Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors
Abstract
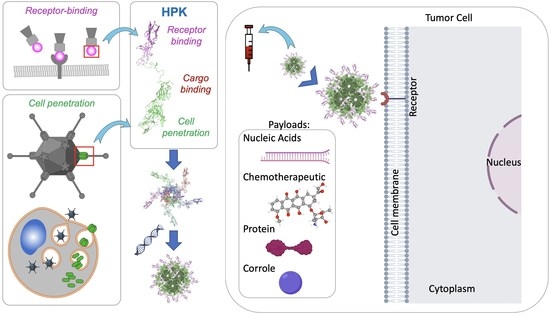
Simple Summary
Abstract
1. Introduction
2. Requirements for Macromolecular Cell Entry
3. The Adenovirus Capsid as a Macromolecular Delivery Vehicle
3.1. Approved Adenovirus-Based Therapeutics for Treating Cancer and Other Indications
3.2. Structure of the Adenovirus Capsid
3.3. Adenovirus Cell Entry
4. Penton Base-Derived Macromolecular Delivery by HerPBK10 (HPK)
4.1. The Penton Base as a Membrane Penetration Platform for HPK
4.2. Nano-Capsid Self-Assembly Nucleated by Therapeutic Cargo
4.3. Tumor Homing through Multivalent Binding with HER3
5. Adenovirus Dodecahedron
6. Cell-Penetrating Peptides
7. Discussion and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Stub, T.; Quandt, S.A.; Arcury, T.A.; Sandberg, J.C.; Kristoffersen, A.E. Conventional and complementary cancer treatments: Where do conventional and complementary providers seek information about these modalities? BMC Health Serv. Res. 2018, 18, 854. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Kolodny, G.; Li, X.; Balk, S. Addressing Cancer Chemotherapeutic Toxicity, Resistance, and Heterogeneity: Novel Theranostic Use of DNA-Encoded Small Molecule Libraries. Bioessays 2018, 40, e1800057. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Kaplan, J.M. Adenovirus-based cancer gene therapy. Curr. Gene Ther. 2005, 5, 595–605. [Google Scholar] [CrossRef]
- Cao, C.; Dong, X.; Wu, X.; Wen, B.; Ji, G.; Cheng, L.; Liu, H. Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction. J. Virol. 2012, 86, 12322–12329. [Google Scholar] [CrossRef]
- Fender, P.; Boussaid, A.; Mezin, P.; Chroboczek, J. Synthesis, cellular localization, and quantification of penton-dodecahedron in serotype 3 adenovirus-infected cells. Virology 2005, 340, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef]
- Fender, P.; Schoehn, G.; Foucaud-Gamen, J.; Gout, E.; Garcel, A.; Drouet, E.; Chroboczek, J. Adenovirus dodecahedron allows large multimeric protein transduction in human cells. J. Virol. 2003, 77, 4960–4964. [Google Scholar] [CrossRef] [PubMed]
- Fender, P. Use of dodecahedron "VLPs" as an alternative to the whole adenovirus. Methods Mol. Biol. 2014, 1089, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Besson, S.; Vragniau, C.; Vassal-Stermann, E.; Dagher, M.C.; Fender, P. The Adenovirus Dodecahedron: Beyond the Platonic Story. Viruses 2020, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Falck-Pedersen, E. Fiber and penton base capsid modifications yield diminished adenovirus type 5 transduction and proinflammatory gene expression with retention of antigen-specific humoral immunity. J. Virol. 2006, 80, 10634–10644. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.C.; Stehle, T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef]
- Medina-Kauwe, L.K.; Maguire, M.; Kasahara, N.; Kedes, L. Nonviral gene delivery to human breast cancer cells by targeted Ad5 penton proteins. Gene Ther. 2001, 8, 1753–1761. [Google Scholar] [CrossRef]
- Medina-Kauwe, L.K.; Kasahara, N.; Kedes, L. 3PO, a novel nonviral gene delivery system using engineered Ad5 penton proteins. Gene Ther. 2001, 8, 795–803. [Google Scholar] [CrossRef]
- Medina-Kauwe, L.K. Development of adenovirus capsid proteins for targeted therapeutic delivery. Ther. Deliv. 2013, 4, 267–277. [Google Scholar] [CrossRef]
