Self-Medication during and after Cancer: A French Nation-Wide Cross-Sectional Study
Abstract
:Simple Summary
Abstract
1. Introduction
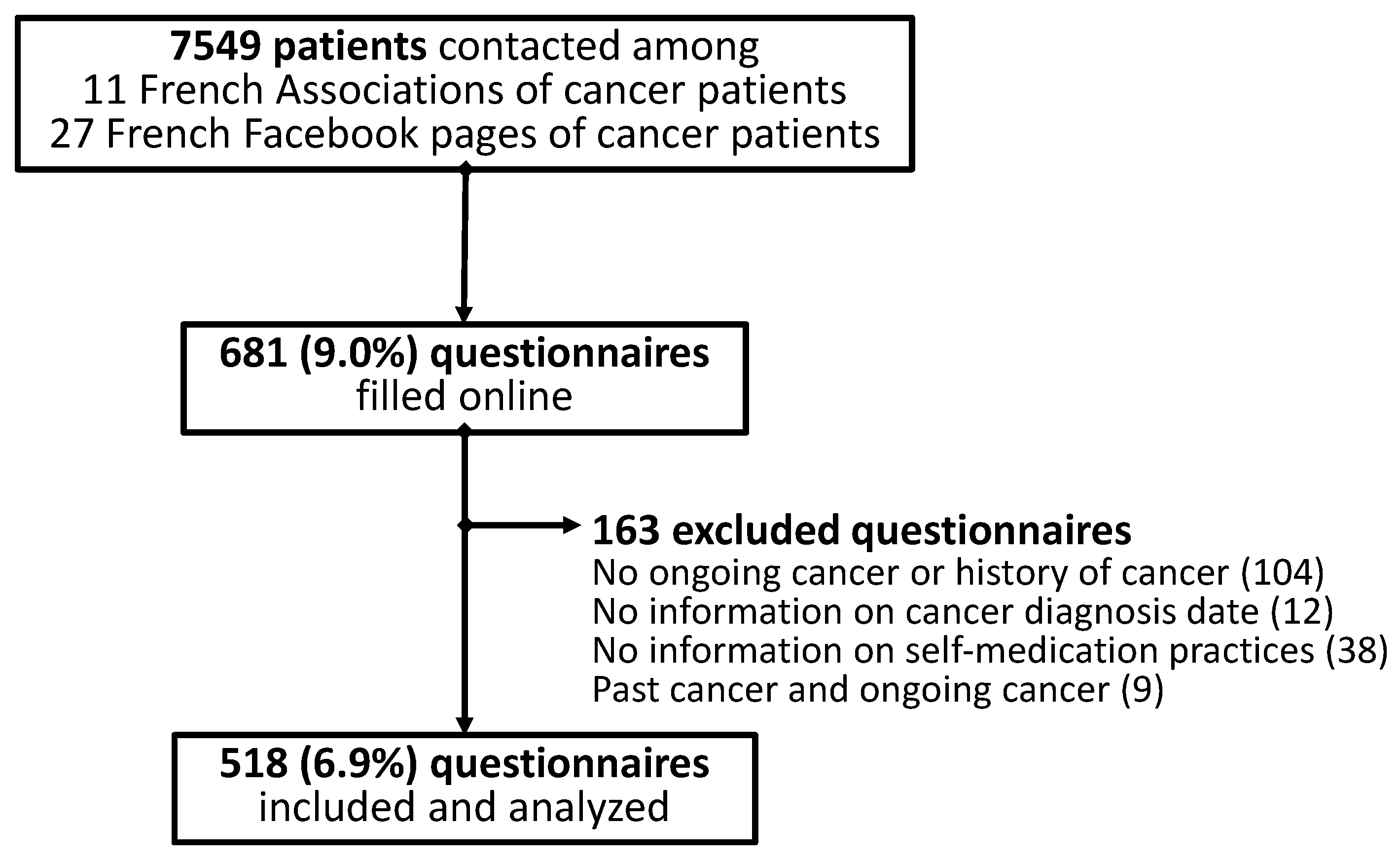
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables
- The modalities: over-the-counter (OTC) pain medication, OTC digestive tract medication, OTC anxiety and sleep disorder medications, OTC mouth and throat medications, OTC venous disorder medications, herbal medicines, aromatherapy medicines, homeopathy medicines, and dietary supplements;
- The indication for the self-medication declared by the patients (management of adverse effects (yes/no; if yes for anxiety/stress, depression, hair/nail disorders, hot flush, constipation, diarrhea, nausea/vomiting, stomach aches, pain, fatigue, mouth disorders, skin disorders, vaginal disorders, sleep disorders, breath disorders, sexual disorders, or others) and/or improvement of anticancer therapies (yes/no));
- The place of purchase (pharmacy, mall, internet, or other);
- The council received for self-medication (oncologist, general practitioner, pharmacist, friend, family, internet, press, or other);
- The disclosure to health professionals involved in cancer management of self-medication practice (oncologist, general practitioner, pharmacist, other, or none);
- The perceived risks related to the self-medication (drug interactions and adverse effects; yes/no);
- The symptoms and HRQoL were assessed with the QLQ-C30 questionnaire [18]. The scoring of the QLQ-C30 questionnaire was carried out according to EORTC recommendations (https://qol.eortc.org/questionnaire/eortc-qlq-c30 (accessed on 6 September 2021)). The QLQ-C30 was divided into 3 subscales with a global health status (0 worst to 100 best), the functional scales (0 worst to 100 best for physical, role, emotional, cognitive, and social functioning), and the symptom scales (0 least to 100 worst for fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties);
- The oncological characteristics of patients (ongoing or past cancer and localization, ongoing or past treatments);
- The socio-demographic characteristics of patients (sex, age, weight, height, French department of residence, monthly income, and education).
