Diagnostic Value of the Texture Analysis Parameters of Retroperitoneal Residual Masses on Computed Tomographic Scan after Chemotherapy in Non-Seminomatous Germ Cell Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Texture Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient and Residual Masses Characteristics
3.2. Radiomics Feature Selection and Radiomics Score Construction
3.3. Choice of Probability Threshold to Maximize Sensitivity
3.4. Performance of the Radiomics-Score on the Test Data Dataset
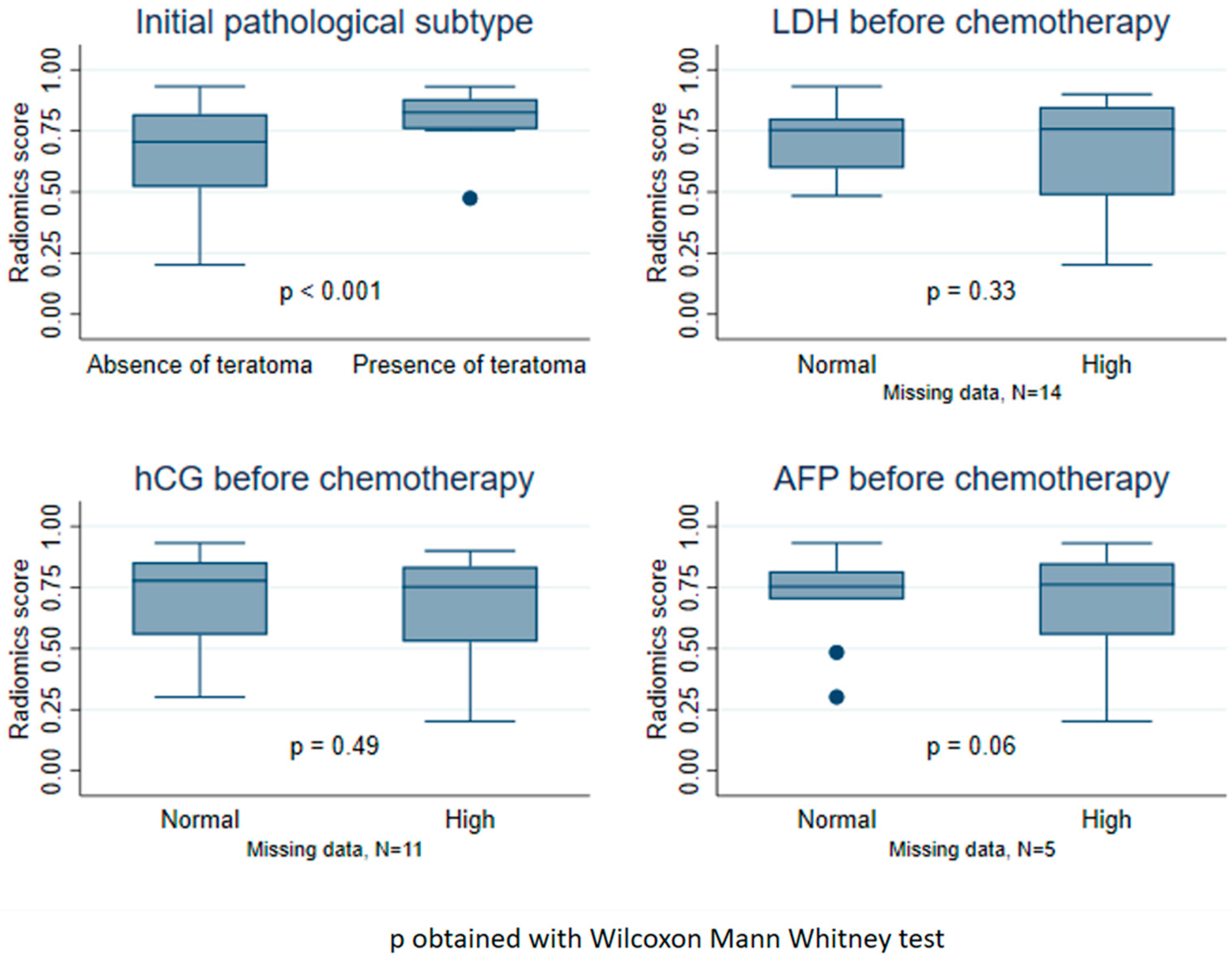
3.5. Distribution of the Radiomics Score According to Clinical Factors
3.6. Evaluation of the Value of Adding Clinical Factors to the Radiomics Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Characteristics | Test (N = 58) | Train (N = 35) | Total (N = 93) | |||
|---|---|---|---|---|---|---|
| Age at diagnosis | N = 58 | N = 35 | N = 93 | |||
| Median-(Range) | 28.5 | (17.0; 46.0) | 28.0 | (17.0; 44.0) | 28.0 | (17.0; 46.0) |
| Mean-SD | 29.5 | 7.5 | 29.2 | 7.3 | 29.4 | 7.4 |
| Initial anatomopathology of the tumor | ||||||
| Presence of teratoma | 19 | 32.8% | 8 | 22.9% | 27 | 29.0% |
| Absence of teratoma | 39 | 67.2% | 27 | 77.1% | 66 | 71.0% |
| Chemotherapy | ||||||
| ≥3 cycles of platinum-based chemotherapy | 58 | 100% | 35 | 100% | 93 | 100% |
| Time between end of chemotherapy and post- chemotherapy scan (MD = 9) | N = 54 | N = 30 | N = 84 | |||
| Median-(Range) | 4.6 | (0.3; 47.3) | 4.7 | (1.3; 364.9) | 4.6 | (0.3; 364.9) |
| Mean-SD | 6.3 | 7.6 | 18.1 | 65.8 | 10.5 | 39.7 |
| Time between post-chemotherapy scan and surgery (in weeks) | N = 58 | N = 35 | N = 93 | |||
| Median-(Range) | 6.7 | (0.0; 27.9) | 6.0 | (1.0; 39.9) | 6.7 | (0.0; 39.9) |
| Mean-SD | 7.6 | 5.1 | 7.6 | 7.0 | 7.6 | 5.9 |
| Number of persistent lesions | ||||||
| 1 | 29 | 50.0% | 16 | 45.7% | 45 | 48.4% |
| 2 à 5 | 26 | 44.8% | 16 | 45.7% | 42 | 45.2% |
| 6 à 10 | 2 | 3.4% | 2 | 5.7% | 4 | 4.3% |
| >10 | 1 | 1.7% | 1 | 2.9% | 2 | 2.2% |
Appendix B. Analysis of Correlation between Radiomics Features

Appendix C. Equation of the Resulting Elastic-Net Logistic Model
- -
- -
References
- Inserm. Cancer et Environnement. Rapport; Expertise Collective 2008; Les éditions Inserm: Paris, France, 2008; p. XVII-889. [Google Scholar]
- Ruf, C.G.; Isbarn, H.; Wagner, W.; Fisch, M.; Matthies, C.; Dieckmann, K.P. Changes in epidemiologic features of testicular germ cell cancer: Age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol. Oncol. 2014, 32, 33.e1–33.e6. [Google Scholar] [CrossRef]
- Murez, T.; Fléchon, A.; Savoie, P.H.; Rocher, L.; Camparo, P.; Morel-Journel, N.; Ferretti, L.; Sèbe, P.; Méjean, A. Recommandations françaises du Comité de Cancérologie de l’AFU—Actualisation 2018–2020: Tumeurs germinales du testicule [French ccAFU guidelines—Update 2018–2020: Testicular germ cell tumors]. Prog. Urol. 2018, 28 (Suppl. S1), R149–R166. [Google Scholar] [CrossRef]
