Gene Expression Analysis Links Autocrine Vasoactive Intestinal Peptide and ZEB1 in Gastrointestinal Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. VIP Expression in Cancer
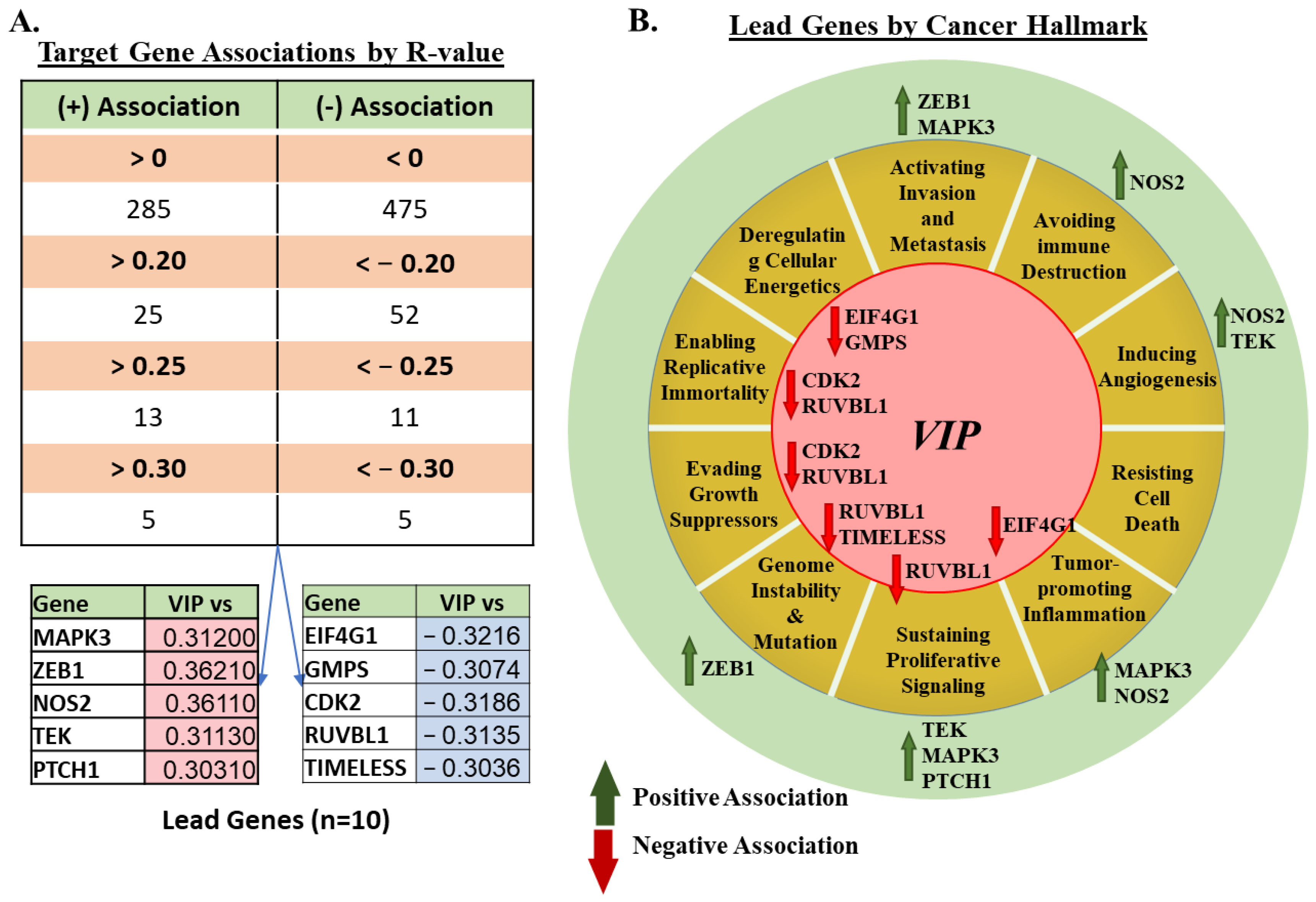
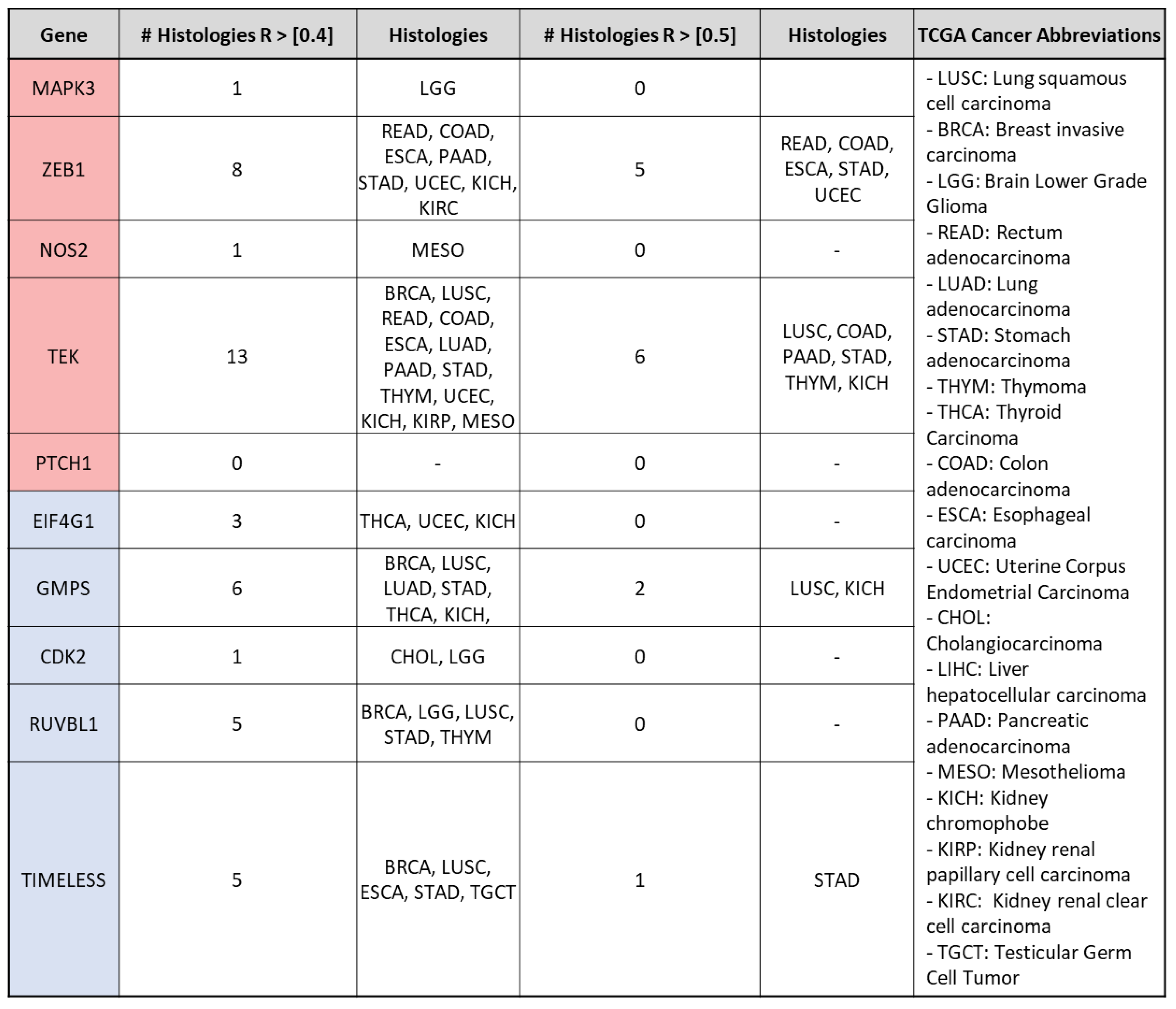