2.5. Data Sources/Measurement
2.6. Study Size
2.7. Statistical Methods
3. Results
3.1. Characteristics of Patients
3.2. Self-Medication during and after Cancer
| Items | All | All | Cancer Survivors | Cancer Patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No SM | SM | p-Value | No SM | SM | p-Values | No SM | SM | p-Values | ||
| Male Female | 87 (19.6) 356 (80.4) | 41 (22.3) 143 (77.7) | 46 (17.8) 213(82.2) | 0.28 | 17 (22.4) 59 (77.6) | 17 (19.8) 69 (80.2) | 0.70 | 24 (22.2) 84 (77.8) | 29 (16.8) 144 (83.2) | 0.28 |
| Age (years) | 52.5 ± 13.0 | 53.6 ± 13.6 | 51.8 ± 12.6 | 0.15 | 52.1 ± 14.5 | 51.5 ± 13.6 | 0.79 | 54.7 ± 12.9 | 51.9 ± 12.1 | 0.07 |
| BMI (kg/m²) | 25.5 ± 5.5 | 24.9 ± 5.0 | 25.9 ± 5.7 | 0.06 | 24.3 ± 4.2 | 25.3 ± 4.8 | 0.20 | 25.3 ± 5.6 | 26.2 ± 6.1 | 0.20 |
| Education | 0.18 | 0.77 | 0.14 | |||||||
| No diploma or middle school | 26 (5.9) | 10 (5.4) | 16 (6.2) | 4 (5.2) | 6 (7.1) | 6 (5.6) | 10 (5.8) | |||
| BTEC first | 63 (14.2) | 35 (18.9) | 28 (10.9) | 13 (16.9) | 12 (14.1) | 22 (20.4) | 16 (9.3) | |||
| High school diploma | 83 (18.7) | 34 (18.4) | 49 (19.0) | 15 (19.5) | 14 (16.5) | 19 (17.6) | 35 (20.2) | |||
| Bachelor’s degree | 84 (19.0) | 30 (16.2) | 54 (20.9) | 10 (13.0) | 17 (20.0) | 20 (18.5) | 37 (21.48) | |||
| Higher than bachelor’s degree | 187 (42.2) | 76 (41.1) | 111 (43.0) | 35 (45.5) | 36 (42.4) | 41 (38.0) | 75 (43.4) | |||
| Income (EUR/month) | 2000 (1400; 2500) | 2000 (1400; 3000) | 2000 (1400; 2500) | 0.41 | 1900 (1400; 2500) | 1900 (1390; 2400) | 0.58 | 2000 (1400; 3000) | 2.0 (1.4; 2.5) | 0.51 |
| Cancer diagnosis (years) | 3.0 (1.0; 5.0) | 3.0 (1.0; 5.0) | 2.5 (1.0; 5.0) | 0.75 | 3.0 (2.0; 5.0) | 3.5 (2.0; 7.5) | 0.45 | 2.0 (1.0; 5.0) | 2.0 (1.0; 4.0) | 0.85 |
| Type of cancer * | 0.21 | 0.76 | 0.07 | |||||||
| Breast | 208 (40.2) | 82 (36.3) | 126 (43.2) | 32 (33.7) | 39 (39.0) | 50 (38.2) | 87 (45.3) | |||
| Kidney | 52 (10.0) | 26 (11.5) | 26 (8.9) | 4 (4.2) | 7 (7.0) | 22 (16.8) | 19 (9.9) | |||
| Prostate | 39 (7.5) | 16 (7.1) | 23 (7.9) | 11 (11.6) | 9 (9.0) | 5 (3.8) | 14 (7.3) | |||
| Ovary | 35 (6.8) | 10 (4.4) | 25 (8.6) | 4 (4.2) | 9 (9.0) | 6 (4.6) | 16 (8.3) | |||
| Lung | 33 (6.4) | 15 (6.6) | 18 (6.2) | 2 (2.1) | 2 (2.0) | 13 (9.9) | 16 (8.3) | |||
| Thyroid | 32 (6.2) | 15 (6.6) | 17 (5.8) | 7 (7.4) | 5 (5.0) | 8 (6.1) | 12 (6.3) | |||
| Cervix | 24 (4.6) | 13 (5.8) | 11 (3.8) | 11 (11.6) | 9 (9.0) | 2 (1.5) | 2 (1.0) | |||
| Bladder | 24 (4.6) | 15 (6.6) | 9 (3.1) | 5 (5.3) | 3 (3.0) | 10 (7.6) | 6 (3.1) | |||
| Colorectal | 22 (4.3) | 11 (4.9) | 11 (3.8) | 5 (5.3) | 9 (9.0) | 6 (4.6) | 2 (1.0) | |||
| Anticancer therapies (ongoing) | nc | nc | nc | |||||||
| No therapy | 46 (8.9) | 24 (10.6) | 22 (7.5) | 0.28 | 24 (18.3) | 22 (11.5) | 0.10 | |||
| Surgery | 14 (2.7) | 6 (2.7) | 8 (2.7) | 1.00 | 6 (4.8) | 8 (4.2) | 1.00 | |||
| Oral anticancer medications | 56 (10.8) | 23 (10.2) | 33 (11.3) | 0.78 | 23 (17.6) | 33 (17.2) | 1.00 | |||
| Injectable chemotherapy | 81 (15.6) | 29 (12.8) | 52 (17.8) | 0.14 | 29 (22.1) | 52 (27.1) | 0.36 | |||
| Radiotherapy | 27 (5.2) | 12 (5.3) | 15 (5.1) | 1.00 | 12 (9.2) | 15 (7.8) | 0.69 | |||
| Anticancer therapies (past) | ||||||||||
| No therapy | 24 (4.6) | 7 (3.1) | 17 (5.8) | 0.21 | 3 (1.5) | 1 (1.01) | 1.00 | 6 (4.6) | 15 (7.8) | 0.36 |
| Surgery | 321 (62.0) | 142 (62.8) | 179 (61.3) | 0.78 | 65 (68.4) | 72 (72.0) | 0.64 | 77 (58.8) | 107 (55.7) | 0.65 |
| Oral anticancer medications | 74 (14.3) | 29 (12.8) | 45 (15.4) | 0.45 | 7 (7.4) | 8 (8.0) | 1.00 | 22 (16.8) | 37 (19.3) | 0.66 |
| Injectable chemotherapy | 252 (48.7) | 110 (48.7) | 142 (48.6) | 1.00 | 51 (53.7) | 49 (49.0) | 0.57 | 59 (45.0) | 93 (48.4) | 0.57 |
| Radiotherapy | 253 (48.8) | 103 (45.6) | 150 (51.4) | 0.22 | 57 (60.0) | 65 (65.0) | 0.55 | 46 (35.1) | 85 (44.3) | 0.11 |
3.3. Self-Medication, Quality of Life and Symptoms
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022.