- Honecker, F.; Aparicio, J.; Berney, D.; Beyer, J.; Bokemeyer, C.; Cathomas, R.; Clarke, N.; Cohn-Cedermark, G.; Daugaard, G.; Dieckmann, K.-P.; et al. ESMO Consensus Conference on testicular germ cell cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 1658–1686. [Google Scholar] [CrossRef]
- Krege, S.; Beyer, J.; Souchon, R.; Albers, P.; Albrecht, W.; Algaba, F.; Bamberg, M.; Bodrogi, I.; Bokemeyer, C.; Cavallin-Ståhl, E.; et al. European Consensus Conference on Diagnosis and Treatment of Germ Cell Cancer: A Report of the Second Meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): Part II. Eur. Urol. 2008, 53, 497–513. [Google Scholar] [CrossRef]
- Rozet, F.; Mongiat-Artus, P.; Hennequin, C.; Beauval, J.B.; Beuzeboc, P.; Cormier, L.; Fromont-Hankard, G.; Mathieu, R.; Ploussard, G.; Renard-Penna, R.; et al. French ccAFU guidelines—Update 2020–2022: Prostate cancer. Progres Urol. 2020, 30, S136–S251. [Google Scholar] [CrossRef] [PubMed]
- Oechsle, K.; Hartmann, M.; Brenner, W.; Venz, S.; Weissbach, L.; Franzius, C.; Kliesch, S.; Mueller, S.; Krege, S.; Heicappell, R.; et al. [18F]Fluorodeoxyglucose positron emission tomography in nonseminomatous germ cell tumors after chemotherapy: The German multicenter positron emission tomography study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5930–5935. [Google Scholar] [CrossRef]
- Fléchon, A.; Rivoire, M.; Berger, N. Surgery of residual masses after chemotherapy in patients with testicular cancer. Rev. Prat. 2007, 57, 389–398. [Google Scholar]
- Chéry, L.; Dash, A. The Role of Postchemotherapy Surgery in Germ Cell Tumors. Urol. Clin. N. Am. 2015, 42, 331–342. [Google Scholar] [CrossRef]
- Nakamura, T.; Oishi, M.; Ueda, T.; Fujihara, A.; Nakanishi, H.; Kamoi, K.; Naya, Y.; Hongo, F.; Okihara, K.; Miki, T. Clinical outcomes and histological findings of patients with advanced metastatic germ cell tumors undergoing post-chemotherapy resection of retroperitoneal lymph nodes and residual extraretroperitoneal masses: RPLND and ExRP resection for advanced GCT. Int. J. Urol. 2015, 22, 663–668. [Google Scholar] [CrossRef]
- Hornák, M.; Ondrus, D.; Matoska, J.; Cársky, S. Postchemotherapy surgery in nonseminomatous testicular tumors. Eur. Urol. 1996, 29, 325–330. [Google Scholar] [CrossRef]
- Tremeau, L.; Mottet, N. Management of residual masses of testis germ cell tumors. Bull. Cancer 2020, 107, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Haidl, F.; Paffenholz, P.; Pape, C.; Neumann, U.; Pfister, D. Surgical management of complex residual masses following systemic chemotherapy for metastatic testicular germ cell tumours. Ann. Oncol. 2017, 28, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Pfister, D.; Busch, J.; Bingöl, C.; Ranft, U.; Schrader, M.; Dieckmann, K.