3.2. mRNA Expression of VIP Associated Genes by Cancer Hallmark and Tissue Histology
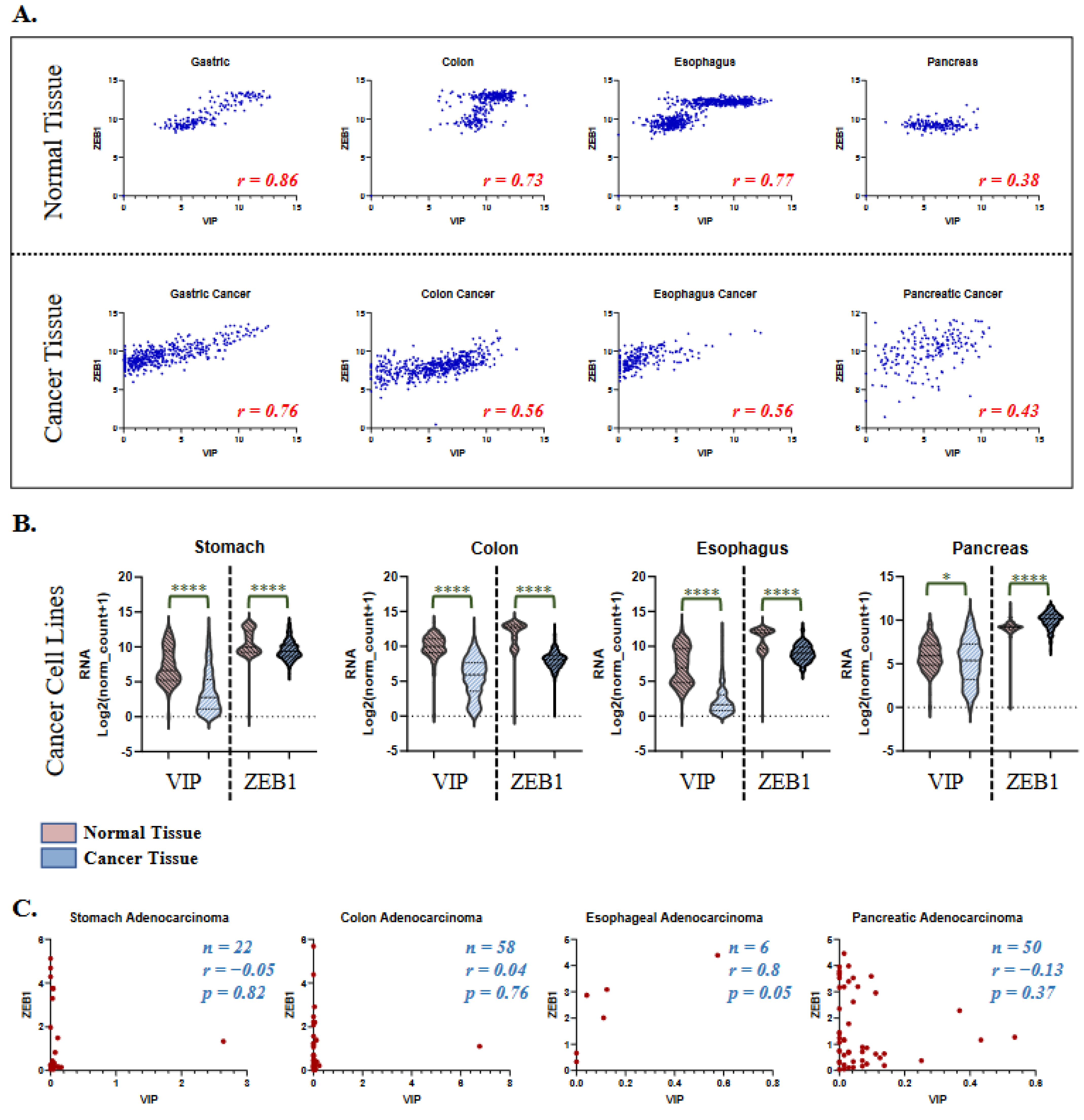