- Sims, J.D.; Hwang, J.Y.; Wagner, S.; Alonso-Valenteen, F.; Hanson, C.; Taguiam, J.M.; Polo, R.; Harutyunyan, I.; Karapetyan, G.; Sorasaenee, K.; et al. A corrole nanobiologic elicits tissue-activated MRI contrast enhancement and tumor-targeted toxicity. J. Control. Release 2015, 217, 92–101. [Google Scholar] [CrossRef]
- Sims, J.D.; Taguiam, J.M.; Alonso-Valenteen, F.; Markman, J.; Agadjanian, H.; Chu, D.; Lubow, J.; Abrol, R.; Srinivas, D.; Jain, A.; et al. Resistance to receptor-blocking therapies primes tumors as targets for HER3-homing nanobiologics. J. Control. Release 2018, 271, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Valenteen, F.; Pacheco, S.; Srinivas, D.; Rentsendorj, A.; Chu, D.; Lubow, J.; Sims, J.; Miao, T.; Mikhael, S.; Hwang, J.Y.; et al. HER3-targeted protein chimera forms endosomolytic capsomeres and self-assembles into stealth nucleocapsids for systemic tumor homing of RNA interference in vivo. Nucleic Acids Res. 2019, 47, 11020–11043. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, J.; Kang, B.J.; Lubow, D.J.; Chu, D.; Farkas, D.L.; Shung, K.K.; Medina-Kauwe, L.K. Multimodality imaging in vivo for preclinical assessment of tumor-targeted doxorubicin nanoparticles. PLoS ONE 2012, 7, e34463. [Google Scholar] [CrossRef]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Chen, M.; Sartorius, R.; Zarrabi, A.; Ashrafizadeh, M.; Dabbagh Moghaddam, F.; Ma, J.; Mattoli, V.; Tay, F.R. Endocytosis of abiotic nanomaterials and nanobiovectors: Inhibition of membrane trafficking. Nano Today 2021, 40, 101279. [Google Scholar] [CrossRef]
- Mullock, B.M.; Bright, N.A.; Fearon, C.W.; Gray, S.R.; Luzio, J.P. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J. Cell Biol. 1998, 140, 591–601. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. Embo. J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Parks, R.J. Human adenovirus type 5 vectors deleted of early region 1 (E1) undergo limited expression of early replicative E2 proteins and DNA replication in non-permissive cells. PLoS ONE 2017, 12, e0181012. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, J.; Zhu, X.; Su, Y.; Bao, L.; Qiu, S.; Zou, C.; Cai, Y.; Wu, J.; Tham, I.W. Recombinant adenovirus-p53 (Gendicine) sensitizes a pancreatic carcinoma cell line to radiation. Chin. J. Cancer Res. 2013, 25, 715–721. [Google Scholar]
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum. Gene Ther. 2018, 29, 160–179. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.; Li, X.; Zhang, J.; Sun, M.; Tang, Z.; Ran, W.; Yang, K.; Huang, G.; Li, L. Expert consensus on the clinical application of recombinant adenovirus human p53 for head and neck cancers. Int. J. Oral Sci. 2021, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Sun, W. Applications of Recombinant Adenovirus-p53 Gene Therapy for Cancers in the Clinic in China. Curr. Gene Ther. 2020, 20, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Liu, J.; Junn, H.J.; Lee, E.J.; Jeong, K.S.; Seol, D.W. No more helper adenovirus: Production of gutless adenovirus (GLAd) free of adenovirus and replication-competent adenovirus (RCA) contaminants. Exp. Mol. Med. 2019, 51, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, A.; Kay, M.A. A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood 2002, 99, 3923–3930. [Google Scholar] [CrossRef]
- Liu, J.; Seol, D.W. Helper virus-free gutless adenovirus (HF-GLAd): A new platform for gene therapy. BMB Rep. 2020, 53, 565–575. [Google Scholar] [CrossRef]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12 (Suppl. 1), S18–S27. [Google Scholar] [CrossRef]
- Yant, S.R.; Ehrhardt, A.; Mikkelsen, J.G.; Meuse, L.; Pham, T.; Kay, M.A. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat. Biotechnol. 2002, 20, 999–1005. [Google Scholar] [CrossRef]