-P.; Heidenreich, A.; Albers, P. Residual tumor size and IGCCCG risk classification predict additional vascular procedures in patients with germ cell tumors and residual tumor resection: A multicenter analysis of the German Testicular Cancer Study Group. Eur. Urol. 2012, 61, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nini, A.; Konieczny, M.; Winter, C.; Lusch, A.; Krauspe, R.; Albers, P. Surgical management and outcomes of patients with bone metastases in germ cell tumors: A case series. Urol. Oncol. 2018, 36, 82.e1–82.e5. [Google Scholar] [CrossRef]
- Klepp, O.; Flodgren, P.; Maartman-Moe, H.; Lindholm, C.E.; Unsgaard, B.; Teigum, H.; Fossa, D.; Paus, E. Early clinical stages (CS1, CS1Mk+ and CS2A) of non-seminomatous testis cancer. Value of pre- and post-orchiectomy serum tumor marker information in prediction of retroperitoneal lymph node metastases. Swedish-Norwegian Testicular Cancer Project (SWENOTECA). Ann. Oncol. 1990, 1, 281–288. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Gerl, A.; Fossá, S.D.; Sleijfer, D.T.; de Wit, R.; Kirkels, W.J.; Clemm, C.; Habbema, J.D.; Keizer, H.J. Validity of predictions of residual retroperitoneal mass histology in nonseminomatous testicular cancer. J. Clin. Oncol. 1998, 16, 269–274. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vergouwe, Y.; Keizer, H.J.; Habbema, J.D.; ReHiT Study Group. Residual mass histology in testicular cancer: Development and validation of a clinical prediction rule. Stat. Med. 2001, 20, 3847–3859. [Google Scholar] [CrossRef]
- Albers, P.; Weissbach, L.; Krege, S.; Kliesch, S.; Hartmann, M.; Heidenreich, A.; Walz, P.; Kuczyk, M.; Fimmers, R.; The German Testicular Cancer Study Group. Prediction of necrosis after chemotherapy of advanced germ cell tumors: Results of a prospective multicenter trial of the German Testicular Cancer Study Group. J. Urol. 2004, 171, 1835–1838. [Google Scholar] [CrossRef]
- Vergouwe, Y.; Steyerberg, E.W.; Foster, R.S.; Sleijfer, D.T.; Fosså, S.D.; Gerl, A.; de Wit, R.; Roberts, J.T.; Habbema, J.D.F. Predicting retroperitoneal histology in postchemotherapy testicular germ cell cancer: A model update and multicentre validation with more than 1000 patients. Eur. Urol. 2007, 51, 424–432. [Google Scholar] [CrossRef]
- Leão, R.; Nayan, M.; Punjani, N.; Jewett, M.A.S.; Fadaak, K.; Garisto, J.; Lewin, J.; Atenafu, E.G.; Sweet, J.; Anson-Cartwright, L.; et al. A New Model to Predict Benign Histology in Residual Retroperitoneal Masses After Chemotherapy in Nonseminoma. Eur. Urol. Focus 2018, 4, 995–1001. [Google Scholar] [CrossRef]
