GTN Enhances Antitumor Effects of Doxorubicin in TNBC by Targeting the Immunosuppressive Activity of PMN-MDSC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Models and Treatments
2.2. Cell Lines and Treatments
2.3. Isolation of Primary Mouse Immune Cells
2.4. RT-qPCR
2.5. Flow Cytometry Analysis
2.6. Immunohistochemistry
2.7. RNAseq
2.8. Biotin Switch Assay (BSA)
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Addition of a NO Donor (GTN) Strongly Increases Doxorubicin Anti-Tumor Efficacy
3.2. CD8 TILs Are Essential for the Anti-Tumor Efficiency of the Doxorubicin/GTN Combination
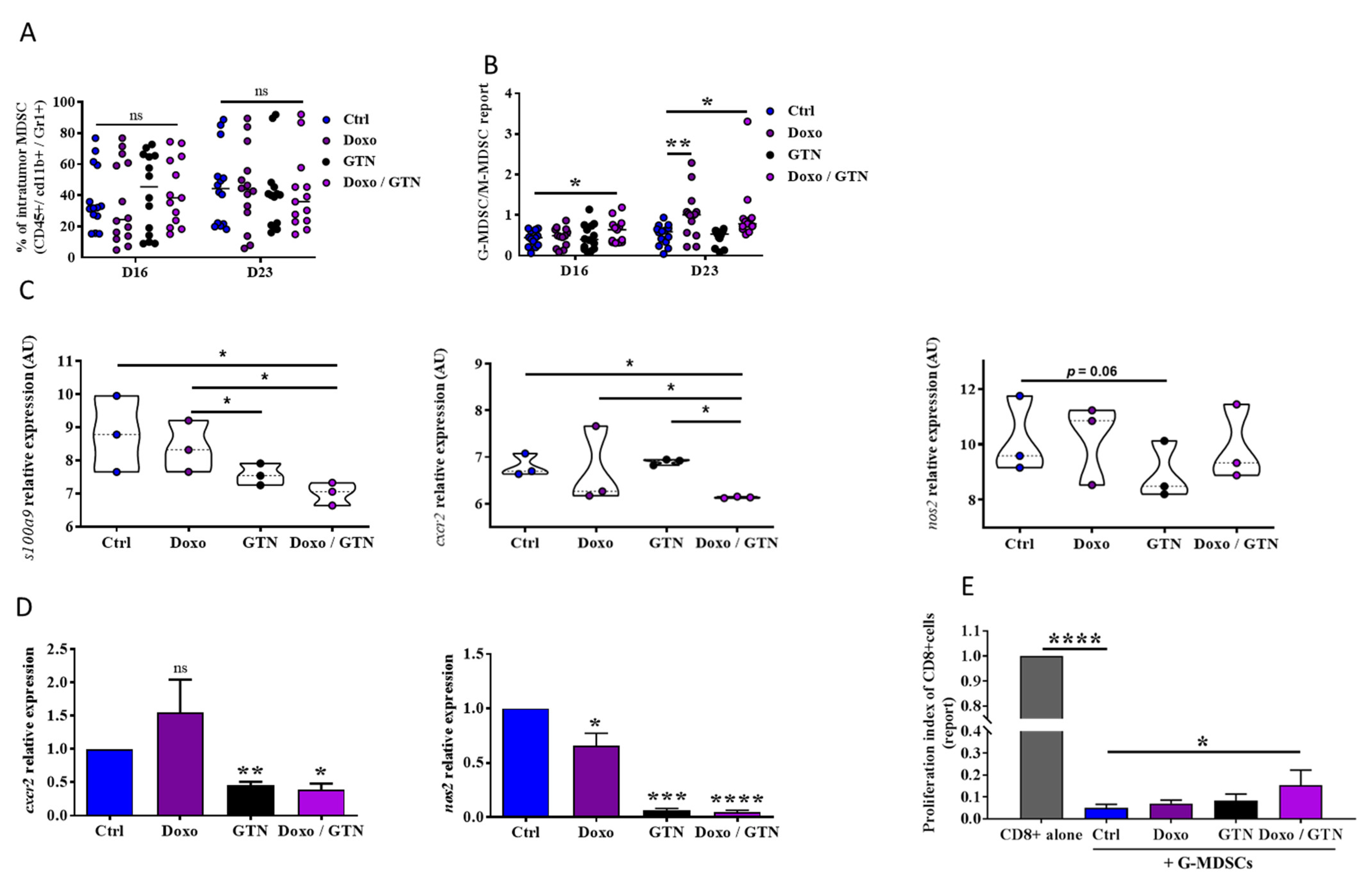
3.3. Doxorubicin/GTN Combination Increases the Intratumor Level of PMN-MDSCs with a Low Immunosuppressive Activity
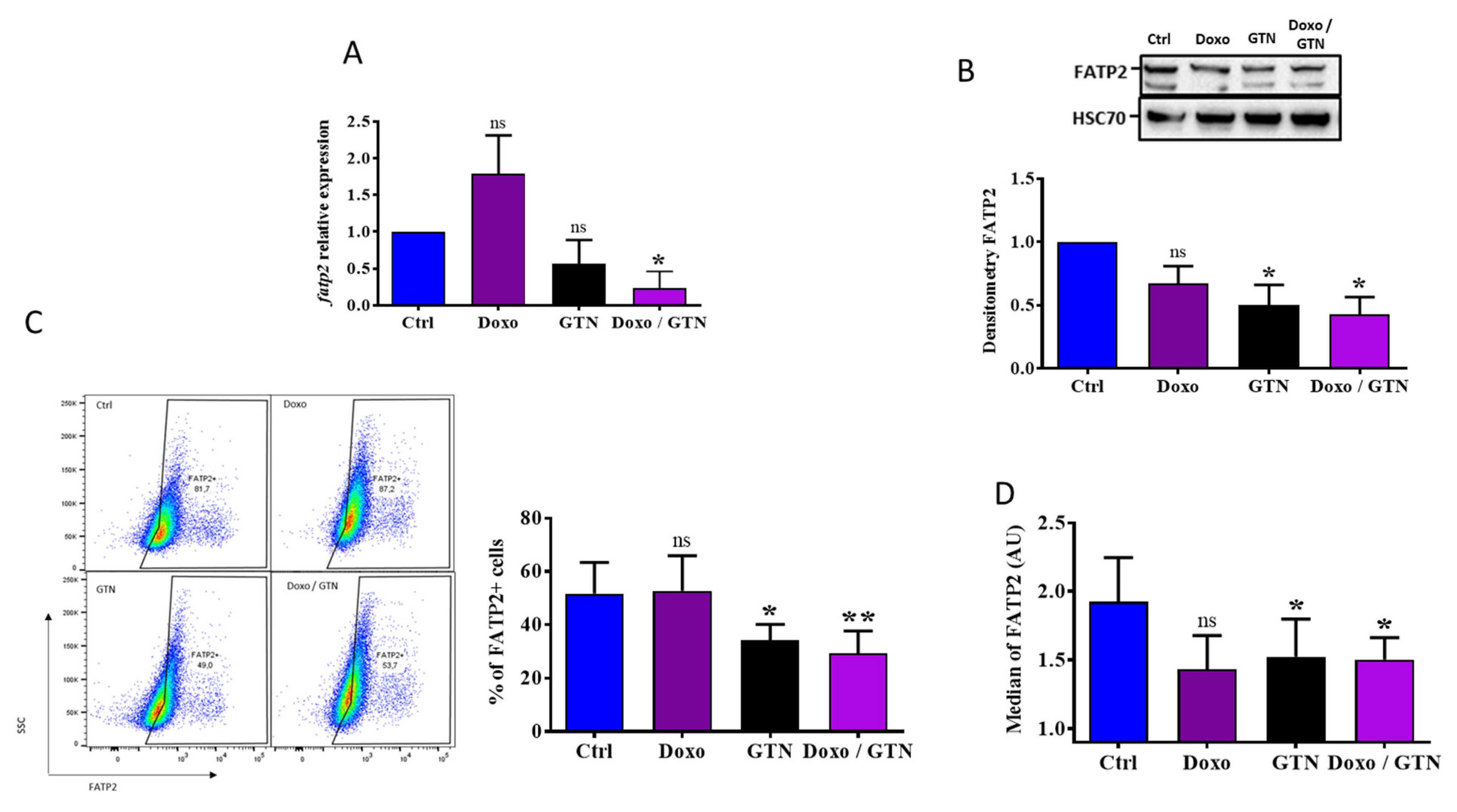
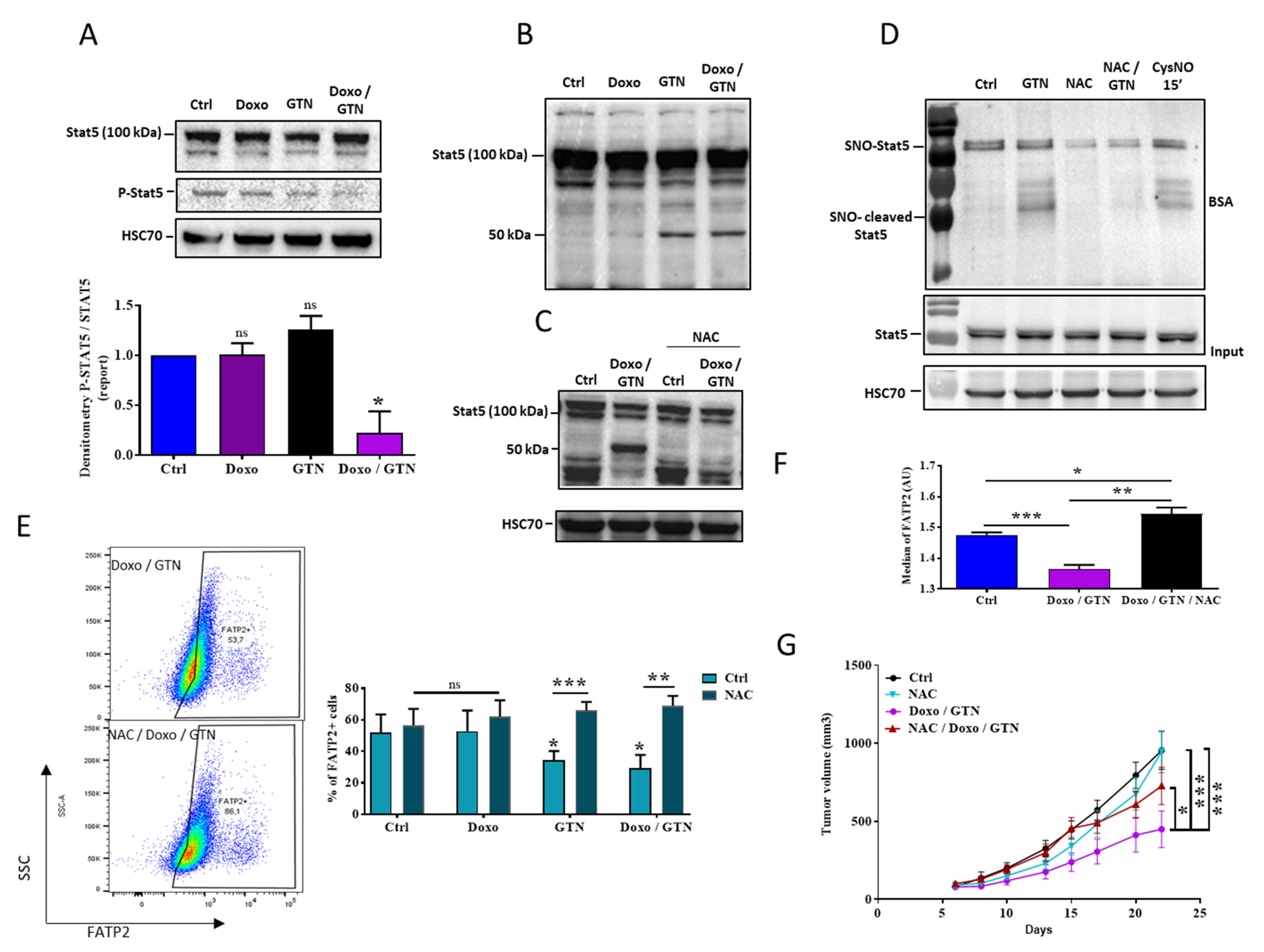
3.4. The Doxorubicin/GTN Combination Regulates the FATP2 Signaling Pathway in PMN-MDSCs
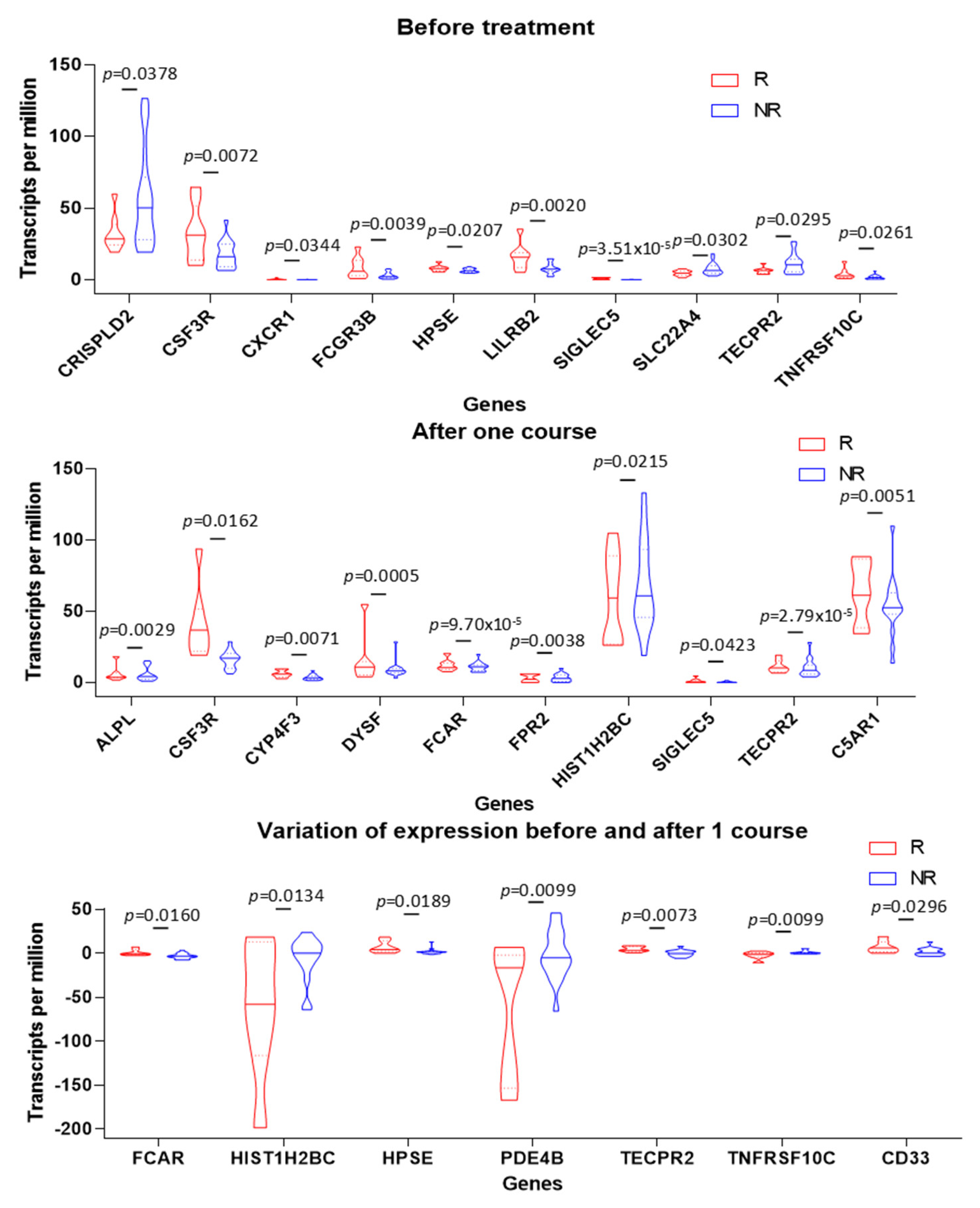
3.5. PMN Gene Signature in Tumors Is Associated with Positive Clinical Outcome in TNBC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergin, A.R.T.; Loi, S. Triple-negative breast cancer: Recent treatment advances. F1000Research 2019, 8, 1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef]
- Ali, H.R.; Glont, S.E.; Blows, F.M.; Provenzano, E.; Dawson, S.J.; Liu, B.; Hiller, L.; Dunn, J.; Poole, C.J.; Bowden, S.; et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann. Oncol. 2015, 26, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Todd, P.A.; Goa, K.L.; Langtry, H.D. Transdermal nitroglycerin (glyceryl trinitrate). A review of its pharmacology and therapeutic use. Drugs 1990, 40, 880–902. