoS ONE 2022, 17, e0270362. [Google Scholar] [CrossRef] [PubMed]
- Brisse, H.J.; on behalf of the European Retinoblastoma Imaging Collaboration (ERIC); de Graaf, P.; Galluzzi, P.; Cosker, K.; Maeder, P.; Göricke, S.; Rodjan, F.; de Jong, M.C.; Savignoni, A.; et al. Assessment of early-stage optic nerve invasion in retinoblastoma using high-resolution 1.5 Tesla MRI with surface coils: A multicentre, prospective accuracy study with histopathological correlation. Eur. Radiol. 2015, 25, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Riechardt, A.I.; Erb-Eigner, K.; Böker, A.; Cordini, D.; Heufelder, J.; Joussen, A.M. Proton Beam Irradiation: A Safe Procedure in Postequatorial Extraocular Extension From Uveal Melanoma. Am. J. Ophthalmol. 2018, 191, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, J.-W.M.; Wezel, J.; Groen, J.; Webb, A.G.; Börnert, P. Silent volumetric multi-contrast 7 Tesla MRI of ocular tumors using Zero Echo Time imaging. PLoS ONE 2019, 14, e0222573. [Google Scholar] [CrossRef]
- Walter, U.; Niendorf, T.; Graessl, A.; Rieger, J.; Krüger, P.-C.; Langner, S.; Guthoff, R.F.; Stachs, O. Ultrahigh field magnetic resonance and colour Doppler real-time fusion imaging of the orbit—A hybrid tool for assessment of choroidal melanoma. Eur. Radiol. 2014, 24, 1112–1117. [Google Scholar] [CrossRef]
- Su, Y.; Xu, X.; Zuo, P.; Xia, Y.; Qu, X.; Chen, Q.; Guo, J.; Wei, W.; Xian, J. Value of MR-based radiomics in differentiating uveal melanoma from other intraocular masses in adults. Eur. J. Radiol. 2020, 131, 109268. [Google Scholar] [CrossRef]
- Tofts, P.S. T1-weighted DCE imaging concepts: Modelling, acquisition and analysis. Signal 2010, 500, 400. [Google Scholar]
- Xu, Q.-G.; Xian, J.-F. Role of Quantitative Magnetic Resonance Imaging Parameters in the Evaluation of Treatment Response in Malignant Tumors. Chin. Med. J. 2015, 128, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Gumeler, E.; Parlak, S.; Yazici, G.; Karabulut, E.; Kiratli, H.; Oguz, K.K. Single shot echo planar imaging (ssEPI) vs single shot turbo spin echo (ssTSE) DWI of the orbit in patients with ocular melanoma. Br. J. Radiol. 2021, 94, 20200825. [Google Scholar] [CrossRef] [PubMed]
- Seeger, A.; Batra, M.; Süsskind, D.; Ernemann, U.; Hauser, T.-K. Assessment of uveal melanomas using advanced diffusion-weighted imaging techniques: Value of reduced field of view DWI (“zoomed DWI”) and readout-segmented DWI (RESOLVE). Acta Radiol. 2019, 60, 977–984. [Google Scholar] [CrossRef]
- Paul, K.; Huelnhagen, T.; Oberacker, E.; Wenz, D.; Kuehne, A.; Waiczies, H.; Schmitter, S.; Stachs, O.; Niendorf, T. Multiband diffusion-weighted MRI of the eye and orbit free of geometric distortions using a RARE-EPI hybrid. NMR Biomed. 2018, 31, e3872. [Google Scholar] [CrossRef]
- Paul-Christian, K.; Graessl, A.; Rieger, J.; Lysiak, D.; Huelnhagen, T.; Winter, L.; Heidemann, R.; Lindner, T.; Hadlich, S.; Zimpfer, A.; et al. Diffusion-Sensitized Ophthalmic Magnetic Resonance Imaging Free of Geometric Distortion at 3.0 and 7.0 T. Investig. Radiol. 2015, 50, 309–321. [Google Scholar] [CrossRef]
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Jackson, E.F.; et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reason. Imaging 2019, 49, e101–e121. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef]