- Xia, D.; Henry, L.J.; Gerard, R.D.; Deisenhofer, J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 1994, 2, 1259–1270. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Hou, W.; Lin, H.; Rebetz, J.; Hong, S.S.; Wang, Y.; Ran, L.; Fan, X. Active and separate secretion of fiber and penton base during the early phase of Ad2 or Ad5 infection. Virology 2017, 505, 172–180. [Google Scholar] [CrossRef]
- Rux, J.J.; Burnett, R.M. Adenovirus structure. Hum. Gene Ther. 2004, 15, 1167–1176. [Google Scholar] [CrossRef]
- Zubieta, C.; Schoehn, G.; Chroboczek, J.; Cusack, S. The structure of the human adenovirus 2 penton. Mol. Cell 2005, 17, 121–135. [Google Scholar] [CrossRef]
- Belin, M.T.; Boulanger, P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J. Gen. Virol. 1993, 74, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, B.; Kidd, A.H. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999, 64, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Persson, B.D.; Frängsmyr, L.; Olofsson, A.; Sandblad, L.; Heino, J.; Takada, Y.; Mould, A.P.; Schnapp, L.M.; Gall, J.; et al. Enteric Species F Human Adenoviruses use Laminin-Binding Integrins as Co-Receptors for Infection of Ht-29 Cells. Sci. Rep. 2018, 8, 10019. [Google Scholar] [CrossRef]
- Tufail, S.; Shah, M.A.; Asif, T.A.; Ullah, R.; Shehzad, A.; Ismat, F.; Shah, M.S.; Habib, M.; Calisto, B.M.; Mirza, O.; et al. Highly soluble and stable ‘insertion domain’ of the capsid penton base protein provides complete protection against infections caused by fowl adenoviruses. Microb. Pathog. 2022, 173, 105835. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M.; Wrigley, N.G.; Russell, W.C.; Martin, S.R.; McLachlan, A.D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983, 2, 1357–1365. [Google Scholar] [CrossRef]
- van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef]
- Law, L.K.; Davidson, B.L. What does it take to bind CAR? Mol. Ther. 2005, 12, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef]
- Baker, A.T.; Mundy, R.M.; Davies, J.A.; Rizkallah, P.J.; Parker, A.L. Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor. Sci. Adv. 2019, 5, eaax3567. [Google Scholar] [CrossRef]
- Rentsendorj, A.; Xie, J.; MacVeigh, M.; Agadjanian, H.; Bass, S.; Kim, D.H.; Rossi, J.; Hamm-Alvarez, S.F.; Medina-Kauwe, L.K. Typical and atypical trafficking pathways of Ad5 penton base recombinant protein: Implications for gene transfer. Gene Ther. 2006, 13, 821–836. [Google Scholar] [CrossRef]
- Scherer, J.; Vallee, R.B. Adenovirus recruits dynein by an evolutionary novel mechanism involving direct binding to pH-primed hexon. Viruses 2011, 3, 1417–1431. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Wodrich, H.; Gerace, L.; Nemerow, G.R. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005, 79, 1992–2000. [Google Scholar] [CrossRef]
- Reddy, V.S.; Nemerow, G.R. Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11715–11720. [Google Scholar] [CrossRef]
- Leopold, P.L.; Kreitzer, G.; Miyazawa, N.; Rempel, S.; Pfister, K.K.; Rodriguez-Boulan, E.; Crystal, R.G. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum. Gene Ther. 2000, 11, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.A.; Pfister, K.K.; Crystal, R.G.; Leopold, P.L. Cytoplasmic dynein mediates adenovirus binding to microtubules. J. Virol. 2004, 78, 10122–10132. [Google Scholar] [CrossRef] [PubMed]