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Blake, M.; de la Mata-Moya, M.D.; Corona, F.; Turcott, J.; Orta, D.; Alexander-Alatorre, J.; Gallardo-Rincón, D. Phase II study. Concurrent chemotherapy and radiotherapy with nitroglycerin in locally advanced non-small cell lung cancer. Radiother. Oncol. 2014, 111, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Siemens, D.R.; Heaton, J.P.; Adams, M.A.; Kawakami, J.; Graham, C.H. Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology 2009, 74, 878–883. [Google Scholar] [CrossRef]
- Millet, A.; Bettaieb, A.; Renaud, F.; Prevotat, L.; Hammann, A.; Solary, E.; Mignotte, B.; Jeannin, J.F. Influence of the nitric oxide donor glyceryl trinitrate on apoptotic pathways in human colon cancer cells. Gastroenterology 2002, 123, 235–246. [Google Scholar] [CrossRef]
- Prévotat, L.; Filomenko, R.; Solary, E.; Jeannin, J.F.; Bettaieb, A. Nitric oxide-induced down-regulation of beta-catenin in colon cancer cells by a proteasome-independent specific pathway. Gastroenterology 2006, 131, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Leon-Bollotte, L.; Subramaniam, S.; Cauvard, O.; Plenchette-Colas, S.; Paul, C.; Godard, C.; Martinez-Ruiz, A.; Legembre, P.; Jeannin, J.F.; Bettaieb, A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology 2011, 140, 2009–2018.e4. [Google Scholar] [CrossRef]
- Bouaouiche, S.; Ghione, S.; Sghaier, R.; Burgy, O.; Racoeur, C.; Derangère, V.; Bettaieb, A.; Plenchette, S. Nitric Oxide-Releasing Drug Glyceryl Trinitrate Targets JAK2/STAT3 Signaling, Migration and Invasion of Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 8449. [Google Scholar] [CrossRef]
