Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Entry Requirement
2.2. c4G12 Treatment
2.3. Radiation Therapy
2.4. Evaluation of Intrathoracic Response
2.5. Evaluation of Survival
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Dogs
3.2. Treatment and Adverse Events
3.3. Intrathoracic Response and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bostock, D.E. Prognosis after surgical excision of canine melanomas. Vet. Pathol. 1979, 16, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Fratini, F.; Corazza, M.; Castagnaro, M.; Zappulli, V.; Poli, A. Proliferation activity in oral and cutaneous canine melanocytic tumours: Correlation with histological parameters, location, and clinical behaviour. Res. Vet. Sci. 2002, 73, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; MacEwen, E.G. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Investig. 2000, 18, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.P.; Hahn, K.A.; Harris, F.D.; King, G.K. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997). J. Vet. Intern. Med. 2003, 17, 96–101. [Google Scholar] [PubMed]
- Murphy, S.; Hayes, A.M.; Blackwood, L.; Maglennon, G.; Pattinson, H.; Sparkes, A.H. Oral malignant melanoma—The effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet. Comp. Oncol. 2005, 3, 222–229. [Google Scholar] [CrossRef]
- Dank, G.; Rassnick, K.M.; Sokolovsky, Y.; Garrett, L.D.; Post, G.S.; Kitchell, B.E.; Sellon, R.K.; Kleiter, M.; Northrup, N.; Segev, G. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet. Comp. Oncol. 2014, 12, 78–84. [Google Scholar] [CrossRef]
- Cancedda, S.; Rohrer Bley, C.; Aresu, L.; Dacasto, M.; Leone, V.F.; Pizzoni, S.; Gracis, M.; Marconato, L. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet. Comp. Oncol. 2016, 14, e146–e157. [Google Scholar] [CrossRef]
- Bateman, K.E.; Catton, P.A.; Pennock, P.W.; Kruth, S.A. 0-7-21 radiation therapy for the treatment of canine oral melanoma. J. Vet. Intern. Med. 1994, 8, 267–272. [Google Scholar] [CrossRef]
- Blackwood, L.; Dobson, J.M. Radiotherapy of oral malignant melanomas in dogs. J. Am. Vet. Med. Assoc. 1996, 209, 98–102. [Google Scholar]
- Kawabe, M.; Mori, T.; Ito, Y.; Murakami, M.; Sakai, H.; Yanai, T.; Maruo, K. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1146–1153. [Google Scholar] [CrossRef]
- Tollett, M.A.; Duda, L.; Brown, D.C.; Krick, E.L. Palliative radiation therapy for solid tumors in dogs: 103 cases (2007–2011). J. Am. Vet. Med. Assoc. 2016, 248, 72–82. [Google Scholar] [CrossRef]
- Proulx, D.R.; Ruslander, D.M.; Dodge, R.K.; Hauck, M.L.; Williams, L.E.; Horn, B.; Price, G.S.; Thrall, D.E. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet. Radiol. Ultrasound 2003, 44, 352–359. [Google Scholar] [CrossRef]
- Rassnick, K.M.; Ruslander, D.M.; Cotter, S.M.; Al-Sarraf, R.; Bruyette, D.S.; Gamblin, R.M.; Meleo, K.A.; Moore, A.S. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989–2000). J. Am. Vet. Med. Assoc. 2001, 218, 1444–1448. [Google Scholar] [CrossRef]