- Agadjanian, H.; Ma, J.; Rentsendorj, A.; Valluripalli, V.; Hwang, J.Y.; Mahammed, A.; Farkas, D.L.; Gray, H.B.; Gross, Z.; Medina-Kauwe, L.K. Tumor detection and elimination by a targeted gallium corrole. Proc. Natl. Acad. Sci. USA 2009, 106, 6105–6110. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lubow, J.; Chu, D.; Ma, J.; Agadjanian, H.; Sims, J.; Gray, H.B.; Gross, Z.; Farkas, D.L.; Medina-Kauwe, L.K. A mechanistic study of tumor-targeted corrole toxicity. Mol. Pharm. 2011, 8, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Agadjanian, H.; Chu, D.; Hwang, J.Y.; Wachsmann-Hogiu, S.; Rentsendorj, A.; Song, L.; Valluripalli, V.; Lubow, J.; Ma, J.; Sharifi, B.; et al. Chemotherapy targeting by DNA capture in viral protein particles. Nanomedicine 2012, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Gay, B.; Karayan, L.; Dabauvalle, M.C.; Boulanger, P. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology 1999, 262, 163–177. [Google Scholar] [CrossRef]
- Karayan, L.; Hong, S.S.; Gay, B.; Tournier, J.; d’Angeac, A.D.; Boulanger, P. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J. Virol. 1997, 71, 8678–8689. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lubow, D.J.; Chu, D.; Sims, J.; Alonso-Valenteen, F.; Gray, H.B.; Gross, Z.; Farkas, D.L.; Medina-Kauwe, L.K. Photoexcitation of tumor-targeted corroles induces singlet oxygen-mediated augmentation of cytotoxicity. J. Control. Release 2012, 163, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Taymaz-Nikerel, H.; Karabekmez, M.E.; Eraslan, S.; Kırdar, B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci. Rep. 2018, 8, 13672. [Google Scholar] [CrossRef]
- Anders, C.K.; Adamo, B.; Karginova, O.; Deal, A.M.; Rawal, S.; Darr, D.; Schorzman, A.; Santos, C.; Bash, R.; Kafri, T.; et al. Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer. PLoS ONE 2013, 8, e61359. [Google Scholar] [CrossRef]
- Garrett, J.T.; Olivares, M.G.; Rinehart, C.; Granja-Ingram, N.D.; Sanchez, V.; Chakrabarty, A.; Dave, B.; Cook, R.S.; Pao, W.; McKinely, E.; et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA 2011, 108, 5021–5026. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.T.; Sutton, C.R.; Kuba, M.G.; Cook, R.S.; Arteaga, C.L. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin. Cancer Res. 2013, 19, 610–619. [Google Scholar] [CrossRef]
- Koumakpayi, I.H.; Diallo, J.S.; Le Page, C.; Lessard, L.; Gleave, M.; Begin, L.R.; Mes-Masson, A.M.; Saad, F. Expression and nuclear localization of ErbB3 in prostate cancer. Clin. Cancer Res. 2006, 12, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Jathal, M.K.; Chen, L.; Mudryj, M.; Ghosh, P.M. Targeting ErbB3: The New RTK(id) on the Prostate Cancer Block. Immunol. Endocr. Metab. Agents Med. Chem. 2011, 11, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Yang, Y.S.; Xu, D.P.; Qu, J.H.; Guo, M.Z.; Gong, Y.; Huang, J. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J. Surg. 2009, 33, 2112–2118. [Google Scholar] [CrossRef]
- Gespach, C. Increasing potential of HER3 signaling in colon cancer progression and therapy. Clin. Cancer Res. 2012, 18, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Chardes, T.; Gaborit, N.; Mollevi, C.; Leconet, W.; Robert, B.; Radosevic-Robin, N.; Penault-Llorca, F.; Gongora, C.; Colombo, P.E.; et al. HER3 as biomarker and therapeutic target in pancreatic cancer: New insights in pertuzumab therapy in preclinical models. Oncotarget 2014, 5, 7138–7148. [Google Scholar] [CrossRef] [PubMed]