- Lamrani, M.; Sassi, N.; Paul, C.; Yousfi, N.; Boucher, J.L.; Gauthier, N.; Labbé, J.; Seignez, C.; Racoeur, C.; Athias, A.; et al. TLR4/IFNγ pathways induce tumor regression via NOS II-dependent NO and ROS production in murine breast cancer models. Oncoimmunology 2016, 5, e1123369. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Seignez, C.; Racoeur, C.; Isambert, N.; Mabrouk, N.; Scagliarini, A.; Reveneau, S.; Arnould, L.; Bettaieb, A.; Jeannin, J.F.; et al. Tumor-derived granzyme B-expressing neutrophils acquire antitumor potential after lipid A treatment. Oncotarget 2018, 9, 28364–28378. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Treger, S.; Ackerman, S.; Kaplan, V.; Ghanem, S.; Nadir, Y. Progestin type affects the increase of heparanase level and procoagulant activity mediated by the estrogen receptor. Hum. Reprod. 2021, 36, 61–69. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Anders, R.A.; Taube, J.M.; Qiu, X.; Mulgaonkar, A.; Liu, X.; Harrington, S.M.; Guo, J.; Xin, Y.; et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J. Clin. Investig. 2018, 128, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 2008, 181, 4666–4675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullock, K.; Richmond, A. Suppressing MDSC Recruitment to the Tumor Microenvironment by Antagonizing CXCR2 to Enhance the Efficacy of Immunotherapy. Cancers 2021, 13, 6293. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Renz, B.W.; Ilmer, M.; Koch, D.; Yang, Y.; Werner, J.