- Boria, P.A.; Murry, D.J.; Bennett, P.F.; Glickman, N.W.; Snyder, P.W.; Merkel, B.L.; Schlittler, D.L.; Mutsaers, A.J.; Thomas, R.M.; Knapp, D.W. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 388–394. [Google Scholar] [CrossRef]
- Almela, R.M.; Ansón, A. A review of immunotherapeutic strategies in canine malignant melanoma. Vet. Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Palata, O.; Hradilova Podzimkova, N.; Nedvedova, E.; Umprecht, A.; Sadilkova, L.; Palova Jelinkova, L.; Spisek, R.; Adkins, I. Radiotherapy in combination with cytokine treatment. Front. Oncol. 2019, 9, 367. [Google Scholar] [CrossRef]
- Frey, B.; Rubner, Y.; Wunderlich, R.; Weiss, E.M.; Pockley, A.G.; Fietkau, R.; Gaipl, U.S. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—Implications for cancer therapies. Curr. Med. Chem. 2012, 19, 1751–1764. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Fan, T.M.; Selting, K.A. Exploring the potential utility of pet dogs with cancer for studying radiation-induced immunogenic cell death strategies. Front. Oncol. 2018, 8, 680. [Google Scholar] [CrossRef]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef]
- Chiang, C.S.; Fu, S.Y.; Wang, S.C.; Yu, C.F.; Chen, F.H.; Lin, C.M.; Hong, J.H. Irradiation promotes an M2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, M.R.; Cottam, B.; Savage, T.; Nguyen, C.; Newell, P.; Gough, M.J. Expression of NF-κB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 2012, 7, e39295. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Ganss, R.; Hanahan, D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998, 58, 4673–4681. [Google Scholar]
- Sznol, M.; Chen, L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Res. 2013, 19, 1021–1034. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Ikebuchi, R.; Okagawa, T.; Adachi, M.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; Murata, S.; et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade. PLoS ONE 2014, 9, e98415. [Google Scholar] [CrossRef]
- Hartley, G.; Faulhaber, E.; Caldwell, A.; Coy, J.; Kurihara, J.; Guth, A.; Regan, D.; Dow, S. Immune regulation of canine tumour and macrophage PD-L1 expression. Vet. Comp. Oncol. 2017, 15, 534–549. [Google Scholar] [CrossRef]
- Shosu, K.; Sakurai, M.; Inoue, K.; Nakagawa, T.; Sakai, H.; Morimoto, M.; Okuda, M.; Noguchi, S.; Mizuno, T. Programmed cell death ligand 1 expression in canine cancer. In Vivo 2016, 30, 195–204. [Google Scholar]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef]
- Kumar, S.R.; Kim, D.Y.; Henry, C.J.; Bryan, J.N.; Robinson, K.L.; Eaton, A.M. Programmed death ligand 1 is expressed in canine B cell lymphoma and downregulated by MEK inhibitors. Vet. Comp. Oncol. 2017, 15, 1527–1536. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef]
- Hartley, G.; Elmslie, R.; Dow, S.; Guth, A. Checkpoint molecule expression by B and T cell lymphomas in dogs. Vet. Comp. Oncol. 2018, 16, 352–360. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. npj Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef]