- Humtsoe, J.O.; Pham, E.; Louie, R.J.; Chan, D.A.; Kramer, R.H. ErbB3 upregulation by the HNSCC 3D microenvironment modulates cell survival and growth. Oncogene 2016, 35, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Y.; Qiao, C.; Liu, G.; Zhao, Q.; Zhou, T.; Chen, G.; Li, Y.; Feng, J.; Li, Y.; et al. IGF-1R and ErbB3/HER3 contribute to enhanced proliferation and carcinogenesis in trastuzumab-resistant ovarian cancer model. Biochem. Biophys. Res. Commun. 2013, 436, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Vera-Badillo, F.; Seruga, B.; Templeton, A.; Pandiella, A.; Amir, E. HER3 overexpression and survival in solid tumors: A meta-analysis. J. Natl. Cancer Inst. 2013, 105, 266–273. [Google Scholar] [CrossRef]
- Clark, P.A.; Iida, M.; Treisman, D.M.; Kalluri, H.; Ezhilan, S.; Zorniak, M.; Wheeler, D.L.; Kuo, J.S. Activation of Multiple ERBB Family Receptors Mediates Glioblastoma Cancer Stem-like Cell Resistance to EGFR-Targeted Inhibition. Neoplasia 2012, 14, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S.; Preziosi, M.; Rothberg, P.G.; Zeitouni, N.; Corson, N.; Xu, L. ERBB3 is required for metastasis formation of melanoma cells. Oncogenesis 2014, 3, e110. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Wilken, J.A.; Harris, L.N.; Baron, A.T.; Kimbler, K.D.; Maihle, N.J. Trastuzumab-induced HER reprogramming in "resistant" breast carcinoma cells. Cancer Res. 2009, 69, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, I.; Barrow, D.; Hasmann, M.; Nicholson, R. Induction of erbB3/EGFR heterodimers mediates resistance to pertuzumab in a tamoxifen-resistant MCF-7 breast cancer cell line. Mol. Cancer Ther. 2007, 6, A118. [Google Scholar]
- Phillips, G.D.L.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris, H.A.; Albain, K.S.; Harbeck, N.; et al. Dual Targeting of HER2-Positive Cancer with Trastuzumab Emtansine and Pertuzumab: Critical Role for Neuregulin Blockade in Antitumor Response to Combination Therapy. Clin. Cancer Res. 2014, 20, 456–468. [Google Scholar] [CrossRef]
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Sanchez, V.; Kuba, M.G.; Rinehart, C.; Arteaga, C.L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Claus, J.; Patel, G.; Autore, F.; Colomba, A.; Weitsman, G.; Soliman, T.N.; Roberts, S.; Zanetti-Domingues, L.C.; Hirsch, M.; Collu, F.; et al. Inhibitor-induced HER2-HER3 heterodimerisation promotes proliferation through a novel dimer interface. eLife 2018, 7, e32271. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ma, J.; Lyu, H.; Huang, J.; Kim, A.; Liu, B. Role of erbB3 receptors in cancer therapeutic resistance. Acta Biochim. Biophys. Sin. 2014, 46, 190–198. [Google Scholar] [CrossRef]
- Dey, N.; Williams, C.; Leyland-Jones, B.; De, P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: Function of a kinase-dead RTK. Am. J. Transl. Res. 2015, 7, 733–750. [Google Scholar]
- Han, X.; Kasahara, N.; Kan, Y.W. Ligand-directed retroviral targeting of human breast cancer cells. Proc. Natl. Acad. Sci. USA 1995, 92, 9747–9751. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Shen, E.; Cao, F.; Li, L.; Li, X.; Wang, X.; Kariminia, S.; Chang, B.; Li, H.; et al. NRG1-dependent activation of HER3 induces primary resistance to trastuzumab in HER2-overexpressing breast cancer cells. Int. J. Oncol. 2017, 51, 1553–1562. [Google Scholar] [CrossRef]
- Rudnick, S.I.; Adams, G.P. Affinity and avidity in antibody-based tumor targeting. Cancer Biother. Radiopharm. 2009, 24, 155–161. [Google Scholar] [CrossRef]
- Mazor, Y.; Sachsenmeier, K.F.; Yang, C.; Hansen, A.; Filderman, J.; Mulgrew, K.; Wu, H.; Dall’Acqua, W.F. Enhanced tumor-targeting selectivity by modulating bispecific antibody binding affinity and format valence. Sci. Rep. 2017, 7, 40098. [Google Scholar] [CrossRef]