- Roelofs, K.A.; O’Day, R.; Al Harby, L.; Arora, A.K.; Cohen, V.M.; Sagoo, M.S.; Damato, B. The MOLES System for Planning Management of Melanocytic Choroidal Tumors: Is It Safe? Cancers 2020, 12, 1311. [Google Scholar] [CrossRef]
- Narang, S.; Pandey, A.K.; Giran, M.; Kaur, R. Plaque brachytherapy for choroidal melanoma with vitreous haemorrhage: A therapeutic challenge. BMJ Case Rep. 2021, 14, e240935. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Damato, B.E.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Arıbal, E.; Toker, E.; Öğüt, M.S. Anterior coloboma with macrophthalmos and cyst: MR findings. Clin. Imaging 2005, 29, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.C.; Wilson, M.W.; Kaste, S.; Helton, K.J.; McCarville, M.B. US and MRI of pediatric ocular masses with histopathological correlation. Pediatr. Radiol. 2012, 42, 738–749. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, X.-H.; Zhang, K.; Hu, R.; Myers, F. Case Report: Adult Retinoblastoma Progression in 19 Months. Optom. Vis. Sci. 2020, 97, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Mukhija, R.; Lomi, N.; Kumar, S.; Sen, S. Retinoblastoma in an adult: A diagnostic dilemma. BMJ Case Rep. 2019, 12, e230537. [Google Scholar] [CrossRef]
- Jansen, R.W.; de Bloeme, C.M.; Brisse, H.J.; Galluzzi, P.; Cardoen, L.; Göricke, S.; Maeder, P.; Cassoux, N.; Gauthier, A.; Schlueter, S.; et al. MR Imaging Features to Differentiate Retinoblastoma from Coats’ Disease and Persistent Fetal Vasculature. Cancers 2020, 12, 3592. [Google Scholar] [CrossRef]
- Meltzer, D.E.; Garner, H.R.; Fazzone, H.E. Kikuchi-Fujimoto Disease with Bilateral Uveitis. J. Radiol. Case Rep. 2009, 3, 1–6. [Google Scholar] [CrossRef]
- Iturralde, J.C.; Bianciotto, C.; Lally, S.E.; Krasnow, M.; Shields, C.L. Massive choroidal effusion and painful secondary glaucoma from underlying uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 627–630. [Google Scholar] [CrossRef]
- Wei, W.-B.; Jie, Y.; Mo, J.; Li, B. Clinical characteristics and treatment of neurofibroma of the choroid. Chin. Med. J. 2012, 125, 1832–1835. [Google Scholar]
- Singh, P.; Sen, S.; Banerjee, M.; Meel, R. Choroidal melanoma masquerading as orbital cellulitis. BMJ Case Rep. 2018, 11, e227486. [Google Scholar] [CrossRef]
- Kıratlı, H. Persistent Intraschisis Hemorrhage Simulating Choroidal Melanoma. Jpn. J. Ophthalmol. 2002, 46, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Honavar, S.G.; Shields, J.A.; Cater, J.; Demirci, H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology 2001, 108, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Bin Won, J.; Byeon, S.H.; Yang, W.I.; Koh, H.J.; Kwon, O.W.; Lee, S.C. A Choroidal Schwannoma Confirmed by Surgical Excision. Korean J. Ophthalmol. 2009, 23, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.; Xu, X.; Wang, Z.; Yang, B.; Li, B.; Man, F.; Chen, Q.; Shi, J.; Zhang, Y. MR Imaging Findings of the Uveal Schwannoma. AJNR Am. J. Neuroradiol. 2009, 30, 769–773. [Google Scholar] [CrossRef]
- Demirci, H.; Shields, C.L.; Honavar, S.; Shields, J.A.; Bardenstein, D.S. Long-term Follow-up of Giant Nodular Posterior Scleritis Simulating Choroidal Melanoma. Arch. Ophthalmol. 2000, 118, 1290–1292. [Google Scholar] [CrossRef]
