Statin Medication Improves Five-Year Survival Rates in Patients with Head and Neck Cancer: A Retrospective Case-Control Study of about 100,000 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Data Acquisition, Inclusion and Exclusion Criteria, and Patient Matching
2.3. Data Analysis
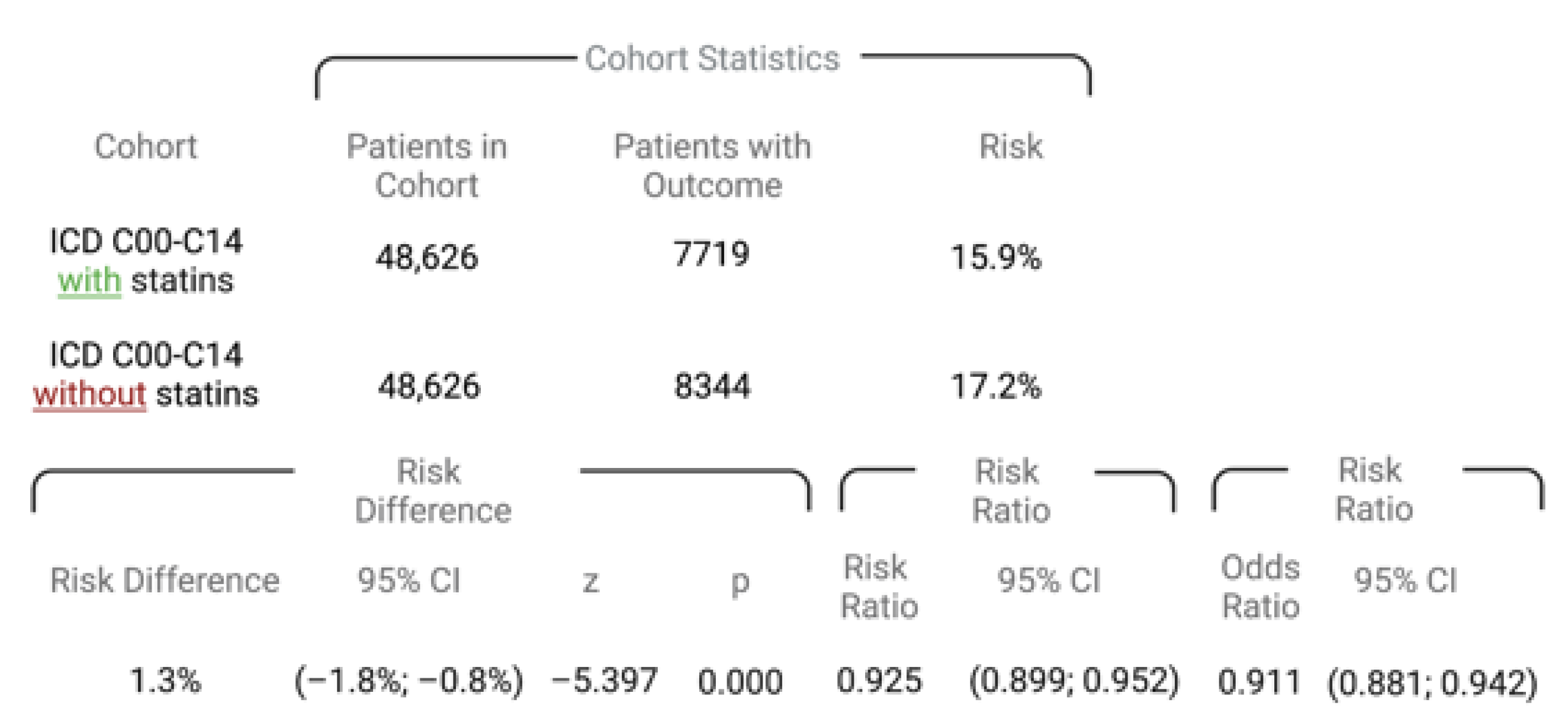
3. Results
3.1. Assessment, Allocation, and Matching
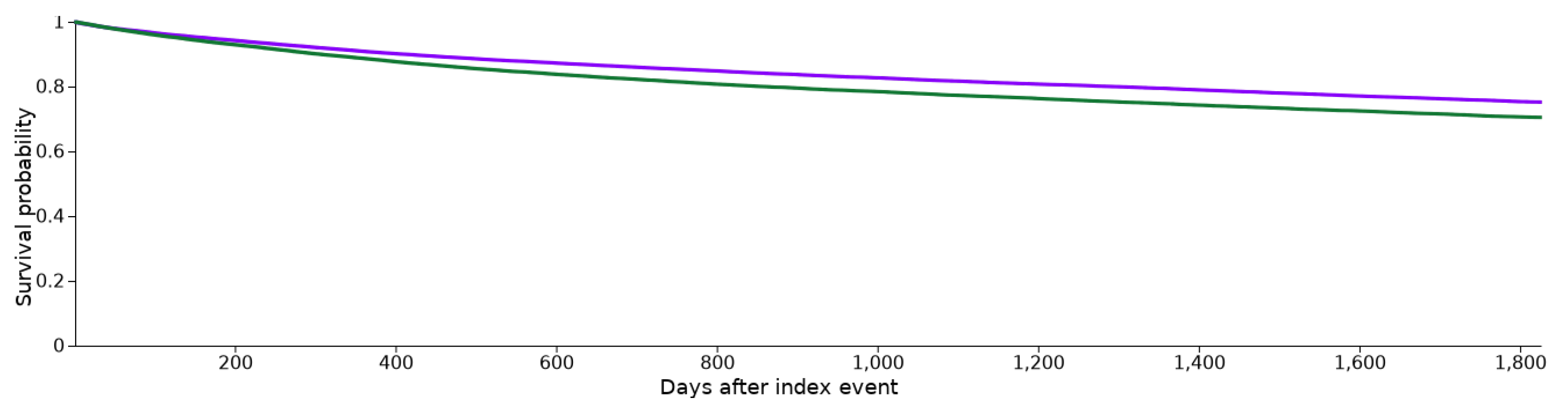
3.2. Patient Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moura, A.C.; Assad, D.X.; Amorim Dos Santos, J.; Porto de Toledo, I.; Barra, G.B.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Worldwide prevalence of PI3K-AKT-mTOR pathway mutations in head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103284. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and-unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, C.; Shen, Y.; Zhou, H.; Shao, Y.; Zhu, W.; Chen, Y. Impact of statin use on cancer-specific mortality and recurrence: A meta-analysis of 60 observational studies. Medicine 2020, 99, 19596. [Google Scholar] [CrossRef] [PubMed]
- Matusewicz, L.; Meissner, J.; Toporkiewicz, M.; Sikorski, A.F. The effect of statins on cancer cells—Review. Tumour Biol. 2015, 36, 4889–4904. [Google Scholar] [CrossRef]
- Brown, M.S.; Faust, J.R.; Goldstein, J.L.; Kaneko, I.; Endo, A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 1978, 253, 1121–1128. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.L.; Kumar, S.; Weyman, C.M.; Liu, W.; Zhou, A. Statins induce apoptosis in ovarian cancer cells through activation of JNK and enhancement of Bim expression. Cancer Chemother. Pharm. 2009, 63, 997–1005. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Chen, H.; Xu, X.C. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2008, 17, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mehibel, M.; Ortiz-Martinez, F.; Voelxen, N.; Boyers, A.; Chadwick, A.; Telfer, B.A.; Mueller-Klieser, W.; West, C.M.; Critchlow, S.E.; Williams, K.J.; et al. Statin-induced metabolic reprogramming in head and neck cancer: A biomarker for targeting monocarboxylate transporters. Sci. Rep. 2018, 8, 16804. [Google Scholar] [CrossRef] [PubMed]
- Galland, S.; Martin, P.; Fregni, G.; Letovanec, I.; Stamenkovic, I. Attenuation of the pro-inflammatory signature of lung cancer-derived mesenchymal stromal cells by statins. Cancer Lett. 2020, 484, 50–64. [Google Scholar] [CrossRef]
- Wang, K.H.; Liu, C.H.; Ding, D.C. Statins as Repurposed Drugs in Gynecological Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13937. [Google Scholar] [CrossRef]