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Solid Tumors. Cells 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Bettaieb, A.; Plenchette, S.; Paul, C.; Laurens, V.; Romagny, S.; Jeannin, J.F. S-nitrosylation of cancer cells. In Nitric Oxide Cancer; Springer: Berlin/Heidelberg, Germany, 2015; pp. 97–109. [Google Scholar]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.; Baay-Guzman, G.; Gonzalez-Bonilla, C.R.; Livingston, E.H.; Huerta-Yepez, S.; Bonavida, B. In vitro and in vivo sensitization of SW620 metastatic colon cancer cells to CDDP-induced apoptosis by the nitric oxide donor DETANONOate: Involvement of AIF. Nitric Oxide 2009, 20, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Bratasz, A.; Selvendiran, K.; Wasowicz, T.; Bobko, A.; Khramtsov, V.V.; Ignarro, L.J.; Kuppusamy, P. NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols. J. Transl. Med. 2008, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Yasuda, H.; Hatachi, Y.; Xue, D.; Sasaki, T.; Yamaya, M.; Sakamori, Y.; Togashi, Y.; Masago, K.; Ito, I.; et al. Nitric oxide (NO) enhances pemetrexed cytotoxicity via NO-cGMP signaling in lung adenocarcinoma cells in vitro and in vivo. Int. J. Oncol. 2012, 41, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef] [Green Version]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Anichini, A.; Mortarini, R.; Maccalli, C.; Squarcina, P.; Fleischhauer, K.; Mascheroni, L.; Parmiani, G. Cytotoxic T cells directed to tumor antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J. Immunol. 1996, 156, 208–217. [Google Scholar] [CrossRef]