- Institut National du Cancer (INCa). Panorama des Cancers en France; Institut National du Cancer: Boulogne-Billancourt, France, 2022. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for the Regulatory Assessment of Medicinal Products for Use in Self-Medication; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Assurance Maladie. Se Soigner Seul avec L’automédication; Assurance Maladie: Nice, France, 2021. [Google Scholar]
- Baracaldo-Santamaría, D.; Trujillo-Moreno, M.J.; Pérez-Acosta, A.M.; Feliciano-Alfonso, J.E.; Calderon-Ospina, C.-A.; Soler, F. Definition of Self-Medication: A Scoping Review. Ther. Adv. Drug Saf. 2022, 13, 20420986221127500. [Google Scholar] [CrossRef] [PubMed]
- Ghasemyani, S.; Benis, M.R.; Hosseinifard, H.; Jahangiri, R.; Aryankhesal, A.; Shabaninejad, H.; Rafiei, S.; Ghashghaee, A. Global, WHO Regional, and Continental Prevalence of Self-Medication from 2000 to 2018: A Systematic Review and Meta-Analysis. Ann. Public Health 2022, 1, 637. [Google Scholar] [CrossRef]
- Carmona-Torres, J.M.; Cobo-Cuenca, A.I.; Recio-Andrade, B.; Laredo-Aguilera, J.A.; Martins, M.M.; Rodríguez-Borrego, M.A. Prevalence and Factors Associated with Polypharmacy in the Older People: 2006–2014. J. Clin. Nurs. 2018, 27, 2942–2952. [Google Scholar] [CrossRef] [PubMed]
- Gazibara, T.; Nurkovic, S.; Kisic-Tepavcevic, D.; Kurtagic, I.; Kovacevic, N.; Gazibara, T.; Pekmezovic, T. Pharmacotherapy and Over-the-Counter Drug Use among Elderly in Belgrade, Serbia. Geriatr. Nur. 2013, 34, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Gautier, S.; Polard, E.; Rabier, M.; Chouchana, L.; Lebrun-Vignes, B.; Faillie, J.; Petitpain, N.; Lagarce, L.; Jonville-Bera, A.; et al. Incidence and Preventability of Hospital Admissions for Adverse Drug Reactions in France: A Prospective Observational Study (IATROSTAT). Br. J. Clin. Pharmacol. 2022, 89, 390–400, bcp.15510. [Google Scholar] [CrossRef]
- Judson, P.L.; Abdallah, R.; Xiong, Y.; Ebbert, J.; Lancaster, J.M. Complementary and Alternative Medicine Use in Individuals Presenting for Care at a Comprehensive Cancer Center. Integr. Cancer Ther. 2017, 16, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.J.; Palmer, C.S.; Healy, K.E.; Desai, K.; Amsterdam, J. Complementary and Alternative Medicine Use among Cancer Survivors: A Population-Based Study. J. Cancer Surviv. Res. Pract. 2011, 5, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Molassiotis, A.; Fernadez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of Complementary and Alternative Medicine in Cancer Patients: A European Survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef]
- Dehghani, M.; Hosseini, S.M.; Molkara, S.; Fazilat-Panah, D.; Mehrpour, O.; Soroosh, D.; Zarei, E.; Welsh, J.S.; Nematshahi, M.; Javadinia, S.A. Opium Poisoning Following Self-medication of Radiation-induced Dermatitis with Topical Use of Opium Latex Traditional Extract; a Teaching Case. Clin. Case Rep. 2021, 9, e04661. [Google Scholar] [CrossRef]
- Bavunoğlu, I.; Balta, M.; Türkmen, Z. Oleander Poisoning as an Example of Self-Medication Attempt. Balk. Med. J. 2016, 33, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Hainer, M.I. Fatal Hepatorenal Failure Associated with Hydrazine Sulfate. Ann. Intern. Med. 2000, 133, 877. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, K.; Pawlik, J.; Czekawy, I.; Kozłowski, M.; Cymbaluk-Płoska, A. Complementary Methods in Cancer Treatment-Cure or Curse? Int. J. Environ. Res. Public. Health 2021, 18, 356. [Google Scholar] [CrossRef]
