Novel Multi-Modal Therapies and Their Prognostic Potential in Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
- ‘laparoscopy’, ‘peritoneal cytology’, ‘cancer staging’, and ‘prognosis’, “gastric” and “stomach”, combined with “cancer or tumo?r* or adenocarcinoma or neoplasm”.
- “gastric” and “stomach” combined with “cancer or tumo?r* or adenocarcinoma or neoplasm”, “peritoneal carcinomatosis”, carcinomato* or carcino* or metast* or neoplas*”, “HIPEC”, “IPHP”, “IHCP”, “CHPP”, “hyperthermic intraperitoneal chemotherapy”, “intraperitoneal hyperthermic perfusion”, “intraperitoneal hyperthermic chemoperfusion”, “continuous hyperthermic peritoneal perfusion”, “cytoreductive surgery”, “cytoreduction”, “CRS”, “prognosis”, “survival”, “survival rate”, and “risk ratio”.
- “gastric” and “stomach” combined with “cancer or tumo?r* or adenocarcinoma or neoplasm”, “peritoneal carcinomatosis”, “carcinomato* or carcino* or metast* or neoplas*”, (pressur* or laparoscopic*); (intra-periton* or intra?periton* or “intra periton*” or intra-abdominal* or intra?abdominal or “intra abdominal*”); (chemo?therap* or chemo or therap* or treat*); PIPAC* or ePIPAC* or PITAC*. The three strings were then combined using the AND modifier.
2.2. Selection of Studies
2.3. Outcome Measures and Data Extraction
2.4. Quality Assessment of Selected Studies
2.5. Statistical Methods
3. Results
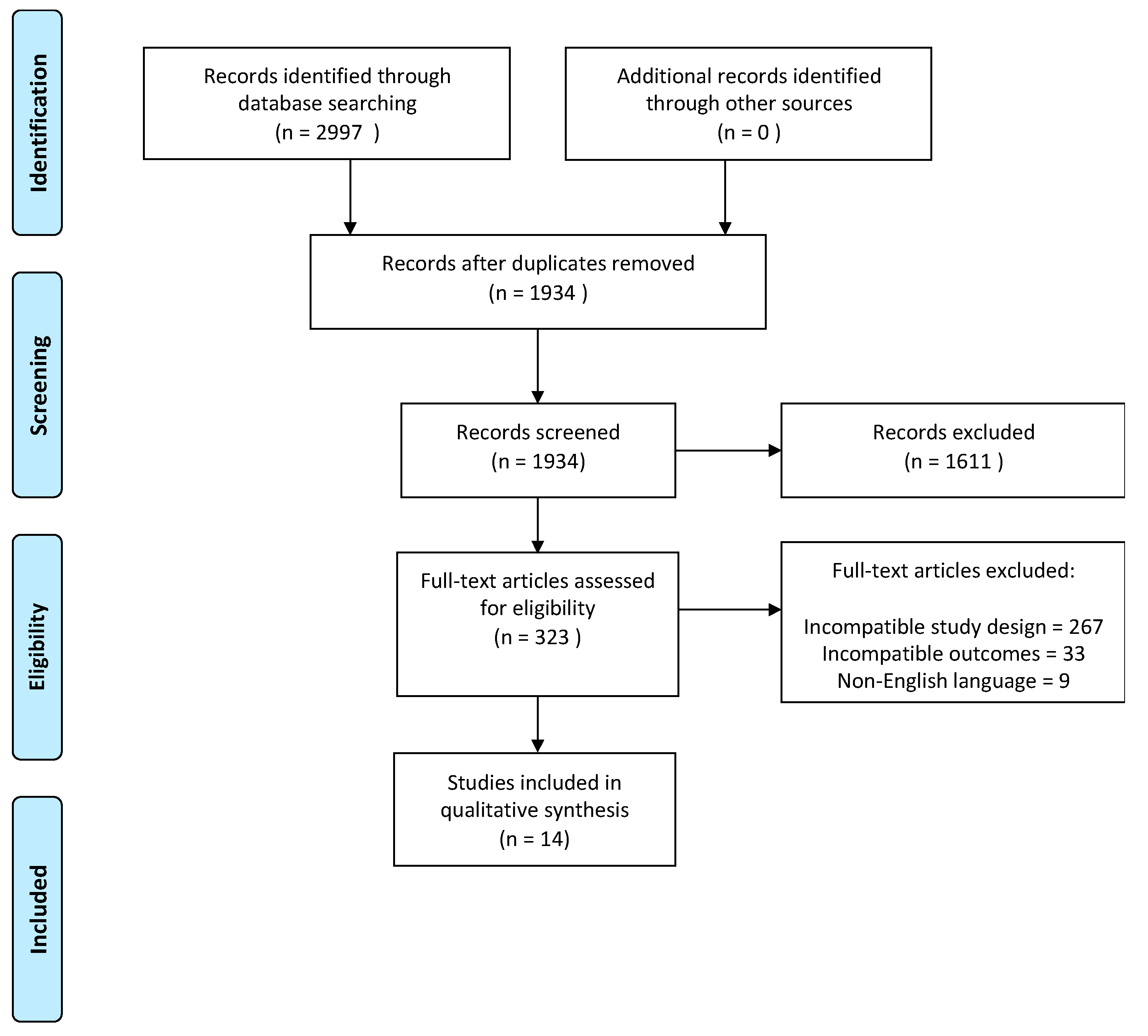
3.1. Search Results
3.2. Patient and Study Characteristics
3.3. HIPEC and Overall Median Survival
3.4. HIPEC and Short-Term Survival
3.5. HIPEC and Long-Term Survival
3.6. PIPAC and Median Survival
3.7. Completeness of Cytoreduction, PCI, and One-Year Survival
4. Discussion
5. Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Maehara, Y.; Hasuda, S.; Koga, T.; Tokunaga, E.; Kakeji, Y.; Sugimachi, K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br. J. Surg. 2000, 87, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Marrelli, D.; De Manzoni, G.; Morgagni, P.; Di Leo, A.; Saragoni, L.; De Stefano, A. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br. J. Surg. 2003, 90, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Endou, Y.; Sasaki, T.; Hirano, M.; Mizumoto, A.; Matsuda, T.; Takao, N.; Ichinose, M.; Miura, M.; Li, Y. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur. J. Surg. Oncol. 2010, 36, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Janunger, K.G.; Hafström, L.; Glimelius, B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur. J. Surg. 2002, 168, 597–608. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Waddell, T.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer+: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi57–vi63. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Prabhu, A.; Mishra, S.; Shah, M.; Shaikh, S.; et al. The Pathologic Peritoneal Cancer Index (PCI) Strongly Differs from the Surgical PCI in Peritoneal Metastases Arising from Various Primary Tumors. Ann. Surg. Oncol. 2020, 27, 2985–2996. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kaibara, N.; Hamazoe, R.; Iitsuka, Y.; Maeta, M.; Koga, S. Hyperthermic peritoneal perfusion combined with anticancer chemotherapy as prophylactic treatment of peritoneal recurrence of gastric cancer. Hepatogastroenterology 1989, 36, 75–78. [Google Scholar]