- Akkilic, N.; Liljeblad, M.; Blaho, S.; Hölttä, M.; Höök, F.; Geschwindner, S. Avidity-Based Affinity Enhancement Using Nanoliposome-Amplified SPR Sensing Enables Low Picomolar Detection of Biologically Active Neuregulin 1. ACS Sens. 2019, 4, 3166–3174. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S. Thinking about Enhanced Permeability and Retention Effect (EPR). J. Pers. Med. 2022, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, S.; Lu, L.; Xu, Q.; Wu, S.; Zhou, J.; Lu, J.; Fan, X.; Meng, N.; Ding, Y.; et al. Influence of lung cancer model characteristics on tumor targeting behavior of nanodrugs. J. Control. Release 2023, 354, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.; Guo, J.; Kiefer, J.R.; Proctor, W.; Bumbaca Yadav, D.; Dybdal, N.; Shen, B.Q. Trastuzumab does not bind rat or mouse ErbB2/neu: Implications for selection of non-clinical safety models for trastuzumab-based therapeutics. Breast Cancer Res. Treat. 2022, 191, 303–317. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, B.; Hothi, S.S. Herceptin-Mediated Cardiotoxicity: Assessment by Cardiovascular Magnetic Resonance. Cardiol. Res. Pract. 2022, 2022, 1910841. [Google Scholar] [CrossRef]
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 2003, 11, 495–505. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Z.; Yang, X.; Li, D.; Lian, W.; Xiang, Z.; Wang, W.; Bu, X.; Lai, W.; Hu, Z.; et al. Structure-based Design of Peptides with High Affinity and Specificity to HER2 Positive Tumors. Theranostics 2015, 5, 1154–1165. [Google Scholar] [CrossRef]
- Honarvar, H.; Calce, E.; Doti, N.; Langella, E.; Orlova, A.; Buijs, J.; D’Amato, V.; Bianco, R.; Saviano, M.; Tolmachev, V.; et al. Evaluation of HER2-specific peptide ligand for its employment as radiolabeled imaging probe. Sci. Rep. 2018, 8, 2998. [Google Scholar] [CrossRef]
- Landgraf, R. HER2 therapy. HER2 (ERBB2): Functional diversity from structurally conserved building blocks. Breast Cancer Res. 2007, 9, 202. [Google Scholar] [CrossRef]
- Yonesaka, K.; Iwama, E.; Hayashi, H.; Suzuki, S.; Kato, R.; Watanabe, S.; Takahama, T.; Tanizaki, J.; Tanaka, K.; Takeda, M.; et al. Heregulin expression and its clinical implication for patients with EGFR-mutant non-small cell lung cancer treated with EGFR-tyrosine kinase inhibitors. Sci. Rep. 2019, 9, 19501. [Google Scholar] [CrossRef]
- Breuleux, M. Role of heregulin in human cancer. Cell Mol. Life Sci. 2007, 64, 2358–2377. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef]
- Khanna, V.; Kim, H.; Zhang, W.; Larson, P.; Shah, M.; Griffith, T.S.; Ferguson, D.; Panyam, J. Novel TLR 7/8 agonists for improving NK cell mediated antibody-dependent cellular cytotoxicity (ADCC). Sci. Rep. 2021, 11, 3346. [Google Scholar] [CrossRef]
- Narvekar, A.; Pardeshi, A.; Jain, R.; Dandekar, P. ADCC enhancement: A conundrum or a boon to mAb therapy? Biologicals 2022, 79, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.W.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022, 12, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Odintsov, I.; Lui, A.J.W.; Sisso, W.J.; Gladstone, E.; Liu, Z.; Delasos, L.; Kurth, R.I.; Sisso, E.M.; Vojnic, M.; Khodos, I.; et al. The Anti-HER3 mAb Seribantumab Effectively Inhibits Growth of Patient-Derived and Isogenic Cell Line and Xenograft Models with Oncogenic NRG1 Fusions. Clin. Cancer Res. 2021, 27, 3154–3166. [Google Scholar] [CrossRef]