- Feise, R.J. Do Multiple Outcome Measures Require P-Value Adjustment? BMC Med. Res. Methodol. 2002, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiol. Camb. Mass 1990, 1, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Nuzzo, R. Scientific Method: Statistical Errors. Nature 2014, 506, 150–152. [Google Scholar] [CrossRef] [Green Version]
- Tran, A.T.-Q.; Soullier, N.; Ankri, J.; Herr, M.; Carcaillon-Bentata, L. Uses and Perceptions of Medications among French Older Adults: Results from the 2020 French Health Barometer Survey. BMC Geriatr. 2022, 22, 602. [Google Scholar] [CrossRef]
- Brandão, G.R.; Teixeira, L.; Araújo, L.; Paúl, C.; Ribeiro, O. Self-Medication in Older European Adults: Prevalence and Predictive Factors. Arch. Gerontol. Geriatr. 2020, 91, 104189. [Google Scholar] [CrossRef] [PubMed]
- Ministère des Solidarités, de L’automonie et des Personnes Handicapées. Le Circuit de Distribution du Médicament en France; Ministère des Solidarités, de L’automonie et des Personnes Handicapées: Paris, France, 2022. [Google Scholar]
- Wang, T.; Molassiotis, A.; Chung, B.P.M.; Tan, J.-Y. Unmet Care Needs of Advanced Cancer Patients and Their Informal Caregivers: A Systematic Review. BMC Palliat. Care 2018, 17, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Prevalence of Pain in Patients with Cancer: A Systematic Review of the Past 40 Years. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Te Boveldt, N.; Vernooij-Dassen, M.; Burger, N.; Ijsseldijk, M.; Vissers, K.; Engels, Y. Pain and Its Interference with Daily Activities in Medical Oncology Outpatients. Pain Physician 2013, 16, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mathie, R.T.; Ramparsad, N.; Legg, L.A.; Clausen, J.; Moss, S.; Davidson, J.R.T.; Messow, C.-M.; McConnachie, A. Randomised, Double-Blind, Placebo-Controlled Trials of Non-Individualised Homeopathic Treatment: Systematic Review and Meta-Analysis. Syst. Rev. 2017, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Ang, L.; Choi, J.; Lee, M.S. Aromatherapy for Managing Menopausal Symptoms: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J. Altern. Complement. Med. 2021, 27, 813–823. [Google Scholar] [CrossRef]
- Calamusa, A.; Di Marzio, A.; Cristofani, R.; Arrighetti, P.; Santaniello, V.; Alfani, S.; Carducci, A. Factors That Influence Italian Consumers’ Understanding of over-the-Counter Medicines and Risk Perception. Patient Educ. Couns. 2012, 87, 395–401. [Google Scholar] [CrossRef]
- Westerlund, T.; Barzi, S.; Bernsten, C. Consumer Views on Safety of Over-the-Counter Drugs, Preferred Retailers and Information Sources in Sweden: After Re-Regulation of the Pharmacy Market. Pharm. Pract. 2017, 15, 894. [Google Scholar] [CrossRef] [Green Version]
- Baracaldo-Santamaría, D.; Pabón-Londoño, S.; Rojas-Rodriguez, L.C. Drug Safety of Frequently Used Drugs and Substances for Self-Medication in COVID-19. Ther. Adv. Drug Saf. 2022, 13, 204209862210941. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction with Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef] [Green Version]
- Ben Kridis, W.; Mnif, A.; Khmiri, S.; Toumi, N.; Khanfir, A. Self-Medication with Herbal Medicine and Breast Cancer Survival: A Prospective Monocentric Study. J. Cancer Res. Clin. Oncol. 2021, 147, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Damery, S.; Gratus, C.; Grieve, R.; Warmington, S.; Jones, J.; Routledge, P.; Greenfield, S.; Dowswell, G.; Sherriff, J.; Wilson, S. The Use of Herbal Medicines by People with Cancer: A Cross-Sectional Survey. Br. J. Cancer 2011, 104, 927–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanimozhi, T.; Hindu, K.; Maheshvari, Y.; Khushnidha, Y.G.; Kumaravel, M.; Srinivas, K.S.; Manickavasagam, M.; Mangathayaru, K. Herbal Supplement Usage among Cancer Patients: A Questionnaire-Based Survey. J. Cancer Res. Ther. 2021, 17, 136–141. [Google Scholar] [CrossRef]