- Kranias, G.; Tyradellis, C.; Krebs, T.; Augsburger, J. Bilateral atypical nodular posterior scleritis. Eur. J. Ophthalmol. 2006, 16, 614–617. [Google Scholar] [CrossRef]
- Branisteanu, D.C.; Bogdanici, C.M.; Branisteanu, D.E.; Maranduca, M.A.; Zemba, M.; Balta, F.; Branisteanu, C.I.; Moraru, A.D. Uveal melanoma diagnosis and current treatment options (Review). Exp. Ther. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Markiewicz, A.; Donizy, P.; Nowak, M.; Krzyziński, M.; Elas, M.; Płonka, P.M.; Orłowska-Heitzmann, J.; Biecek, P.; Hoang, M.P.; Romanowska-Dixon, B. Amelanotic Uveal Melanomas Evaluated by Indirect Ophthalmoscopy Reveal Better Long-Term Prognosis Than Pigmented Primary Tumours—A Single Centre Experience. Cancers 2022, 14, 2753. [Google Scholar] [CrossRef]
- Almarzooqi, S.; Reyes-Múgica, M.; Ali, B.R.; Habbal, A.; Asha, M.J.; AlShamsi, E.T. Congenital Teratocarcinosarcoma with CTNNB1 Gene Mutation Presenting as an Ocular Mass. Pediatr. Dev. Pathol. 2022, 25, 10935266221111127. [Google Scholar] [CrossRef]
- Hada, M.; Chawla, B.; Seth, R.; Khurana, S.; Kashyap, S. Primary intraocular malignant rhabdoid tumor presenting as orbital mass with intracranial extension in an adolescent. Can. J. Ophthalmol. 2017, 52, e3–e5. [Google Scholar] [CrossRef]
- Richter, M.N.; Bechrakis, N.E.; Stoltenburg-Didinger, G.; Foerster, M.H. Transscleral resection of a ciliary body leiomyoma in a child: Case report and review of the literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Kwon, B.J.; Han, M.H.; Hwang, P.G.; Kim, C.J.; Na, D.G.; Chang, K.-H. MR Imaging Findings of Uveal Leiomyoma: Three Cases. AJNR Am. J. Neuroradiol. 2005, 26, 100–103. [Google Scholar] [PubMed]
- Küker, W.; Herrlinger, U.; Grönewäller, E.; Rohrbach, J.M.; Weller, M. Ocular manifestation of primary nervous system lymphoma: What can be expected from imaging? J. Neurol. 2002, 249, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma-Coes, M.G.; Ferreira, T.A.; Luyten, G.P.M.; Beenakker, J.W.M. Reaction on “Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma”. BMC Ophthalmol. 2019, 19, 193. [Google Scholar] [CrossRef]
- Fleury, E.; Trnkova, P.; Erdal, E.; Hassan, M.; Stoel, B.; Jaarma-Coes, M.; Luyten, G.; Herault, J.; Webb, A.; Hoogeman, M.; et al. Three-dimensional MRI-based treatment planning approach for non-invasive ocular proton therapy. Med. Phys. 2021, 48, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-G.; Sznitman, R.; Maeder, P.; Schalenbourg, A.; Peroni, M.; Hrbacek, J.; Weber, D.C.; Pica, A.; Cuadra, M.B. Personalized Anatomic Eye Model from T1-Weighted Volume Interpolated Gradient Echo Magnetic Resonance Imaging of Patients with Uveal Melanoma. Int. J. Radiat. Oncol. 2018, 102, 813–820. [Google Scholar] [CrossRef]
- Ciller, C.; De Zanet, S.; Kamnitsas, K.; Maeder, P.; Glocker, B.; Munier, F.L.; Rueckert, D.; Thiran, J.-P.; Cuadra, M.B.; Sznitman, R. Multi-channel MRI segmentation of eye structures and tumors using patient-specific features. PLoS ONE 2017, 12, e0173900. [Google Scholar] [CrossRef]