- Topalian, S.L.; Solomon, D.; Rosenberg, S.A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J. Immunol. 1989, 142, 3714–3725. [Google Scholar] [CrossRef]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Simons, D.L.; Jimenez, G.; Jung, J.Y.; Melstrom, L.; Margolin, K.; Yim, J.H.; Kruper, L.; et al. Human breast tumor-infiltrating CD8. Nat. Commun. 2018, 9, 4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Li, H.; Xie, Z.; Jin, T.; Chen, Y.; Lv, Z.; Tan, X.; Li, J.; Han, G.; He, W.; et al. Quantitative multiplex immunofluorescence analysis identifies infiltrating PD1. Thorac. Cancer 2020, 11, 2941–2954. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.; Miska, J.; Wainwright, D.A.; Dey, M.; Rivetta, C.V.; Yu, D.; Kanojia, D.; Pituch, K.C.; Qiao, J.; Pytel, P.; et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5671–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Ma, X.L.; Wei, Y.Q.; Wei, X.W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef]
- Zhou, J.; Nefedova, Y.; Lei, A.; Gabrilovich, D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin. Immunol. 2018, 35, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Urueña, C.; Lasso, P.; Bernal-Estevez, D.; Rubio, D.; Salazar, A.J.; Olaya, M.; Barreto, A.; Tawil, M.; Torregrosa, L.; Fiorentino, S. The breast cancer immune microenvironment is modified by neoadjuvant chemotherapy. Sci. Rep. 2022, 12, 7981. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shu, X.; Xu, J.; Su, S.M.; Chan, U.I.; Mo, L.; Liu, J.; Zhang, X.; Adhav, R.; Chen, Q.; et al. S100A9-CXCL12 activation in BRCA1-mutant breast cancer promotes an immunosuppressive microenvironment associated with resistance to immunotherapy. Nat. Commun. 2022, 13, 1481. [Google Scholar] [CrossRef]