- Theuser, A.-K.; Hack, C.C.; Fasching, P.A.; Antoniadis, S.; Grasruck, K.; Wasner, S.; Knoll, S.; Sievers, H.; Beckmann, M.W.; Thiel, F.C. Patterns and Trends of Herbal Medicine Use among Patients with Gynecologic Cancer. Geburtshilfe Frauenheilkd. 2021, 81, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Dulal, S.; Paudel, B.D.; Wood, L.A.; Neupane, P.; Shah, A.; Acharya, B.; Shilpakar, R.; Acharya, S.C.; Karn, A.; Poudel, B.; et al. Reliance on Self-Medication Increase Delays in Diagnosis and Management of GI Cancers: Results from Nepal. JCO Glob. Oncol. 2020, 6, 1258–1263. [Google Scholar] [CrossRef]
- Tata, M.D.; Dharmendran, R.; Ramesh, G.; Kandasami, P. Delay in Diagnosis of Upper Gastrointestinal Cancer: Whose Fault Is It? Med. J. Malays. 2013, 68, 275–277. [Google Scholar]
- Dapkevičiūtė, A.; Šapoka, V.; Martynova, E.; Pečeliūnas, V. Time from Symptom Onset to Diagnosis and Treatment among Haematological Malignancies: Influencing Factors and Associated Negative Outcomes. Med. Kaunas Lith. 2019, 55, 238. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.; McGrail, M.R. Disclosure of CAM Use to Medical Practitioners: A Review of Qualitative and Quantitative Studies. Complement. Ther. Med. 2004, 12, 90–98. [Google Scholar] [CrossRef]
- Fereidouni, Z.; Kameli Morandini, M.; Najafi Kalyani, M. Experiences of Self-Medication among People: A Qualitative Meta-Synthesis. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 83–89. [Google Scholar] [CrossRef]
- Balneaves, L.G.; Watling, C.Z.; Hayward, E.N.; Ross, B.; Taylor-Brown, J.; Porcino, A.; Truant, T.L.O. Addressing Complementary and Alternative Medicine Use Among Individuals With Cancer: An Integrative Review and Clinical Practice Guideline. JNCI J. Natl. Cancer Inst. 2022, 114, 25–37. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Mariotto, A.; Tangka, F.; Zhao, J.; Islami, F.; Sung, H.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Ward, E.M. Annual Report to the Nation on the Status of Cancer, Part 2: Patient Economic Burden Associated with Cancer Care. JNCI J. Natl. Cancer Inst. 2021, 113, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Noone, J.; Blanchette, C.M. The Value of Self-Medication: Summary of Existing Evidence. J. Med. Econ. 2018, 21, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attal, N.; Lanteri-Minet, M.; Laurent, B.; Fermanian, J.; Bouhassira, D. The Specific Disease Burden of Neuropathic Pain: Results of a French Nationwide Survey. Pain 2011, 152, 2836–2843. [Google Scholar] [CrossRef] [PubMed]

| Products for Self-Medication | All N (%) | Cancer Survivors N (%) | Cancer Patients N (%) |
|---|---|---|---|
| Dietary supplements | 175 (59.9) | 66 (66.0) | 109 (56.8) |
| Pain medications | 171 (58.6) | 64 (64.0) | 107 (55.7) |
| Herbal medicines | 103 (35.3) | 40 (40.0) | 63 (32.8) |
| Digestive tract medications | 98 (33.6) | 38 (38.0) | 60 (31.3) |
| Essential oils | 86 (29.5) | 29 (29.0) | 57 (29.7) |
| Homeopathy | 80 (27.4) | 28 (28.0) | 52 (27.1) |
| Anxiety and sleep disorder medications | 69 (23.6) | 28 (28.0) | 41 (21.4) |
| Mouth and throat medications | 45 (15.4) | 19 (19.0) | 26 (13.5) |
| Venous disorder medications | 26 (8.9) | 7 (7.0) | 19 (9.9) |
| Adverse Effects | All N (%) | Cancer Survivors N (%) | Cancer Patients N (%) |
|---|---|---|---|
| Pain | 114 (39.0) | 36 (36.0) | 78 (40.6) |
| Fatigue | 74 (25.3) | 26 (26.0) | 48 (25.0) |
| Anxiety and/or stress | 63 (21.6) | 23 (23.0) | 40 (20.8) |
| Nausea and/or vomiting | 61 (20.9) | 18 (18.0) | 43 (22.4) |
| Sleep disorders | 52 (17.8) | 20 (20.0) | 32 (16.7) |
| Stomach aches | 50 (17.1) | 15 (15.0) | 35 (18.2) |