- Ma, L.; Niknejad, N.; Gorn-Hondermann, I.; Dayekh, K.; Dimitroulakos, J. Lovastatin induces multiple stress pathways including LKB1/AMPK activation that regulate its cytotoxic effects in squamous cell carcinoma cells. PLoS ONE 2012, 7, e46055. [Google Scholar] [CrossRef]
- Dimitroulakos, J.; Marhin, W.H.; Tokunaga, J.; Irish, J.; Gullane, P.; Penn, L.Z.; Kamel-Reid, S. Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia 2002, 4, 337–346. [Google Scholar] [CrossRef]
- Niknejad, N.; Morley, M.; Dimitroulakos, J. Activation of the integrated stress response regulates lovastatin-induced apoptosis. J. Biol. Chem. 2007, 282, 29748–29756. [Google Scholar] [CrossRef]
- Takeda, I.; Maruya, S.; Shirasaki, T.; Mizukami, H.; Takahata, T.; Myers, J.N.; Kakehata, S.; Yagihashi, S.; Shinkawa, H. Simvastatin inactivates beta1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007, 98, 890–899. [Google Scholar] [CrossRef]
- Wang, W.; Le, W.; Cho, D.Y.; Hwang, P.H.; Upadhyay, D. Novel effects of statins in enhancing efficacy of chemotherapy in vitro in nasopharyngeal carcinoma. Int. Forum Allergy Rhinol. 2011, 1, 284–289. [Google Scholar] [CrossRef]
- Gabryś, D.; Dörfler, A.; Yaromina, A.; Hessel, F.; Krause, M.; Oertel, R.; Baumann, M. Effects of lovastatin alone or combined with irradiation on tumor cells in vitro and in vivo. Strahlenther Onkol. 2008, 184, 48–53. [Google Scholar] [CrossRef]
- Mantha, A.J.; Hanson, J.E.; Goss, G.; Lagarde, A.E.; Lorimer, I.A.; Dimitroulakos, J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin. Cancer Res. 2005, 11, 2398–2407. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, M.; Sun, C.; Qu, G.; Shi, T.; Min, M.; Wu, Y.; Sun, Y. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas 2019, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Zhang, R.; Xia, Y.; Shao, Z.; Mei, Z. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharm. Res. 2019, 141, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Stokes, W.; Eguchi, M.; Hararah, M.; Amini, A.; Mueller, A.; Morgan, R.; Bradley, C.; Raben, D.; McDermott, J.; et al. Statin use associated with improved overall and cancer specific survival in patients with head and neck cancer. Oral Oncol. 2019, 90, 54–66. [Google Scholar] [CrossRef]
- Bourguillon, R.O.; Stokes, W.A.; Dorth, J.; Schmitt, N.C. Repurposing Statin Drugs to Decrease Toxicity and Improve Survival Outcomes in Head and Neck Cancer. OTO Open 2021, 5, 2473974X211065715. [Google Scholar] [CrossRef] [PubMed]
- Lebo, N.L.; Griffiths, R.; Hall, S.; Dimitroulakos, J.; Johnson-Obaseki, S. Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck 2018, 40, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Getz, K.R.; Bellile, E.; Zarins, K.R.; Rullman, C.; Chinn, S.B.; Taylor, J.M.G.; Rozek, L.S.; Wolf, G.T.; Mondul, A.M. Statin use and head and neck squamous cell carcinoma outcomes. Int. J. Cancer 2021, 148, 2440–2448. [Google Scholar] [CrossRef]