- Dagvadorj, A.; Tan, S.H.; Liao, Z.; Xie, J.; Nurmi, M.; Alanen, K.; Rui, H.; Mirtti, T.; Nevalainen, M.T. N-terminal truncation of Stat5a/b circumvents PIAS3-mediated transcriptional inhibition of Stat5 in prostate cancer cells. Int. J. Biochem. Cell Biol. 2010, 42, 2037–2046. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Nakajima, H.; Ikeda, K.; Tamachi, T.; Hiwasa, T.; Saito, Y.; Iwamoto, I. Stat6-protease but not Stat5-protease is inhibited by an elastase inhibitor ONO-5046. Biochem. Biophys. Res. Commun. 2003, 309, 768–773. [Google Scholar] [CrossRef]
- Oda, A.; Wakao, H.; Fujita, H. Calpain is a signal transducer and activator of transcription (STAT) 3 and STAT5 protease. Blood 2002, 99, 1850–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Moriggl, R.; Stravopodis, D.; Carpino, N.; Marine, J.C.; Teglund, S.; Feng, J.; Ihle, J.N. A small amphipathic alpha-helical region is required for transcriptional activities and proteasome-dependent turnover of the tyrosine-phosphorylated Stat5. EMBO J. 2000, 19, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Guarneri, V.; Gennari, A. Myelopoiesis, metabolism and therapy: A crucial crossroads in cancer progression. Cell Stress 2019, 3, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.; Nair, L.C.R.; Johnson, B.S.; Thomas, P.L.; Padmanabhan, R.A.; Puthumadathil, N.; Laloraya, M. Transcriptional Regulation of Nos2 via STAT5B Binding to Nos2 Gene Promoter Mediates Nitric Oxide Production: Relevance in β-Cell Maintenance. Cell. Physiol. Biochem. 