| Hot flushes | 48 (16.4) | 21 (21.0) | 27 (14.1) |
| Constipation | 46 (15.8) | 16 (16.0) | 30 (15.6) |
| Diarrhea | 42 (14.4) | 9 (9.0) | 33 (17.2) |
| Oral lesions | 36 (12.3) | 11 (11.0) | 25 (13.0) |
| Nail and/or hair disorders | 32 (11.0) | 12 (12.0) | 20 (10.4) |
| Skin disorders | 32 (11.0) | 11 (11.0) | 21 (10.9) |
| Vaginal disorders | 27 (9.3) | 12 (12.0) | 15 (7.8) |
| Depression | 24 (8.2) | 11 (11.0) | 13 (6.8) |
| Sexual disorders | 10 (3.4) | 4 (4.0) | 6 (3.1) |
| Respiratory disorders | 8 (2.7) | 1 (1.0) | 7 (3.7) |
| QLQ-C30 | All | No SM | SM | Effect Size | p-Values |
|---|---|---|---|---|---|
| Global health status | 60.6 ± 19.5 | 62.3 ± 20.8 | 59.2 ± 18.4 | −0.06(−0.24; 0.13) | 0.07 |
| Physical functioning | 77.3 ± 21.1 | 78.6 ± 21.4 | 76.3 ± 20.9 | −0.04 (−0.22; 0.14) | 0.12 |
| Role functioning | 59.0 ± 32.3 | 59.6 ± 33.9 | 58.5 ± 31.1 | 0.06 (−0.11; 0.24) | 0.60 |
| Emotional functioning | 57.6 ± 28.8 | 60.2 ± 29.6 | 55.7 ± 28.1 | −0.07 (−0.25; 0.12) | 0.07 |
| Cognitive functioning | 64.3 ± 30.2 | 65.6 ± 31.3 | 63.3 ± 29.4 | 0.03 (−0.15; 0.21) | 0.26 |
| Social functioning | 58.2 ± 32.1 | 63.0 ± 32.0 | 54.6 ± 31.8 | −0.14 (−0.32; 0.05) | 0.003 |
| Fatigue | 54.5 ± 29.3 | 53.1 ± 29.7 | 55.6 ± 29.0 | 0.08 (−0.10; 0.26) | 0.33 |
| Nausea and vomiting | 16.7 ± 26.2 | 19.0 ± 29.8 | 14.9 ± 23.0 | −0.02 (−0.20; 0.16) | 0.74 |
| Pain | 42.0 ± 32.2 | 36.9 ± 31.2 | 46.0 ± 32.4 | 0.22 (−0.04; 0.40) | 0.001 |
| Dyspnea | 30.7 ± 31.6 | 29.9 ± 32.2 | 31.2 ± 31.2 | 0.09 (−0.10; 0.27) | 0.51 |
| Insomnia | 49.5 ± 36.8 | 43.4 ± 35.1 | 54.2 ± 37.5 | 0.20 (−0.02; 0.38) | 0.002 |
| Appetite loss | 24.6 ± 33.6 | 25.2 ± 34.2 | 24.1 ± 33.2 | −0.02 (−0.20; 0.16) | 0.78 |
| Constipation | 29.1 ± 35.1 | 27.8 ± 34.7 | 30.1 ± 35.4 | 0.06 (−0.12; 0.24) | 0.47 |
| Diarrhea | 20.2 ± 29.6 | 19.5 ± 30.8 | 20.8 ± 28.8 | 0.12 (−0.06; 0.31) | 0.26 |
| Financial difficulties | 25.0 ± 34.2 | 19.2 ± 31.5 | 29.4 ± 35.5 | 0.33 (0.15; 0.52) | <0.001 |
| QLQ-C30 | Dietary Supplements | Pain Medications | Herbal Medicines |
|---|---|---|---|
| Scores (No vs. Yes) Effect Size (95 CI) | Scores (No vs. Yes) Effect Size (95 CI) | Scores (No vs. Yes) Effect Size (95 CI) | |
| Global health status | 57.7 ± 18.8 vs. 60.1 ± 18.1 0.13 (−0,12; 0.38) | 63.8 ± 18.3 vs. 56.0 ± 17.8 *** −0.43 (−0.68; −0.18) | 58.5 ± 19.0 vs. 60.5 ± 17.2 0.11 (−0.14; 0.35) |
| Physical functioning | 74.7 ± 20.8 vs. 77.4 ± 20.9 0.13 (−0.11; 0.37) | 81.5 ± 18.0 vs. 72.7 ± 22.1 *** −0.42 (−0.66; −0.18) | 75.3 ± 21.4 vs. 78.2 ± 20.0 0.14 (−0.11; 0.38) |
| Role functioning | 54.4 ± 29.5 vs. 60.9 ± 31.8 0.21 (−0.03; 0.45) | 67.1 ± 29.2 vs. 52.5 ± 31.1 *** −0.48 (−0.72; −0.24) | 56.8 ± 31.8 vs. 61.4 ± 29.8 0.15 (−0.10; 0.39) |
| Emotional functioning | 56.3 ± 28.0 vs. 55.3 ± 28.3 −0.04 (−0.29; 0.21) | 65.2 ± 25.6 vs. 49.0 ± 28.0 *** −0.60 (−0.85; −0.35) | 55.1 ± 29.2 vs. 56.6 ± 26.3 0.05 (−0.19; 0.30) |
| Cognitive functioning | 62.1 ± 27.1 vs. 64.0 ± 30.8 0.06 (−0.18; 0.31) | 68.5 ± 29.6 vs. 59.7 ± 28.9 ** −0.30 (−0.54; −0.06) | 63.0 ± 30.6 vs. 63.8 ± 27.5 0.03 (−0.22; 0.27) |
| Social functioning | 52.9 ± 31.6 vs. 55.6 ± 32.0 0.08 (−0.16; 0.33) | 60.6 ± 32.0 vs. 50.3 ± 31.1 * −0.33 (−0.57; −0.08) | 54.6 ± 32.9 vs. 54.4 ± 29.9 −0.01 (−0.25; 0.24) |
| Fatigue | 57.1 ± 27.7 vs. 54.7 ± 29.9 −0.08 (−0.32; 0.16) | 45.9 ± 28.3 vs. 62.4 ± 27.7 *** 0.59 (0.34; 0.83) | 56.8 ± 30.8 vs. 53.6 ± 25.8 −0.11 (−0.35; 0.14) |
| Nausea and vomiting | 14.6 ± 22.0 vs. 15.1 ± 23.7 0.02 (−0.22; 0.26) | 11.4 ± 19.2 vs. 17.4 ± 25.1 0.26 (0.02; 0.50) | 16.1 ± 24.8 vs. 12.9 ± 19.7 −0.14 (−0.38; 0.11) |
| Pain | 49.2 ± 29.8 vs. 44.0 ± 33.9 −0.16 (−0.40; 0.08) | 34.2 ± 30.0 vs. 54.1 ± 31.6 *** 0.64 (0.39; 0.88) | 45.8 ± 33.6 vs. 46.2 ± 30.4 0.01 (−0.23; 0.26) |