- Hassan, M.K.; Fleury, E.; Shamonin, D.; Fonk, L.G.; Marinkovic, M.; Jaarsma-Coes, M.G.; Luyten, G.P.; Webb, A.; Beenakker, J.-W.; Stoel, B. An Automatic Framework to Create Patient-specific Eye Models From 3D Magnetic Resonance Images for Treatment Selection in Patients with Uveal Melanoma. Adv. Radiat. Oncol. 2021, 6, 100697. [Google Scholar] [CrossRef] [PubMed]
- Hrbacek, J.; Mishra, K.K.; Kacperek, A.; Dendale, R.; Nauraye, C.; Auger, M.; Herault, J.; Daftari, I.K.; Trofimov, A.V.; Shih, H.A.; et al. Practice Patterns Analysis of Ocular Proton Therapy Centers: The International OPTIC Survey. Int. J. Radiat. Oncol. 2016, 95, 336–343. [Google Scholar] [CrossRef]
- Thomson, R.M.; Furutani, K.M.; Kaulich, T.W.; Mourtada, F.; Rivard, M.J.; Soares, C.G.; Vanneste, F.M.; Melhus, C.S. AAPM recommendations on medical physics practices for ocular plaque brachytherapy: Report of task group 221. Med. Phys. 2020, 47, e92–e124. [Google Scholar] [CrossRef]
- Ares, W.J.; Flickinger, J.C.; Lunsford, L.D. Leksell Radiosurgery for Orbital, Uveal, and Choroidal Tumors. Prog. Neurol. Surg. 2019, 34, 298–305. [Google Scholar] [CrossRef]
- Sarici, A.M.; Pazarli, H. Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Miralbell, R.; Caro, M.; Weber, D.C.; Elizalde, J.; Perez-Ochoa, A.; Villà, S.; IgnacioToscas, J.; Martinez, P.; Linero, D.; Nouet, P.; et al. Stereotactic Radiotherapy for Ocular Melanoma: Initial Experience Using Closed Eyes for Ocular Target Immobilization. Technol. Cancer Res. Treat. 2007, 6, 413–417. [Google Scholar] [CrossRef]
- Zorlu, F.; Selek, U.; Kiratli, H. Initial results of fractionated CyberKnife radiosurgery for uveal melanoma. J. Neuro-Oncol. 2009, 94, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.; Georg, D.; Zehetmayer, M.; Rottenfusser, A.; Pötter, R. Stereotactic Photon Beam Irradiation of Uveal Melanoma: Indications and Experience at the University of Vienna since 1997. Strahlenther. Onkol. 2007, 183, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, C.M.; Chan, M.; Mignano, J.; Duker, J.; Melhus, C.S.; Williams, L.B.; Wu, J.K.; Yao, K.C. Dose De-Escalation with Gamma Knife Radiosurgery in the Treatment of Choroidal Melanoma. Int. J. Radiat. Oncol. 2009, 75, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Furdova, A.; Furda, R.; Sramka, M.; Chorvath, M.; Rybar, J.; Vesely, P.; Valaskova, J.; Siska, V. Stereotactic irradiation on linear accelerator—Ultrasound versus MRI in choroidal melanoma volume calculation. BMC Ophthalmol. 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Schmelter, V.; Hofmann, T.; Schneider, F.; Weber, C.; Fuerweger, C.; Muacevic, A.; Priglinger, S.G.; Foerster, P.; Liegl, R. Robotic CyberKnife radiosurgery for small choroidal melanomas. Melanoma Res. 2022, 32, 192–199. [Google Scholar] [CrossRef]
- Via, R.; Hennings, F.; Pica, A.; Fattori, G.; Beer, J.; Peroni, M.; Baroni, G.; Lomax, A.; Weber, D.C.; Hrbacek, J. Potential and pitfalls of 1.5T MRI imaging for target volume definition in ocular proton therapy. Radiother. Oncol. 2021, 154, 53–59. [Google Scholar] [CrossRef]
- Daftari, I.; Aghaian, E.; O’Brien, J.M.; Dillon, W.; Phillips, T.L. 3D MRI-based tumor delineation of ocular melanoma and its comparison with conventional techniques. Med. Phys. 2005, 32, 3355–3362. [Google Scholar] [CrossRef]