- Ikeguchi, M.; Kondou, A.; Oka, A.; Tsujitani, S.; Maeta, M.; Kaibara, N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur. J. Surg. Acta Chir. 1995, 161, 581–586. [Google Scholar]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999, 85, 529–534. [Google Scholar] [CrossRef]
- Fujimura, T.; Yonemura, Y.; Muraoka, K.; Takamura, H.; Hirono, Y.; Sahara, H.; Ninomiya, I.; Matsumoto, H.; Tsugawa, K.; Nishimura, G.; et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: Randomized controlled study. World J. Surg. 1994, 18, 150–155. [Google Scholar] [CrossRef]
- Cui, H.B.; Ge, H.E.; Bai, X.Y.; Zhang, W.; Zhang, Y.Y.; Wang, J.; Li, X.; Xing, L.P.; Guo, S.H.; Wang, Z.Y. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp. Ther. Med. 2014, 7, 1083–1088. [Google Scholar] [CrossRef]
- Yu, W.; Whang, I.; Chung, H.Y.; Averbach, A.; Sugarbaker, P.H. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: Results of a prospective randomized trial. World J. Surg. 2001, 25, 985–990. [Google Scholar] [CrossRef]
- Rau, B.; Loeffler, M.; Rau, H.-G.; Sulkowski, U.; Kuhlmann, J.; Weimann, A.; Keck, T.; Angele, M.; Topp, S.A.; Koenigsrainer, A.; et al. Perioperative chemotherapy and cytoreductive surgery with versus without HIPEC in gastric cancer with limited peritoneal metastases: A randomized phase III study (GASTRIPEC). J. Clin. Oncol. 2015, 33, TPS4132. [Google Scholar] [CrossRef]
- Beeharry, M.K.; Zhu, Z.L.; Liu, W.T.; Yao, X.X.; Yan, M.; Zhu, Z.G. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study. BMC Cancer 2019, 19, 932. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Hamazoe, R.; Maeta, M.; Kaibara, N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994, 73, 2048–2052. [Google Scholar] [CrossRef] [PubMed]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Königsrainer, A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: A phase II study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846402. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: A descriptive cohort study. Clin. Exp. Metastasis 2020, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Khomyakov, V.M.; Ryabov, A.B.; Kolobaev, I.V.; Bolotina, L.V.; Utkina, A.B.; Sobolev, D.D.; Kuznetsova, O.S.; Kaprin, A.D. Pressurized intraperitoneal aerosol chemotherapy combined with system chemotherapy—A new approach to treatment of gastric cancer patients with peritonal carcinomatosis. Sib. J. Oncol. 2020, 19, 49–58. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef]
- Glehen, O.; Schreiber, V.; Cotte, E.; Sayag-Beaujard, A.C.; Osinsky, D.; Freyer, G.; François, Y.; Vignal, J.; Gilly, F.N. Cytoreductive Surgery and Intraperitoneal Chemohyperthermia for Peritoneal Carcinomatosis Arising from Gastric Cancer. Arch. Surg. 2004, 139, 20–26. [Google Scholar] [CrossRef]