2019, 52, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, D.; Angulo, P.; Ponnurangam, S.; Dandawate, P.; Ramamoorthy, P.; Srinivasan, P.; Iwakuma, T.; Weir, S.J.; Chastain, K.; Anant, S. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabrouk, N.; Racoeur, C.; Shan, J.; Massot, A.; Ghione, S.; Privat, M.; Dondaine, L.; Ballot, E.; Truntzer, C.; Boidot, R.; et al. GTN Enhances Antitumor Effects of Doxorubicin in TNBC by Targeting the Immunosuppressive Activity of PMN-MDSC. Cancers 2023, 15, 3129. https://doi.org/10.3390/cancers15123129
Mabrouk N, Racoeur C, Shan J, Massot A, Ghione S, Privat M, Dondaine L, Ballot E, Truntzer C, Boidot R, et al. GTN Enhances Antitumor Effects of Doxorubicin in TNBC by Targeting the Immunosuppressive Activity of PMN-MDSC. Cancers. 2023; 15(12):3129. https://doi.org/10.3390/cancers15123129
Chicago/Turabian StyleMabrouk, Nesrine, Cindy Racoeur, Jingxuan Shan, Aurélie Massot, Silvia Ghione, Malorie Privat, Lucile Dondaine, Elise Ballot, Caroline Truntzer, Romain Boidot, and et al. 2023. "GTN Enhances Antitumor Effects of Doxorubicin in TNBC by Targeting the Immunosuppressive Activity of PMN-MDSC" Cancers 15, no. 12: 3129. https://doi.org/10.3390/cancers15123129
APA StyleMabrouk, N., Racoeur, C., Shan, J., Massot, A., Ghione, S., Privat, M., Dondaine, L., Ballot, E., Truntzer, C., Boidot, R., Hermetet, F., Derangère, V., Bruchard, M., Végran, F., Chouchane, L., Ghiringhelli, F., Bettaieb, A., & Paul, C. (2023). GTN Enhances Antitumor Effects of Doxorubicin in TNBC by Targeting the Immunosuppressive Activity of PMN-MDSC. Cancers, 15(12), 3129. https://doi.org/10.3390/cancers15123129