- Gulay, K.C.M.; Aoshima, K.; Maekawa, N.; Suzuki, T.; Konnai, S.; Kobayashi, A.; Kimura, T. Hemangiosarcoma cells induce M2 polarization and PD-L1 expression in macrophages. Sci. Rep. 2022, 12, 2124. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Sato, H.; Okonogi, N.; Nakano, T. Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int. J. Clin. Oncol. 2020, 25, 801–809. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yasui, T.; Tamari, K.; Minami, K.; Otani, K.; Isohashi, F.; Seo, Y.; Kambe, R.; Koizumi, M.; Ogawa, K.; et al. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS ONE 2017, 12, e0189697. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Veterinary Cooperative Oncology Group (VCOG). Veterinary cooperative oncology group—Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Simeone, E.; Giannarelli, D.; Muto, P.; Falivene, S.; Borzillo, V.; Giugliano, F.M.; Sandomenico, F.; Petrillo, A.; Curvietto, M.; et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014, 3, e28780. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.; Finet, A.; Boru, B.; Beauchet, A.; Mazeron, J.J.; Otzmeguine, Y.; Blom, A.; Longvert, C.; de Maleissye, M.F.; Fort, M.; et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology 2018, 7, e1442166. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Kaira, K.; Hashimoto, K.; Mouri, A.; Miura, Y.; Shiono, A.; Nishihara, F.; Murayama, Y.; Noda, S.E.; Kato, S.; et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac. Cancer 2019, 10, 992–1000. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, Y.; Zhao, X.; Pan, J.; Xu, Z.; Zhang, L. Optimizing the treatment schedule of radiotherapy combined with anti-PD-1/PD-L1 immunotherapy in metastatic cancers. Front. Oncol. 2021, 11, 638873. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Liniker, E.; Menzies, A.M.; Kong, B.Y.; Cooper, A.; Ramanujam, S.; Lo, S.; Kefford, R.F.; Fogarty, G.B.; Guminski, A.; Wang, T.W.; et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016, 5, e1214788. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-year overall survival with durvalumab after chemoradiotherapy in Stage III NSCLC-update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef]
- Wei, J.; Montalvo-Ortiz, W.; Yu, L.; Krasco, A.; Ebstein, S.; Cortez, C.; Lowy, I.; Murphy, A.J.; Sleeman, M.A.; Skokos, D. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci. Immunol. 2021, 6, eabg0117. [Google Scholar] [CrossRef]
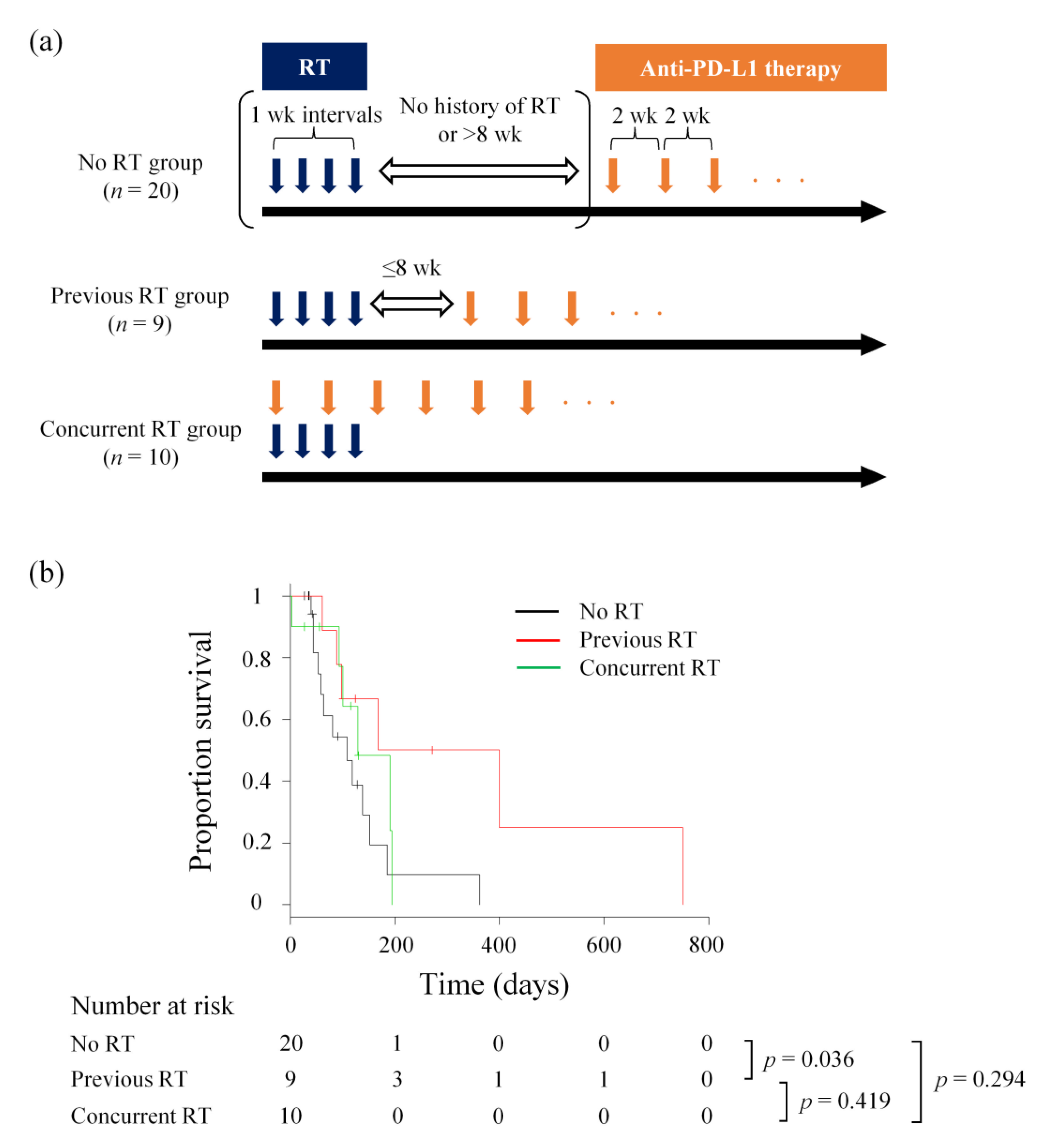
| No RT (n = 20) | Previous RT (n = 9) | Concurrent RT (n = 10) | |
|---|---|---|---|
| Breed | |||
| American cocker spaniel | 1 (5) | 1 (11) | 0 |
| Beagle | 1 (5) | 0 | 0 |
| Chihuahua | 1 (5) | 0 | 1 (10) |
| Flat-coated retriever | 1 (5) | 0 | 0 |
| Golden retriever | 1 (5) | 2 (22) | 0 |
| Kaninchen dachshund | 1 (5) | 0 | 0 |
| Labrador retriever | 1 (5) | 1 (11) | 0 |
| Miniature dachshund | 6 (30) | 2 (22) | 4 (40) |
| Papillon | 1 (5) | 0 | 0 |
| Pekingese | 0 | 1 (11) | 0 |
| Pomeranian | 0 | 1 (11) | 0 |
| Pug | 2 (10) | 0 | 1 (10) |
| Toy poodle | 1 (5) | 0 | 2 (20) |
| Yorkshire terrier | 1 (5) | 0 | 0 |