- Paffenholz, P.; Nestler, T.; Hoier, S.; Pfister, D.; Hellmich, M.; Heidenreich, A. External validation of 2 models to predict necrosis/fibrosis in postchemotherapy residual retroperitoneal masses of patients with advanced testicular cancer. Urol. Oncol. 2019, 37, 809.e9–809.e18. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, D.A.; Das, S.; Jacobs, P.M.; Jeraj, R.; Lambin, P. How Advances in Imaging Will Affect Precision Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 292–298. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Liu, Z.-Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Bayanati, H.; Thornhill, R.E.; Souza, C.A.; Sethi-Virmani, V.; Gupta, A.; Maziak, D.; Amjadi, K.; Dennie, C. Quantitative CT texture and shape analysis: Can it differentiate benign and malignant mediastinal lymph nodes in patients with primary lung cancer? Eur. Radiol. 2015, 25, 480–487. [Google Scholar] [CrossRef]
- Andersen, M.B.; Harders, S.W.; Ganeshan, B.; Thygesen, J.; Torp Madsen, H.H.; Rasmussen, F. CT texture analysis can help differentiate between malignant and benign lymph nodes in the mediastinum in patients suspected for lung cancer. Acta Radiol. 2016, 57, 669–676. [Google Scholar] [CrossRef]
- Tan, X.; Ma, Z.; Yan, L.; Ye, W.; Liu, Z.; Liang, C. Radiomics nomogram outperforms size criteria in discriminating lymph node metastasis in resectable esophageal squamous cell carcinoma. Eur. Radiol. 2019, 29, 392–400. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, J.; Li, Y.; Yu, H.; Shi, S.; Xie, W.; Liu, H.; Su, Y.; Huang, J.; Lin, T. A Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Bladder Cancer. Clin. Cancer Res. 2017, 23, 6904–6911. [Google Scholar] [CrossRef]
- Lewin, J.; Dufort, P.; Halankar, J.; O’Malley, M.; Jewett, M.A.S.; Hamilton, R.J.; Gupta, A.; Lorenzo, A.; Traubici, J.; Nayan, M.; et al. Applying Radiomics to Predict Pathology of Postchemotherapy Retroperitoneal Nodal Masses in Germ Cell Tumors. JCO Clin. Cancer Inform. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Baessler, B.; Nestler, T.; Pinto Dos Santos, D.; Paffenholz, P.; Zeuch, V.; Pfister, D.; Maintz, D.; Heidenreich, A. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur. Radiol. 2020, 30, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Collewet, G.; Strzelecki, M.; Mariette, F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn. Reason. Imaging 2004, 22, 81–91. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; Bogowicz, M.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Duron, L.; Heraud, A.; Charbonneau, F.; Zmuda, M.; Savatovsky, J.; Fournier, L.; Lecler, A. A Magnetic Resonance Imaging Radiomics Signature to Distinguish Benign From Malignant Orbital Lesions. Investig. Radiol. 2021, 56, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vandendorpe, B.; Durot, C.; Lebellec, L.; Le Deley, M.C.; Sylla, D.; Bimbai, A.-M.; Amroun, K.; Ramiandrisoa, F.; Cordoba, A.; Mirabel, X.; et al. Prognostic value of the texture analysis parameters of the initial computed tomographic scan for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Radiother. Oncol. 2019, 135, 153–160. [Google Scholar] [CrossRef]