- Heym, M.; Heiland, M.; Preissner, R.; Huebel, C.; Nahles, S.; Schmidt-Westhausen, A.M.; Preissner, S.; Hertel, M. The risk of oral squamous cell carcinoma in patients with and without somatoform disorders including bruxism: A retrospective evaluation of 309,278 individuals. Front. Oncol. 2022, 12, 1080492. [Google Scholar] [CrossRef]
- Hertel, M.; Hagedorn, L.; Schmidt-Westhausen, A.M.; Dommisch, H.; Heiland, M.; Preissner, R.; Preissner, S. Comparison of five-year survival rates among patients with oral squamous cell carcinoma with and without association with syphilis: A retrospective case-control study. BMC Cancer 2022, 22, 454. [Google Scholar] [CrossRef]
- Zhao, G.; Ji, Y.; Ye, Q.; Ye, X.; Wo, G.; Chen, X.; Shao, X.; Tang, J. Effect of statins use on risk and prognosis of breast cancer: A meta-analysis. Anticancer Drugs 2022, 33, e507–e518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, X.; Chen, L.; Ma, T.; Tang, J.; Zhao, J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015, 41, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Ding, Y.; Chen, H.; Sun, L. Statin uses and mortality in colorectal cancer patients: An updated systematic review and meta-analysis. Cancer Med. 2019, 8, 3305–3313. [Google Scholar] [CrossRef]
- Raval, A.D.; Thakker, D.; Negi, H.; Vyas, A.; Kaur, H.; Salkini, M.W. Association between statins and clinical outcomes among men with prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2016, 19, 151–162. [Google Scholar] [CrossRef]
- Gray, R.T.; Coleman, H.G.; Hughes, C.; Murray, L.J.; Cardwell, C.R. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016, 45, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Nayan, M.; Punjani, N.; Juurlink, D.N.; Finelli, A.; Austin, P.C.; Kulkarni, G.S.; Uleryk, E.; Hamilton, R.J. Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis. Cancer Treat Rev. 2017, 52, 105–116. [Google Scholar] [CrossRef]
- Wang, J.; Li, X. Impact of statin use on the risk and prognosis of hepatocellular carcinoma: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1603–1609. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Wang, Z.; Luo, Z.; Zhou, X. Relationship between the use of statins and patient survival in colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126944. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, N.; Li, H.F.; Zhang, C.J.; Gong, Y.Z.; Liao, D.F.; Qin, L. Ezetimibe and Cancer: Is There a Connection? Front. Pharm. 2022, 13, 831657. [Google Scholar] [CrossRef]