- Marnitz, S.; Cordini, D.; Bendl, R.; Lemke, A.J.; Heufelder, J.; Simiantonakis, I.; Kluge, H.; Bechrakis, N.E.; Foerster, M.H.; Hinkelbein, W. Proton therapy of uveal melanomas: Intercomparison of MRI-based and conventional treatment planning. Strahlenther. Onkol. 2006, 182, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Zoberi, J.E.; Garcia-Ramirez, J.; Hedrick, S.; Rodriguez, V.; Bertelsman, C.G.; Mackey, S.; Hu, Y.; Gach, H.M.; Rao, P.K.; Grigsby, P.W. MRI-based treatment planning and dose delivery verification for intraocular melanoma brachytherapy. Brachytherapy 2018, 17, 31–39. [Google Scholar] [CrossRef]
- Dobler, B.; Bendl, R. Precise modelling of the eye for proton therapy of intra-ocular tumours. Phys. Med. Biol. 2002, 47, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Hennings, F.; Lomax, A.; Pica, A.; Weber, D.C.; Hrbacek, J. Automated Treatment Planning System for Uveal Melanomas Treated with Proton Therapy: A Proof-of-Concept Analysis. Int. J. Radiat. Oncol. 2018, 101, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J.; Koska, B.; Heufelder, J.; Janson, M.; Bäcker, C.M.; Siregar, H.; Behrends, C.; Bäumer, C.; Foerster, A.; Bechrakis, N.E.; et al. Commissioning and validation of a novel commercial TPS for ocular proton therapy. Med. Phys. 2022, 50, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Astrahan, M.A. Improved treatment planning for COMS eye plaques. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1227–1242. [Google Scholar] [CrossRef]
- Jaarsma-Coes, M.G.; Marinkovic, M.; Astreinidou, E.; Schuurmans, M.S.; Peters, F.P.; Luyten, G.P.; Rasch, C.R.; Beenakker, J.-W.M. Measuring eye deformation between planning and proton beam therapy position using magnetic resonance imaging. Phys. Imaging Radiat. Oncol. 2020, 16, 33–36. [Google Scholar] [CrossRef]
- Detorakis, E.T.; Perisinakis, K.; Drakonaki, E.; Liakopoulos, D.; Tzedakis, A.; Papadaki, E.; Tsilimbaris, M.K. MRI and dual-energy CT fusion anatomic imaging in Ru-106 ophthalmic brachytherapy. Brachytherapy 2021, 20, 828–834. [Google Scholar] [CrossRef]
- Fionda, B.; Pagliara, M.M.; Lancellotta, V.; Caputo, C.G.; Casà, C.; Sammarco, M.G.; Placidi, E.; Cornacchione, P.; Boselli, F.; Iezzi, R.; et al. Radiological and clinical findings in uveal melanoma treated by plaque interventional radiotherapy (brachytherapy): Visual atlas and literature review on response assessment. J. Contemp. Brachyther. 2022, 14, 96–106. [Google Scholar] [CrossRef]
- Jampol, L.M.; Moy, C.S.; Murray, T.G.; Reynolds, S.M.; Albert, D.M.; Schachat, A.P.; Diddie, K.R.; E Engstrom, R.; Finger, P.T.; Hovland, K.R.; et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002, 109, 2197–2206. [Google Scholar] [CrossRef]
- Maschi, C.; Thariat, J.; Herault, J.; Caujolle, J.P. Tumour Response in Uveal Melanomas Treated with Proton Beam Therapy. Clin. Oncol. 2015, 28, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Lee, S.C.; Park, Y.G.; Chang, J.H. Long-term results of Gamma Knife surgery for uveal melanomas. J. Neurosurg. 2012, 117, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, F.B.C.S.N.; Bitencourt, A.G.V.; Chojniak, M.M.M.; Souza, J.O.; Castro, D.G.; Pellizzon, A.C.A.; Chojniak, R. Response Evaluation of Choroidal Melanoma After Brachytherapy Using Diffusion-Weighted Magnetic Resonance Imaging (DW-MRI): Preliminary Findings. Front. Oncol. 2020, 10, 825. [Google Scholar] [CrossRef]