| Mix | 2 (10) | 1 (11) | 2 (20) |
| Sex | |||
| Male | 10 (50) | 7 (78) | 4 (40) |
| Castrated | 6 (30) | 5 (56) | 4 (40) |
| Intact | 4 (20) | 2 (22) | 0 |
| Female | 10 (50) | 2 (22) | 6 (60) |
| Spayed | 8 (40) | 1 (11) | 4 (40) |
| Intact | 2 (10) | 1 (11) | 2 (20) |
| Age | |||
| ≤10 years | 2 (10) | 2 (22) | 1 (10) |
| >10 years | 18 (90) | 7 (78) | 9 (90) |
| Body Weight | |||
| ≤5 kg | 6 (30) | 2 (22) | 1 (10) |
| 5–10 kg | 10 (50) | 3 (33) | 9 (90) |
| >10 kg | 4 (20) | 4 (44) | 0 |
| Primary tumour site | |||
| Mandible | 10 (50) | 2 (22) | 5 (50) |
| Maxilla | 8 (40) | 7 (78) | 5 (50) |
| Unspecified | 2 (10) | 0 | 0 |
| No RT (n = 20) | Previous RT (n = 9) | Concurrent RT (n = 10) | ||||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Any Grade | Grade 3 | Any Grade | Grade 3 | |
| Any | 12 (60) | 2 (10) | 4 (44) | 3 (33) | 6 (60) | 2 (20) |
| Anorexia | 0 | 0 | 1 (11) | 0 | 0 | 0 |
| Diarrhoea | 2 (10) | 0 | 1 (11) | 0 | 2 (20) | 0 |
| Vomiting | 3 (15) | 0 | 2 (22) | 0 | 2 (20) | 0 |
| Alkaline phosphatase | 1 (5) | 0 | 0 | 0 | 2 (20) | 1 (10) |
| ALT | 5 (25) | 0 | 3 (33) | 1 (11) | 1 (10) | 0 |
| AST | 0 | 0 | 3 (33) | 1 (11) | 1 (10) | 1 (10) |
| BUN | 0 | 0 | 0 | 0 | 2 (20) | 1 (10) |
| Albumin, low | 0 | 0 | 1 (11) | 0 | 0 | 0 |
| Glucose, low | 0 | 0 | 1 (11) | 0 | 0 | 0 |
| Lipase | 2 (10) | 0 | 1 (11) | 1 (11) | 1 (10) | 0 |
| CPK | 0 | 0 | 1 (11) | 0 | 0 | 0 |
| Conjunctivitis/ocular surface disease | 1 (5) | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 2 (10) | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 1 (10) | 0 |
| Pneumonitis/pulmonary infiltrates | 1 (5) | 1 (5) | 0 | 0 | 0 | 0 |
| Allergic reaction | 0 | 0 | 0 | 0 | 1 (10) | 0 |
| Anaphylaxis | 1 (5) | 1 (5) | 0 | 0 | 0 | 0 |
| No RT (n = 20) | Previous RT (n = 9) | Concurrent RT (n = 10) | |
|---|---|---|---|
| CR | 1 (5) | 4 (44) | 0 |
| SD or SD/PR * | 1 (5) | 1 (11) | 2 (20) |
| PD | 15 (75) | 4 (44) | 6 (60) |
| NE | 3 (15) | 0 | 2 (20) |
| Clinical benefit ** | 2 (10) | 5 (56) † | 2 (20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers 2023, 15, 3013. https://doi.org/10.3390/cancers15113013
Deguchi T, Maekawa N, Konnai S, Owaki R, Hosoya K, Morishita K, Nakamura M, Okagawa T, Takeuchi H, Kim S, et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers. 2023; 15(11):3013. https://doi.org/10.3390/cancers15113013
Chicago/Turabian StyleDeguchi, Tatsuya, Naoya Maekawa, Satoru Konnai, Ryo Owaki, Kenji Hosoya, Keitaro Morishita, Motoji Nakamura, Tomohiro Okagawa, Hiroto Takeuchi, Sangho Kim, and et al. 2023. "Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma" Cancers 15, no. 11: 3013. https://doi.org/10.3390/cancers15113013
APA StyleDeguchi, T., Maekawa, N., Konnai, S., Owaki, R., Hosoya, K., Morishita, K., Nakamura, M., Okagawa, T., Takeuchi, H., Kim, S., Kinoshita, R., Tachibana, Y., Yokokawa, M., Takagi, S., Kato, Y., Suzuki, Y., Murata, S., & Ohashi, K. (2023). Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers, 15(11), 3013. https://doi.org/10.3390/cancers15113013