- Ouchi, Y.; Sasaki, J.; Arai, H.; Yokote, K.; Harada, K.; Katayama, Y.; Urabe, T.; Uchida, Y.; Hayashi, M.; Yokota, N.; et al. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation 2019, 140, 992–1003. [Google Scholar] [CrossRef]
- Mourikis, P.; Zako, S.; Dannenberg, L.; Nia, A.M.; Heinen, Y.; Busch, L.; Richter, H.; Hohlfeld, T.; Zeus, T.; Kelm, M.; et al. Lipid lowering therapy in cardiovascular disease: From myth to molecular reality. Pharmacol. Ther. 2020, 213, 107592. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe is an inhibitor of tumor angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef]
- He, J.; Shin, H.; Wei, X.; Kadegowda, A.K.; Chen, R.; Xie, S.K. NPC1L1 knockout protects against colitis-associated tumorigenesis in mice. BMC Cancer 2015, 15, 189. [Google Scholar] [CrossRef]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Chowdhury, A.; Chaudhury, K.; Shukla, P.C. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188581. [Google Scholar] [CrossRef] [PubMed]
- Pavan, L.M.; Rêgo, D.F.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N. In vitro Anti-Tumor Effects of Statins on Head and Neck Squamous Cell Carcinoma: A Systematic Review. PLoS ONE 2015, 10, e0130476. [Google Scholar] [CrossRef]
- Winquist, E.; Agbassi, C.; Meyers, B.M.; Yoo, J.; Chan, K.K.W. Systemic therapy in the curative treatment of head and neck squamous cell cancer: A systematic review. J. Otolaryngol. Head Neck Surg. 2017, 46, 29. [Google Scholar] [CrossRef]
- Wushou, A.; Hou, J.; Zhao, Y.J.; Miao, X.C. Postoperative adjuvant radiotherapy improves loco-regional recurrence of head and neck mucosal melanoma. J. Cranio-Maxillo-Facial Surg. 2015, 43, 553–558. [Google Scholar] [CrossRef]
- Habib, A. Management of advanced hypopharyngeal carcinoma: Systematic review of survival following surgical and non-surgical treatments. J. Laryngol. Otol. 2018, 132, 385–400. [Google Scholar] [CrossRef]
- Reiersen, D.A.; Pahilan, M.E.; Devaiah, A.K. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol. Head Neck Surg. 2012, 147, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; MacNeil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region With Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef]
- Russo, D.P.; Tham, T.; Bardash, Y.; Kraus, D. The effect of race in head and neck cancer: A meta-analysis controlling for socioeconomic status. Am. J. Otolaryngol. 2020, 41, 102624. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Takemoto, N.; Oya, R.; Inohara, H. Prognostic impact of sarcopenia in patients with head and neck cancer treated with surgery or radiation: A meta-analysis. PLoS ONE 2021, 16, e0259288. [Google Scholar] [CrossRef] [PubMed]