- Hall, J.J.; Loggie, B.W.; Shen, P.; Beamer, S.; Case, L.D.; McQuellon, R.; Geisinger, K.R.; Levine, E.A. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J. Gastrointest. Surg. 2004, 8, 454–463. [Google Scholar] [CrossRef]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Takahashi, S.; Sawa, T.; Matsuki, N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br. J. Surg. 2005, 92, 370–375. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Alyami, M.; Sgarbura, O.; Khomyakov, V.; Horvath, P.; Vizzielli, G.; So, J.; Torrent, J.; Delgadillo, X.; Martin, D.; Ceelen, W.; et al. Standardizing training for Pressurized Intraperitoneal Aerosol Chemotherapy. Eur. J. Surg. Oncol. 2020, 46, 2270–2275. [Google Scholar] [CrossRef]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Schena, C.A.; El Halabieh, M.A.; Abatini, C.; Vita, E.; Strippoli, A.; Inzani, F.; Rodolfino, E.; Romanò, B.; Pacelli, F.; et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A bidirectional approach for gastric cancer peritoneal metastasis. Surg. Oncol. 2020, 34, 270–275. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Larbre, V.; Alyami, M.; Mercier, F.; Vantard, N.; Bonnefoy, I.; Opsomer, M.A.; Villeneuve, L.; Bakrin, N.; Rioufol, C.; Glehen, O.; et al. No renal toxicity after repeated treatment with pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with unresectable peritoneal metastasis. Anticancer Res. 2018, 38, 6869–6875. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Yonemura, Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J. Gastrointest. Oncol. 2010, 2, 85. [Google Scholar] [CrossRef]
- Coccolini, F.; Catena, F.; Glehen, O.; Yonemura, Y.; Sugarbaker, P.H.; Piso, P.; Montori, G.; Ansaloni, L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2015, 41, 911–919. [Google Scholar] [CrossRef]
- Canbay, E.; Mizumoto, A.; Ichinose, M.; Ishibashi, H.; Sako, S.; Hirano, M.; Takao, N.; Yonemura, Y. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann. Surg. Oncol. 2014, 21, 1147–1152. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A propensity score analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Goetze, T.O.; Mueller, D.W.; Vogel, A.; Winkler, M.; Lorenzen, S.; Novotny, A.; Pauligk, C.; Homann, N.; Jungbluth, T.; et al. The RENAISSANCE (AIO-FLOT5) trial: Effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—A phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017, 17, 893. [Google Scholar] [CrossRef]
- Jamel, S.; Markar, S.R.; Malietzis, G.; Acharya, A.; Athanasiou, T.; Hanna, G.B. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: Systematic review and meta-analysis. Gastric Cancer 2018, 21, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Takiguchi, S.; Nakajima, K.; Miyata, H.; Yamasaki, M.; Kurokawa, Y.; Okada, K.; Mori, M.; Doki, Y. Neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal dissemination. Ann. Surg. Oncol. 2011, 18, 3726–3731. [Google Scholar] [CrossRef]
- Aizawa, M.; Matsuki, A.; Yabusaki, H.; Nashimoto, A. Clinical impact of induction chemotherapy followed by surgery for gastric cancer with positive peritoneal cytology. J. Clin. Oncol. 2014, 32, 4050. [Google Scholar] [CrossRef]
- Shim, H.J.; Kim, H.J.; Lee, S.H.; Bae, W.K.; Hwang, E.C.; Cho, S.H.; Chung, I.J.; Bang, H.J.; Hwang, J.E. Observational Study of Peritoneal Washing Cytology-Positive Gastric Cancer without Gross Peritoneal Metastasis in Patients who Underwent Radical D2 Gastrectomy. Sci. Rep. 2020, 10, 9549. [Google Scholar] [CrossRef]