- Xue, J.; Kong, D.; Yao, Y.; Yang, L.; Yao, Q.; Zhu, Y.; Ding, Y.; Yang, F.; Gong, J.; Shen, L.; et al. Prediction of Human Pharmacokinetics and Clinical Effective Dose of SI-B001, an EGFR/HER3 Bi-specific Monoclonal Antibody. J. Pharm. Sci. 2020, 109, 3172–3180. [Google Scholar] [CrossRef]
- Thakkar, D.; Paliwal, S.K.; Kar, S.; Gandhi, N.; Paszkiewicz, K.; Ingram, P.; Boyd-Kirkup, J. Abstract P197: An anti-HER3 antibody, HMBD-001, that uniquely binds to and blocks the HER3 heterodimerization interface, shows superior tumor growth inhibition in biomarker-defined preclinical cancer models including NRG1-fusion driven cancers. Mol. Cancer Ther. 2021, 20, P197. [Google Scholar] [CrossRef]
- Hong, M.; Yoo, Y.; Kim, M.; Kim, J.Y.; Cha, J.S.; Choi, M.K.; Kim, U.; Kim, K.; Sohn, Y.; Bae, D.; et al. A Novel Therapeutic Anti-ErbB3, ISU104 Exhibits Potent Antitumorigenic Activity by Inhibiting Ligand Binding and ErbB3 Heterodimerization. Mol. Cancer Ther. 2021, 20, 1142–1152. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, C.; Chen, Z.; Gao, Y.; Li, Z.; Wang, L.; Li, Y.; Shen, L.; Gao, J. CAN017, a novel anti-HER3 antibody, exerted great potency in mouse avatars of esophageal squamous cell carcinoma with NRG1 as a biomarker. Am. J. Cancer Res. 2021, 11, 1697–1708. [Google Scholar]
- Beckford-Vera, D.; Li, J.; McCloskey, M.; Jennings, C.; Chin, A.; Liang, Q.; Hwang, J.; Roy, M.; Chen, M.; Kotanides, H. Targeting HER3 receptor positive cancers with a novel anti-HER3 antibody radioconjugate (ARC). Cancer Res. 2022, 82, 3306. [Google Scholar] [CrossRef]
- Gandullo-Sánchez, L.; Capone, E.; Ocaña, A.; Iacobelli, S.; Sala, G.; Pandiella, A. HER3 targeting with an antibody-drug conjugate bypasses resistance to anti-HER2 therapies. EMBO Mol. Med. 2020, 12, e11498. [Google Scholar] [CrossRef]
- Burgess, A.W.; Cho, H.S.; Eigenbrot, C.; Ferguson, K.M.; Garrett, T.P.; Leahy, D.J.; Lemmon, M.A.; Sliwkowski, M.X.; Ward, C.W.; Yokoyama, S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell 2003, 12, 541–552. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef]
- Norrby, E. The relationship between the soluble antigens and the virion of adenovirus type 3. I. Morphological characteristics. Virology 1966, 28, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Fender, P.; Ruigrok, R.W.; Gout, E.; Buffet, S.; Chroboczek, J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat. Biotechnol. 1997, 15, 52–56. [Google Scholar] [CrossRef]
- Fender, P. Recombinant adenoviruses and adenovirus penton vectors: From DNA transfer to direct protein delivery into cell. Gene Ther. Mol. Biol. 2004, 8, 85–90. [Google Scholar]
- Villegas-Méndez, A.; Fender, P.; Garin, M.I.; Rothe, R.; Liguori, L.; Marques, B.; Lenormand, J.L. Functional characterisation of the WW minimal domain for delivering therapeutic proteins by adenovirus dodecahedron. PLoS ONE 2012, 7, e45416. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.L. Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Gräslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Green, M.; Ishino, M.; Loewenstein, P.M. Mutational analysis of HIV-1 Tat minimal domain peptides: Identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell 1989, 58, 215–223. [Google Scholar] [CrossRef]
- Rice, A.P. The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies. Curr. Pharm. Des. 2017, 23, 4098–4102. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nicol, F.; Szoka, F.C., Jr. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar] [CrossRef]