- Van der Elst, S.; Bardash, Y.; Wotman, M.; Kraus, D.; Tham, T. The prognostic impact of depression or depressive symptoms on patients with head and neck cancer: A systematic review and meta-analysis. Head Neck 2021, 43, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef]
- Koyanagi, Y.N.; Matsuo, K.; Ito, H.; Wakai, K.; Nagata, C.; Nakayama, T.; Sadakane, A.; Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; et al. Cigarette smoking and the risk of head and neck cancer in the Japanese population: A systematic review and meta-analysis. Jpn. J. Clin. Oncol. 2016, 46, 580–595. [Google Scholar] [CrossRef]

| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients (n) | Cohort I | Cohort II | p-Value | Standardized Mean Difference | Cohort I | Cohort II | p-Value | Standardized Mean Difference |
| Total | 54,238 | 122,588 | <0.001 | 0.582 | 48,626 | 48,626 | 0.189 | 0.008 |
| Female | 16,966 (31.3%) | 37,309 (33.0%) | <0.001 | 0.038 | 15,409 (31.7%) | 15,432 (31.7%) | 0.874 | 0.001 |
| Male | 37,309 (68.7%) | 82,100 (67.0%) | <0.001 | 0.038 | 33,212 (68.3%) | 33,187 (68.2%) | 0.863 | 0.001 |
| Mean age at diagnosis (years) | 66.7 | 58.5 | 66.3 | 66.4 | ||||
| Standard deviation | 11.2 | 16.7 | 11.4 | 11.5 | ||||
| ICD-10 Z87.891 | 11,307 (20.8%) | 5935 (4.8%) | <0.001 | 0.0492 | 5710 (11.7%) | 5596 (11.5%) | 0.254 | 0.007 |
| ICD-10 F10.1 | 2839 (5.2%) | 2935 (2.4%) | <0.001 | 0.149 | 2088 (4.3%) | 2.047 (4.2%) | 0.515 | 0.004 |
| ICD-10 F10.2 | 2348 (4.3%) | 2520 (2.1%) | <0.001 | 0.129 | 1689 (3.5%) | 1658 (3.4%) | 0.586 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wüster, J.; Heiland, M.; Nahles, S.; Preissner, R.; Preissner, S. Statin Medication Improves Five-Year Survival Rates in Patients with Head and Neck Cancer: A Retrospective Case-Control Study of about 100,000 Patients. Cancers 2023, 15, 3093. https://doi.org/10.3390/cancers15123093
Wüster J, Heiland M, Nahles S, Preissner R, Preissner S. Statin Medication Improves Five-Year Survival Rates in Patients with Head and Neck Cancer: A Retrospective Case-Control Study of about 100,000 Patients. Cancers. 2023; 15(12):3093. https://doi.org/10.3390/cancers15123093
Chicago/Turabian StyleWüster, Jonas, Max Heiland, Susanne Nahles, Robert Preissner, and Saskia Preissner. 2023. "Statin Medication Improves Five-Year Survival Rates in Patients with Head and Neck Cancer: A Retrospective Case-Control Study of about 100,000 Patients" Cancers 15, no. 12: 3093. https://doi.org/10.3390/cancers15123093
APA StyleWüster, J., Heiland, M., Nahles, S., Preissner, R., & Preissner, S. (2023). Statin Medication Improves Five-Year Survival Rates in Patients with Head and Neck Cancer: A Retrospective Case-Control Study of about 100,000 Patients. Cancers, 15(12), 3093. https://doi.org/10.3390/cancers15123093