| Dyspnea | 32.4 ± 31.5 vs. 30.6 ± 31.0 −0.06 (−0.30; 0.19) | 28.9 ± 32.1 vs. 32.9 ± 30.5 0.13 (−0.11; 0.37) | 32.4 ± 31.5 vs. 29.3 ± 30.6 −0.10 (−0.34; 0.15) |
| Insomnia | 54.0 ± 37.4 vs. 54.3 ± 37.7 0.01 (−0.24; 0.25) | 43.2 ± 37.1 vs. 61.9 ± 35.9 *** 0.51 (0.27; 0.76) | 52.4 ± 38.7 vs. 57.3 ± 35.2 0.13 (−0.11; 0.38) |
| Appetite loss | 29.8 ± 35.8 vs. 20.5 ± 31.1 * −0.28 (−0.53; −0.04) | 19.3 ± 29.9 vs. 27.4 ± 35.0 0.24 (0.00; 0.48) | 26.2 ± 35.1 vs. 20.5 ± 29.4 −0.17 (−0.42; 0.07) |
| Constipation | 26.6 ± 33.9 vs. 32.2 ± 36.1 0.16 (−0.09; 0.40) | 26.8 ± 33.9 vs. 32.3 ± 36.3 0.15 (−0.09; 0.39) | 30.8 ± 35.5 vs. 28.7 ± 35.3 −0.06 (−0.30; 0.18) |
| Diarrhea | 26.6 ± 32.3 vs. 17.4 ± 25.9 −0.32 (−0.57; −0.07) | 17.9 ± 28.2 vs. 22.9 ± 29.0 0.17 (−0.07; 0.42) | 24.2 ± 30.5 vs. 15.1 ± 24.5 * −0.32 (−0.57; −0.06) |
| Financial difficulties | 31.3 ± 34.8 vs. 28.3 ± 35.9 −0.08 (−0.33; 0.17) | 25.1 ± 34.2 vs. 32.3 ± 36.1 0.20 (−0.04; 0.45) | 29.1 ± 33.4 vs. 29.9 ± 38.9 0.02 (−0.23; 0.27) |
| QLQ-C30 | Digestive Tract Medications | Essential Oils | Homeopathy |
|---|---|---|---|
| Scores (No vs. Yes) Effect Size (95 CI)) | Scores (No vs. Yes) Effect Size (95 CI) | Scores (No vs. Yes) Effect Size (95 CI) | |
| Global health status | 61.2 ± 17.8 vs. 55.4 ± 19.3 0.31 (−0.57; −0.06) | 59.0 ± 18.7 vs. 59.8 ± 17.7 −0.04 (−0.22; 0.30) | 58.6 ± 18.9 vs. 60.7 ± 17.1 −0.11 (−0.15; 0.38) |
| Physical functioning | 77.8 ± 20.3 vs. 73.5 ± 21.8 0.21 (−0.45; 0.04) | 76.4 ± 21.2 vs. 76.2 ± 20.2 0.01 (−0.26; 0.25) | 75.6 ± 21.3 vs. 78.2 ± 19.7 −0.12 (−0.14; 0.38) |
| Role functioning | 61.8 ± 30.1 vs. 51.6 ± 32.2 * 0.33 (−0.58; −0.08) | 59.5 ± 31.2 vs. 56.0 ± 30.9 0.12 (−0.37; 0.14) | 57.9 ± 31.0 vs. 59.8 ± 31.6 −0.06 (−0.20; 0.32) |
| Emotional functioning | 59.0 ± 27.6 vs. 49.0 ± 28.2 ** 0.36 (−0.61; −0.10) | 56.8 ± 27.9 vs. 53.1 ± 28.8 0.13 (−0.39; 0.13) | 54.2 ± 29.6 vs. 59.2 ± 24.0 −0.18 (−0.09; 0.44) |
| Cognitive functioning | 66.2 ± 28.2 vs. 57.6 ± 31.1 * 0.29 (−0.55; −0.04) | 66.0 ± 28.0 vs. 57.2 ± 31.9 * 0.30 (−0.56; −0.04) | 62.3 ± 30.0 vs. 65.8 ± 28.0 −0.12 (−0.15; 0.38) |
| Social functioning | 57.3 ± 32.0 vs. 49.3 ± 30.9 * 0.25 (−0.51; 0.00) | 57.2 ± 32.3 vs. 48.3 ± 29.8 * 0.28 (−0.54; −0.02) | 53.8 ± 32.1 vs. 56.4 ± 31.1 −0.08 (−0.19; 0.34) |
| Fatigue | 52.6 ± 29.3 vs. 61.8 ± 27.6 * −0.32 (0.07; 0.57) | 54.1 ± 29.7 vs. 59.1 ± 27.2 −0.17 (−0.08; 0.43) | 55.6 ± 29.9 vs. 55.7 ± 26.8 −0.00 (−0.26; 0.26) |
| Nausea and vomiting | 11.1 ± 19.4 vs. 22.6 ± 27.6 *** −0.52 (0.26; 0.77) | 14.1 ± 22.8 vs. 16.7 ± 23.6 −0.11 (−0.15; 0.36) | 15.0 ± 23.7 vs. 14.7 ± 21.5 0.01 (−0.27; 0.25) |
| Pain | 43.3 ± 31.5 vs. 51.3 ± 33.7 −0.25 (−0.00; 0.50) | 43.9 ± 32.3 vs. 50.6 ± 32.4 −0.21 (−0.05; 0.46) | 44.9 ± 32.1 vs. 48.7 ± 33.2 −0.12 (−0.14; 0.38) |
| Dyspnea | 28.5 ± 30.5 vs. 36.7 ± 32.0 * −0.26 (0.01; 0.51) | 29.6 ± 30.7 vs. 35.0 ± 32.0) −0.17 (−0.09; 0.43) | 31.6 ± 31.6 vs. 30.3 ± 30.2 0.04 (−0.31; 0.22) |
| Insomnia | 51.0 ± 38.6 vs. 60.5 ± 34.6 −0.25 (0.00; 0.50) | 52.4 ± 37.4 vs. 58.3 ± 37.6 −0.16 (−0.10; 0.42) | 54.7 ± 38.4 vs. 53.0 ± 35.4 0.05 (−0.31; 0.22) |
| Appetite loss | 20.7 ± 31.6 vs. 30.8 ± 35.4 * −0.31 (0.05; 0.56) | 24.4 ± 33.0 vs. 23.4 ± 33.8 0.03 (−0.29; 0.23) | 22.2 ± 32.6 vs. 29.0 ± 34.3 −0.21 (−0.06; 0.47) |
| Constipation | 28.4 ± 35.4 vs. 33.3 ± 35.3 −0.14 (−0.11; 0.39) | 29.0 ± 35.2 vs. 32.5 ± 35.8 −0.10 (−0.16; 0.36) | 31.3 ± 36.7 vs. 26.9 ± 31.8 0.12 (−0.39; 0.14) |
| Diarrhea | 16.2 ± 27.4 vs. 30.0 ± 29.3 *** −0.49 (0.23; 0.75) | 22.7 ± 30.5 vs. 16.5 ± 23.8 0.22 (−0.48; 0.05) | 21.0 ± 29.3 vs. 20.3 ± 27.7 0.02 (−0.29; 0.24) |