- Nakamura, M.; Ojima, T.; Nakamori, M.; Katsuda, M.; Tsuji, T.; Hayata, K.; Kato, T.; Yamaue, H. Conversion Surgery for Gastric Cancer with Peritoneal Metastasis Based on the Diagnosis of Second-Look Staging Laparoscopy. J. Gastrointest. Surg. 2019, 23, 1758–1766. [Google Scholar] [CrossRef]
- Yasufuku, I.; Nunobe, S.; Ida, S.; Kumagai, K.; Ohashi, M.; Hiki, N.; Sano, T. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer 2020, 23, 319–327. [Google Scholar] [CrossRef]
- Leake, P.A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Law, C.; Coburn, N.G. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012, 15, 38–47. [Google Scholar] [CrossRef]
- Gęca, K.; Rawicz-Pruszyński, K.; Mlak, R.; Sędłak, K.; Skórzewska, M.; Pelc, Z.; Małecka-Massalska, T.; Polkowski, W.P. Molecular cytology by one-step nucleic acid amplification (Osna) assay of peritoneal washings during d2 gastrectomy in advanced gastric cancer patients: Preliminary results. J. Clin. Med. 2021, 10, 5230. [Google Scholar] [CrossRef]
- Gęca, K.; Rawicz-Pruszyński, K.; Mielko, J.; Mlak, R.; Sędłak, K.; Polkowski, W.P. Rapid Detection of Free Cancer Cells in Intraoperative Peritoneal Lavage Using One-Step Nucleic Acid Amplification (OSNA) in Gastric Cancer Patients. Cells 2020, 9, 2168. [Google Scholar] [CrossRef]
- Deng, K.; Zhu, H.; Chen, M.; Wu, J.; Hu, R.; Tang, C. Prognostic significance of molecular analysis of peritoneal fluid for patients with gastric cancer: A meta-analysis. PLoS ONE 2016, 11, e0151608. [Google Scholar] [CrossRef]
- Kumagai, K.; Yamamoto, N.; Miyashiro, I.; Tomita, Y.; Katai, H.; Kushima, R.; Tsuda, H.; Kitagawa, Y.; Takeuchi, H.; Mukai, M.; et al. Multicenter study evaluating the clinical performance of the OSNA assay for the molecular detection of lymph node metastases in gastric cancer patients. Gastric Cancer 2014, 17, 273–280. [Google Scholar] [CrossRef]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef]
- Koemans, W.J.; Van Der Kaaij, R.T.; Boot, H.; Buffart, T.; Veenhof, A.A.F.A.; Hartemink, K.J.; Grootscholten, C.; Snaebjornsson, P.; Retel, V.P.; Van Tinteren, H.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 2019, 19, 420. [Google Scholar] [CrossRef]
- Eveno, C.; Jouvin, I.; Pocard, M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 2018, 3, 20180116. [Google Scholar] [CrossRef]
- Glehen, O.; Horvath, P.; Hübner, M.; Hyung-Ho, K.; Königsrainer, A.; Pocard, M.; Reymond, M.A.; So, J.; Fristrup, C.W.; Mortensen, M.B. The ISSPP PIPAC database: Design, process, access, and first interim analysis. Pleura Peritoneum 2021, 6, 91–97. [Google Scholar] [CrossRef]
| Study | HIPEC/CRS (n) | CRS (n) | Age (HIPEC; CRS) | Sex | T2 (%) | T3–T4 (%) | N0–N1 | HIPEC Characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agents | Temperature (°C) | Duration (mins) | |||||||||
| Kaibara et al. (1989) | 42 | 40 | - | - | - | 100 | - | - | MMC | 44–45 | 60 |
| Fujimura et al. (1994) | 22 | 18 | 60.2 63.2 | 12 9 | 45 | 55 | 23 | 16 | MMC CDDP | 41 | 60 |
| Hamazoe et al. (1994) | 42 | 40 | 56.5 (10.4) 63.4 (9.6) | 25 31 | 19.5 | 80.5 | 26 | MMC | 48 | 60 | |