- Mali, S.A.; Ibrahim, A.; Woodruff, H.C.; Andrearczyk, V.; Müller, H.; Primakov, S.; Salahuddin, Z.; Chatterjee, A.; Lambin, P. Making Radiomics More Reproducible across Scanner and Imaging Protocol Variations: A Review of Harmonization Methods. J. Pers. Med. 2021, 11, 842. [Google Scholar] [CrossRef]
- Refaee, T.; Salahuddin, Z.; Widaatalla, Y.; Primakov, S.; Woodruff, H.C.; Hustinx, R.; Mottaghy, F.M.; Ibrahim, A.; Lambin, P. CT Reconstruction Kernels and the Effect of Pre- and Post-Processing on the Reproducibility of Handcrafted Radiomic Features. J. Pers. Med. 2022, 12, 553. [Google Scholar] [CrossRef]
- Leão, R.; van Agthoven, T.; Figueiredo, A.; Jewett, M.A.S.; Fadaak, K.; Sweet, J.; Ahmad, A.E.; Anson-Cartwright, L.; Chung, P.; Hansen, A.; et al. Serum miRNA Predicts Viable Disease after Chemotherapy in Patients with Testicular Nonseminoma Germ Cell Tumor. J. Urol. 2018, 200, 126–135. [Google Scholar] [CrossRef]


| Characteristics | No Malignant Lesion | At Least One Malignant Lesion (N = 47) | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| (N = 29) | (N = 76) | ||||||
| Age at diagnosis (years) | N = 29 | N = 47 | N = 76 | 0.051 (1) | |||
| Median-(Range) | 26 | (17.0; 45.0) | 31 | (17.0; 46.0) | 28 | (17.0; 46.0) | |
| Mean-SD | 27.3 | 7 | 30.1 | 7.4 | 29.1 | 7.4 | |
| Initial pathology of the tumor | 0.02 (2) | ||||||
| Presence of teratoma | 4 | 13.80% | 18 | 38.30% | 22 | 28.90% | |
| Predominantly teratoma | 2 | 6.90% | 8 | 17% | 10 | 13.20% | |
| Mixed with teratoma | 2 | 6.90% | 10 | 21.30% | 12 | 15.80% | |
| Absence of teratoma | 25 | 86.20% | 29 | 61.70% | 54 | 71.10% | |
| Predominantly embryonal carcinoma | 17 | 58.60% | 25 | 53.20% | 42 | 55.30% | |
| Choriocarcinoma | 1 | 3.40% | 0 | 1 | 1.30% | ||
| Predominantly vitelline tumor | 2 | 6.90% | 1 | 2.10% | 3 | 3.90% | |
| Predominantly seminoma | 3 | 10.30% | 0 | 3 | 3.90% | ||
| Burn-out tumor | 0 | 2 | 4.30% | 2 | 2.60% | ||
| Mixed without teratoma | 2 | 6.90% | 1 | 2.10% | 3 | 3.90% | |
| Chemotherapy | |||||||
| ≥3 cycles of platinum-based chemotherapy | 29 | 100% | 47 | 100% | 76 | 100% | |
| Time between end of chemotherapy and post-chemotherapy scan (weeks) (MD = 8) | N = 24 | N = 44 | N = 68 | 0.95 (1) | |||
| Median-(Range) | 4.6 | (1.3; 18.4) | 4.4 | (0.3; 364.9 *) | 4.5 | (0.3; 364.9) | |
| Mean-SD | 5.6 | 3.8 | 14.5 | 54.6 | 11.4 | 44 | |
| Time between post-chemotherapy scan and surgery (weeks) | N = 29 | N = 47 | N = 76 | 0.72 (1) | |||
| Median-(Range) | 6.9 | (0.7; 27.9) | 6.7 | (0.0; 39.9) | 6.7 | (0.0; 39.9) | |
| Mean-SD | 7.2 | 5.5 | 7.9 | 6.5 | 7.6 | 6.1 | |
| Number of persistent lesions | 0.80 (3) | ||||||
| 1 | 19 | 65.50% | 26 | 55.30% | 45 | 59.20% | |
| 2 to 5 | 9 | 31.00% | 19 | 40.40% | 28 | 36.80% | |