| Financial difficulties | 24.5 ± 32.7 vs. 39.0 ± 38.7 ** −0.41 (0.16; 0.67) | 29.0 ± 35.7 vs. 30.3 ± 35.1 −0.04 (−0.23; 0.30) | 27.7 ± 34.7 vs. 33.8 ± 37.2 −0.17 (−0.10; 0.44) |
| QLQ-C30 | Anxiety and Sleep Disorder Medications | Mouth and Throat Medications | Venous Disorder Medications |
|---|---|---|---|
| Scores (No vs. Yes) Effect Size (95 CI) | Scores (No vs. Yes) Effect Size (95 CI) | Scores (No vs. Yes) Effect Size (95 CI) | |
| Global health status | 59.1 ± 18.8 vs. 59.8 ± 17.0 −0.04 (−0.24; 0.33) | 59.4 ± 19.2 vs. 58.1 ± 13.2 0.07 (−0.41; 0.27) | 59.3 ± 18.6 vs. 59.0 ± 17.0 0.01 (−0.43; 0.41) |
| Physical functioning | 76.8 ± 20.2 vs. 74.9 ± 23.2 0.09 (−0.37; 0.18) | 76.1 ± 21.5 vs. 77.6 ± 17.3 −0.07 (−0.26; 0.40) | 76.4 ± 20.9 vs. 76.0 ± 21.3 0.02 (−0.43; 0.39) |
| Role functioning | 60.0 ± 30.8 vs. 53.3 ± 31.8 0.22 (−0.49; 0.06) | 58.6 ± 31.4 vs. 57.5 ± 29.5 0.03 (−0.36; 0.29) | 58.3 ± 31.2 vs. 60.0 ± 30.8 −0.05 (−0.36; 0.46) |
| Emotional functioning | 58.78 ± 26.6 vs. 45.3 ± 30.7 ** 0.49 (−0.77; −0.20) | 55.6 ± 28.6 vs. 56.2 ± 25.6 −0.02 (−0.31; 0.36) | 56.0 ± 28.4 vs. 52.0 ± 25.5 0.14 (−0.56; 0.27) |
| Cognitive functioning | 65.5 ± 28.8 vs. 56.2 ± 30.5 * 0.32 (−0.60; −0.03) | 64.6 ± 29.7 vs. 55.8 ± 26.8 * 0.30 (−0.63; 0.04) | 63.9 ± 29.4 vs. 56.9 ± 29.5 0.24 (−0.66; 0.18) |
| Social functioning | 54.9 ± 31.9 vs. 53.3 ± 31.7 0.05 (−0.34; 0.23) | 56.1 ± 32.2 vs. 45.8 ± 28.4 * 0.32 (−0.66; 0.01) | 54.4 ± 32.1 vs. 56.3 ± 29.4 −0.06 (−0.36; 0.48) |
| Fatigue | 53.6 ± 29.4 vs. 62.1 ± 26.9 * −0.29 (0.02; 0.57) | 55.3 ± 29.6 vs. 57.4 ± 25.6 −0.07 (−0.26; 0.40) | 55.0 ± 29.3 vs. 62.0 ± 26.4 −0.24 (−0.17; 0.65) |
| Nausea and vomiting | 14.8 ± 22.9 vs. 15.4 ± 23.8 −0.03 (−0.25; 0.30) | 14.1 ± 22.2 vs. 19.4 ± 27.0 −0.23 (−0.10; 0.56) | 14.9 ± 23.6 vs. 15.3 ± 17.3 −0.02 (−0.39; 0.43) |
| Pain | 43.5 ± 32.0 vs. 53.8 ± 32.9 * −0.32 (0.04; 0.60) | 45.3 ± 32.3 vs. 49.6 ± 33.2 −0.13 (−0.20; 0.46) | 45.0 ± 32.7 vs. 55.3 ± 28.8 −0.32 (−0.09; 0.73) |
| Dyspnea | 30.0 ± 30.7 vs. 35.5 ± 32.7 −0.18 (−0.11; 0.46) | 30.3 ± 31.5 vs. 36.6 ± 28.7 −0.20 (−0.13; 0.53) | 29.3 ± 29.5 vs. 51.4 ± 40.5 ** −0.72 (0.30; 1.14) |
| Insomnia | 47.7 ± 37. vs. 75.5 ± 29.2 *** −0.78 (0.49; 1.07) | 52.6 ± 37.6 vs. 63.4 ± 35.6 −0.29 (−0.04; 0.62) | 52.6 ± 38.0 vs. 70.7 ± 27.8 * −0.49 (0.07; 0.90) |
| Appetite loss | 23.8 ± 33. vs. 25.0 ± 33.1 −0.04 (−0.24; 0.31) | 22.8 ± 32.8 vs. 31.0 ± 34.8 −0.24 (−0.08; 0.57) | 23.3 ± 32.5 vs. 32.0 ± 39.1 −0.26 (−0.15; 0.67) |
| Constipation | 27.9 ± 35. vs. 36.9 ± 35.4 * −0.25 (−0.02; 0.53) | 28.8 ± 35.1 vs. 37.3 ± 36.2 −0.24 (−0.09; 0.57) | 28.8 ± 35.1 vs. 42.7 ± 36.7* −0.39 (−0.02; 0.80) |
| Diarrhea | 22.5 ± 30.3 vs. 15.3 ± 22.4 0.25 (−0.54; 0.04) | 20.4 ± 29.0 vs. 23.3 ± 27.4 −0.10 (−0.23; 0.44) | 21.0 ± 29.0 vs. 18.8 ± 26.3 0.08 (−0.50; 0.35) |
| Financial difficulties | 28.0 ± 34.7 vs. 33.8 ± 37.8 −0.16 (−0.12; 0.45) | 26.8 ± 34.5 vs. 44.4 ± 37.7 ** −0.50 (0.16; 0.85) | 29.4 ± 35.9 vs. 29.2 ± 31.6 0.01 (−0.43; 0.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraud, J.; Bedhomme, S.; Pereira, B.; Trévis, S.; Jary, M.; Balayssac, D. Self-Medication during and after Cancer: A French Nation-Wide Cross-Sectional Study. Cancers 2023, 15, 3190. https://doi.org/10.3390/cancers15123190
Maraud J, Bedhomme S, Pereira B, Trévis S, Jary M, Balayssac D. Self-Medication during and after Cancer: A French Nation-Wide Cross-Sectional Study. Cancers. 2023; 15(12):3190. https://doi.org/10.3390/cancers15123190
Chicago/Turabian StyleMaraud, Julie, Sabrina Bedhomme, Bruno Pereira, Sophie Trévis, Marine Jary, and David Balayssac. 2023. "Self-Medication during and after Cancer: A French Nation-Wide Cross-Sectional Study" Cancers 15, no. 12: 3190. https://doi.org/10.3390/cancers15123190