| Ikeguchi et al. (1995) | 78 | 96 | 100 | MMC | 44–45 | 60 | |||||
| Takahashi et al. (1995) | 56 | 57 | 54.5 55.7 | 34 34 | - | - | - | - | MMC | 180 | |
| Fujimoto et al. (1991) | 71 | 70 | 58.5 (8.1) 59.2 (9.1) | 50 51 | 17 | 83 | 11 | 130 | MMC | 45 | 120 |
| Yu et al. (2001) | 125 | 123 | 54 55 | 84 81 | 30.6 | 69.4 | 165 | 83 | MMC 5-FU | 37 | |
| Yang et al. (2011) | 34 | 34 | 50 (24–74) 51 (28–75) | 16 19 | - | - | - | - | CDDP MMC | 43 | 60–90 |
| Cui et al. (2014) | 96 | 96 | 39–72 39–70 | 22 21 | - | - | - | - | CDDP 5-FU | 41–43 | 90 |
| Beeharry et al. (2019) | 40 | 40 | 59 (10) 58 (10) | 23 23 | 100 | 27 | 53 | CDDP | 42 | 60 | |
| Rau et al. (2015) | 53 | 52 | - | - | - | - | - | - | MMC CDDP | 42 | 60 |
| Study | Age | Sex | Number of PIPAC Sessions | Chemotherapy Used for PIPAC | Interval between PIPAC Sessions | Bidirectional with SACT (%) | Systemic Regimen | Median OS (Months) | One-Year OS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Khomyakov et al. (2016) | - | - | 56 | Cisplatin Doxorubicin | 6 weeks | Yes (100%) | XELOX | 13.0 | 49.80% |
| Struller et al. (2019) | 55.1 (13) | 10 | 43 | Cisplatin (7.5) + Doxorubicin (1.5) | 6 weeks | No | N/A | NS | NS |
| Ellebᴂk et al. (2020) | 58.5 (31–70) | 7 | 52 (11 ePIPAC) | Cisplatin (7.5) + Doxorubicin (1.5) | 4–6 weeks | Yes (n = 9, 45%) | NS | 11.5 | NS |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessments | Incomplete Outcome Data | Selective Reporting | Other Biases |
|---|---|---|---|---|---|---|---|
| Kaibara et al. (1989) | + | + | + | + | - | + | ? |
| Fujimura et al. (1994) | + | + | + | + | - | + | ? |
| Hamazoe et al. (1994) | + | + | + | + | - | - | ? |
| Ikeguchi et al. (1995) | + | + | + | + | - | - | ? |
| Takahashi et al. (1995) | + | + | + | + | + | - | ? |
| Fujimoto et al. (1991) | + | + | + | + | + | - | ? |
| Yu et al. (2001) | + | + | + | + | + | + | + |
| Yang et al. (2011) | + | + | + | + | + | + | + |
| Cui et al. (2014) | + | + | + | + | + | + | + |
| Beeharry et al. (2019) | + | + | + | + | + | + | + |
| Rau et al. (2015) | + | + | + | + | + | + | + |
| Khomyakov et al. (2016) | + | + | + | + | + | + | ? |
| Struller et al. (2019) | + | + | + | + | + | + | ? |
| Ellebᴂk et al. (2020) | + | + | + | + | + | + | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chidambaram, S.; Guiral, D.C.; Markar, S.R. Novel Multi-Modal Therapies and Their Prognostic Potential in Gastric Cancer. Cancers 2023, 15, 3113. https://doi.org/10.3390/cancers15123113
Chidambaram S, Guiral DC, Markar SR. Novel Multi-Modal Therapies and Their Prognostic Potential in Gastric Cancer. Cancers. 2023; 15(12):3113. https://doi.org/10.3390/cancers15123113
Chicago/Turabian StyleChidambaram, Swathikan, Delia Cortés Guiral, and Sheraz Rehan Markar. 2023. "Novel Multi-Modal Therapies and Their Prognostic Potential in Gastric Cancer" Cancers 15, no. 12: 3113. https://doi.org/10.3390/cancers15123113
APA StyleChidambaram, S., Guiral, D. C., & Markar, S. R. (2023). Novel Multi-Modal Therapies and Their Prognostic Potential in Gastric Cancer. Cancers, 15(12), 3113. https://doi.org/10.3390/cancers15123113