- Toktas, Z.O.; Bicer, A.; Demirci, G.; Pazarli, H.; Abacioglu, U.; Peker, S.; Kilic, T. Gamma knife stereotactic radiosurgery yields good long-term outcomes for low-volume uveal melanomas without intraocular complications. J. Clin. Neurosci. 2010, 17, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, E.; Park, J.; Shin, D.-H.; Jung, S.K.; Seok, S.; Cho, K.H.; Kim, J.-Y.; Kim, D.Y.; Suh, Y.K.; et al. Clinical Outcomes of Proton Beam Therapy for Choroidal Melanoma at a Single Institute in Korea. Cancer Res. Treat. 2018, 50, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Daftari, I.K. Proton therapy for the management of uveal melanoma and other ocular tumors. Chin. Clin. Oncol. 2016, 5, 50. [Google Scholar] [CrossRef]
- Egger, E.; Schalenbourg, A.; Zografos, L.; Bercher, L.; Boehringer, T.; Chamot, L.; Goitein, G. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int. J. Radiat. Oncol. 2001, 51, 138–147. [Google Scholar] [CrossRef]
- Knutsen, S.; Hafslund, R.; Monge, O.R.; Valen, H.; Muren, L.P.; Rekstad, B.L.; Krohn, J.; Dahl, O. Dosimetric verification of a dedicated 3D treatment planning system for episcleral plaque therapy. Int. J. Radiat. Oncol. 2001, 51, 1159–1166. [Google Scholar] [CrossRef]
- Results of a survey across 17 ocular proton therapy centers. Presented at the OPTIC subcommittee meeting at PTCOG 2022. Miami, FL, USA, 14 July 2022.
- Najjar, Y.G.; Navrazhina, K.; Ding, F.; Bhatia, R.; Tsai, K.; Abbate, K.; Durden, B.; Eroglu, Z.; Bhatia, S.; Park, S.; et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: A multicenter, retrospective study. J. Immunother. Cancer 2020, 8, e000331. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Grech Fonk, L.; Ferreira, T.A.; Webb, A.G.; Luyten, G.P.M.; Beenakker, J.M. The Economic Value of MR-Imaging for Uveal Melanoma. Clin. Ophthalmol. 2020, 14, 1135–1143. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaarsma-Coes, M.G.; Klaassen, L.; Marinkovic, M.; Luyten, G.P.M.; Vu, T.H.K.; Ferreira, T.A.; Beenakker, J.-W.M. Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients—A Systematic Review from an Ophthalmic Perspective. Cancers 2023, 15, 2995. https://doi.org/10.3390/cancers15112995
Jaarsma-Coes MG, Klaassen L, Marinkovic M, Luyten GPM, Vu THK, Ferreira TA, Beenakker J-WM. Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients—A Systematic Review from an Ophthalmic Perspective. Cancers. 2023; 15(11):2995. https://doi.org/10.3390/cancers15112995
Chicago/Turabian StyleJaarsma-Coes, Myriam G., Lisa Klaassen, Marina Marinkovic, Gregorius P. M. Luyten, T. H. Khanh Vu, Teresa A. Ferreira, and Jan-Willem M. Beenakker. 2023. "Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients—A Systematic Review from an Ophthalmic Perspective" Cancers 15, no. 11: 2995. https://doi.org/10.3390/cancers15112995
APA StyleJaarsma-Coes, M. G., Klaassen, L., Marinkovic, M., Luyten, G. P. M., Vu, T. H. K., Ferreira, T. A., & Beenakker, J.-W. M. (2023). Magnetic Resonance Imaging in the Clinical Care for Uveal Melanoma Patients—A Systematic Review from an Ophthalmic Perspective. Cancers, 15(11), 2995. https://doi.org/10.3390/cancers15112995