- Haas, D.H.; Murphy, R.M. Templated assembly of the pH-sensitive membrane-lytic peptide GALA. J. Pept. Res. 2004, 63, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef] [PubMed]
- Warso, M.A.; Richards, J.M.; Mehta, D.; Christov, K.; Schaeffer, C.; Rae Bressler, L.; Yamada, T.; Majumdar, D.; Kennedy, S.A.; Beattie, C.W.; et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br. J. Cancer 2013, 108, 1061–1070. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Su, Z.; Huang, Y. Bacterial cupredoxin azurin hijacks cellular signaling networks: Protein-protein interactions and cancer therapy. Protein Sci. 2017, 26, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J.; et al. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro. Oncol. 2016, 18, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Zender, L.; Kühnel, F.; Köck, R.; Manns, M.; Kubicka, S. VP22-mediated intercellular transport of p53 in hepatoma cells in vitro and in vivo. Cancer Gene Ther. 2002, 9, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Johansson, M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem. Biophys. Res. Commun. 2002, 291, 367–371. [Google Scholar] [CrossRef]
- Medina-Kauwe, L.K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 2003, 55, 1485–1496. [Google Scholar] [CrossRef]




| Name of Nanobiologic | Therapeutic Cargo (Type of Therapeutic) | Mechanism of Action | In Vitro Disease Model | In Vivo Disease Model |
| HerGa/H3-G [21,67,68] | Gallium corrole (Small molecule) | Mitochondrial and cytoskeletal disruption; Fluorescence imaging | Human HER2+ breast tumor lines: BT-474, BT-474 TR *, SKBR3, SKBR3 TR * | Female NU/NU mice with BT474 or BT474 TR * xenografts; Particle dosage per injection, route: 0.004 mg/kg corrole dose, IV |
| HerMn [21] | Manganese corrole (small molecule) | Mitochondrial and cytoskeletal disruption; Paramagnetism; MR detection and MRI | Human HER2+ tumor lines: BT474, MDA-MB-435; Human HER2 low tumor sub-line of MDA-MB-231; Human cardiosphere-derived cells (CDCs) | Female NU/NU mice with MDA-MB-435 xenografts; Particle dosage per injection, route: 0.00022 mg/kg corrole dose, IV |
| HerDox/H3-D [22,69] | Doxorubicin (Chemotherapeutic) | DNA-intercalating agent | Human HER2+ breast tumor lines: BT-474, BT-474 TR *, SKBR3, SKBR3 TR *, JIMT-1 | Female NU/NU mice with JIMT-1 xenografts; Particle dosage per injection, route: 0.02 mg/kg doxorubicin dose, IV |
| HerSi [23] | siRNA (nucleic acid) | RNA interference | Human HER3+ melanoma tumor lines: MDA-MB-435, MDA-MB-435-Br4 | Female NU/NU mice with MDA-MB-435 xenografts; Particle dosage per injection, route: 0.087 mg/kg siRNA, IV; Female BALB/c mice with 4T1-Luc orthotopic implants; Particle dosage per injection, route: 0.087 mg/kg siRNA, IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhaghnazar, R.L.; Medina-Kauwe, L. Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors. Cancers 2023, 15, 3240. https://doi.org/10.3390/cancers15123240
Benhaghnazar RL, Medina-Kauwe L. Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors. Cancers. 2023; 15(12):3240. https://doi.org/10.3390/cancers15123240
Chicago/Turabian StyleBenhaghnazar, Rebecca Leah, and Lali Medina-Kauwe. 2023. "Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors" Cancers 15, no. 12: 3240. https://doi.org/10.3390/cancers15123240
APA StyleBenhaghnazar, R. L., & Medina-Kauwe, L. (2023). Adenovirus-Derived Nano-Capsid Platforms for Targeted Delivery and Penetration of Macromolecules into Resistant and Metastatic Tumors. Cancers, 15(12), 3240. https://doi.org/10.3390/cancers15123240