| 6 to 10 | 1 | 3.40% | 1 | 2.10% | 2 | 2.60% | |
| >10 | 0 | 0.00% | 1 | 2.10% | 1 | 1.30% | |
| Anatomopathology of Residual Masses | Train (N = 99) | Test (N = 50) | Total (N = 149) | |||
|---|---|---|---|---|---|---|
| Malignant lesion | ||||||
| No (Necrosis or fibrosis) | 34 | 34.3% | 18 | 36.0% | 52 | 34.9% |
| Yes | 65 | 65.7% | 32 | 64.0% | 97 | 65.1% |
| 10 | 10.1% | 2 | 4.0% | 12 | 8.1% |
| 55 | 55.6% | 30 | 60.0% | 85 | 57.0% |
| Training Dataset | Test Dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC = 0.856 (0.772–0.939) | AUC = 0.823 (0.693–0.953) | |||||||
| Threshold | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) |
| (1) 0.5621 | 89.2% (79.1–95.6) | 67.6% (49.5–82.6) | 84.1% (73.3–91.8) | 76.7% (57.7–90.1) | 90.6% (75.0–98.0) | 61.1% (35.7–82.7) | 80.6% (64.0–91.8) | 78.6% (49.2–95.3) |
| (2) 0.5966 (Youden) | 86.2% (75.3–93.5) | 76.5% (58.8–89.3) | 87.5% (76.8–94.4) | 74.3% (56.7–87.5) | 84.4% (67.2–94.7) | 66.7% (41.0–86.7) | 81.8% (64.5–93.0) | 70.6% (44.0–89.7) |
| (3) 0.5095 | 92.3% (83.0–97.5) | 64.7% (46.5–80.3) | 83.3% (72.7–91.1) | 81.5% (61.9–93.7) | 93.8% (79.2–99.2) | 44.4% (21.5–69.2) | 75.0% (58.8–87.3) | 80.0% (44.4–97.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fournier, C.; Leguillette, C.; Leblanc, E.; Le Deley, M.-C.; Carnot, A.; Pasquier, D.; Escande, A.; Taieb, S.; Ceugnart, L.; Lebellec, L. Diagnostic Value of the Texture Analysis Parameters of Retroperitoneal Residual Masses on Computed Tomographic Scan after Chemotherapy in Non-Seminomatous Germ Cell Tumors. Cancers 2023, 15, 2997. https://doi.org/10.3390/cancers15112997
Fournier C, Leguillette C, Leblanc E, Le Deley M-C, Carnot A, Pasquier D, Escande A, Taieb S, Ceugnart L, Lebellec L. Diagnostic Value of the Texture Analysis Parameters of Retroperitoneal Residual Masses on Computed Tomographic Scan after Chemotherapy in Non-Seminomatous Germ Cell Tumors. Cancers. 2023; 15(11):2997. https://doi.org/10.3390/cancers15112997
Chicago/Turabian StyleFournier, Clémence, Clémence Leguillette, Eric Leblanc, Marie-Cécile Le Deley, Aurélien Carnot, David Pasquier, Alexandre Escande, Sophie Taieb, Luc Ceugnart, and Loïc Lebellec. 2023. "Diagnostic Value of the Texture Analysis Parameters of Retroperitoneal Residual Masses on Computed Tomographic Scan after Chemotherapy in Non-Seminomatous Germ Cell Tumors" Cancers 15, no. 11: 2997. https://doi.org/10.3390/cancers15112997
APA StyleFournier, C., Leguillette, C., Leblanc, E., Le Deley, M.-C., Carnot, A., Pasquier, D., Escande, A., Taieb, S., Ceugnart, L., & Lebellec, L. (2023). Diagnostic Value of the Texture Analysis Parameters of Retroperitoneal Residual Masses on Computed Tomographic Scan after Chemotherapy in Non-Seminomatous Germ Cell Tumors. Cancers, 15(11), 2997. https://doi.org/10.3390/cancers15112997