3.3. VIP and ZEB1 Expression in Healthy Tissue and Cancer Cell Lines
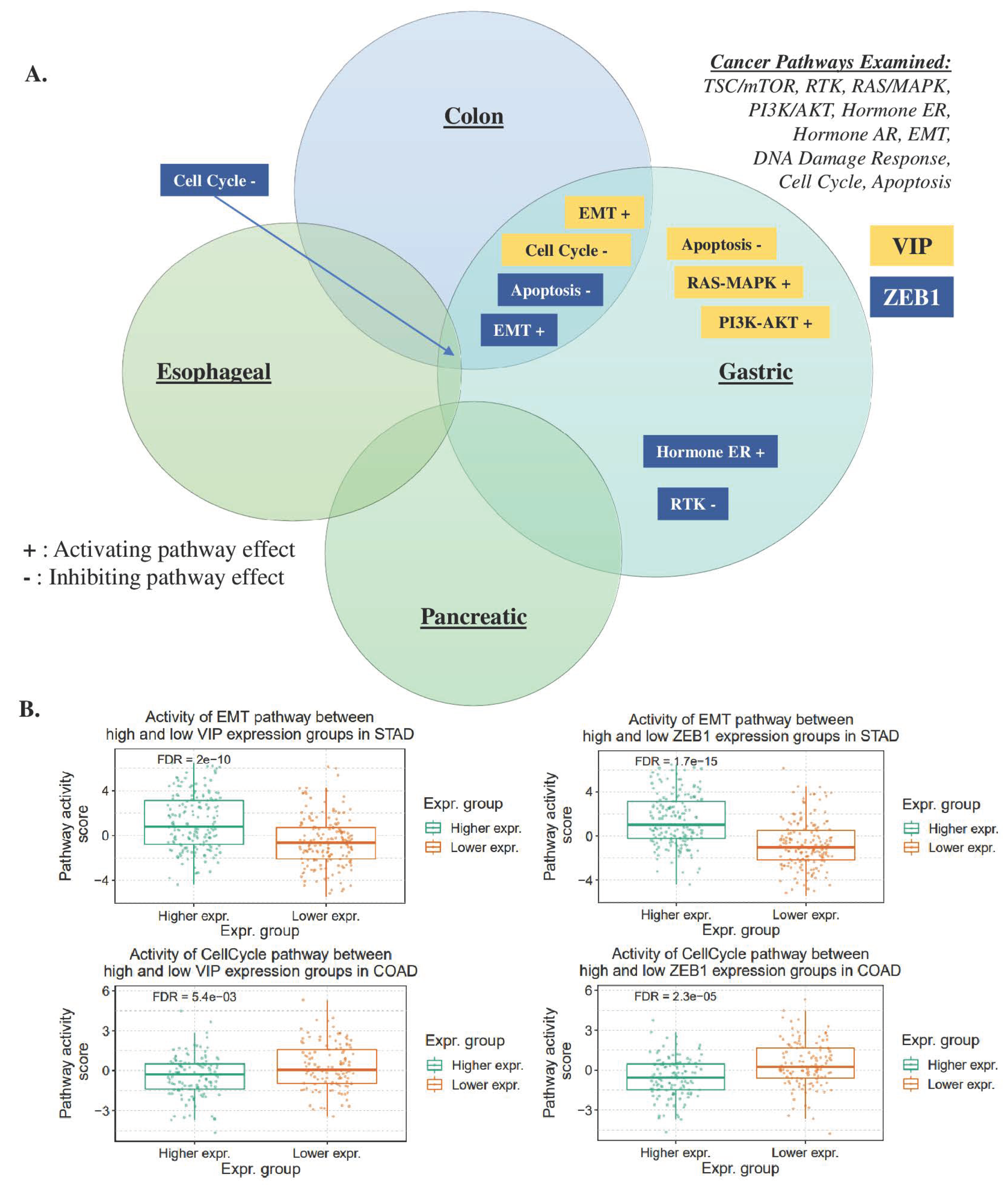
3.4. Gene Set Analysis of VIP and ZEB1
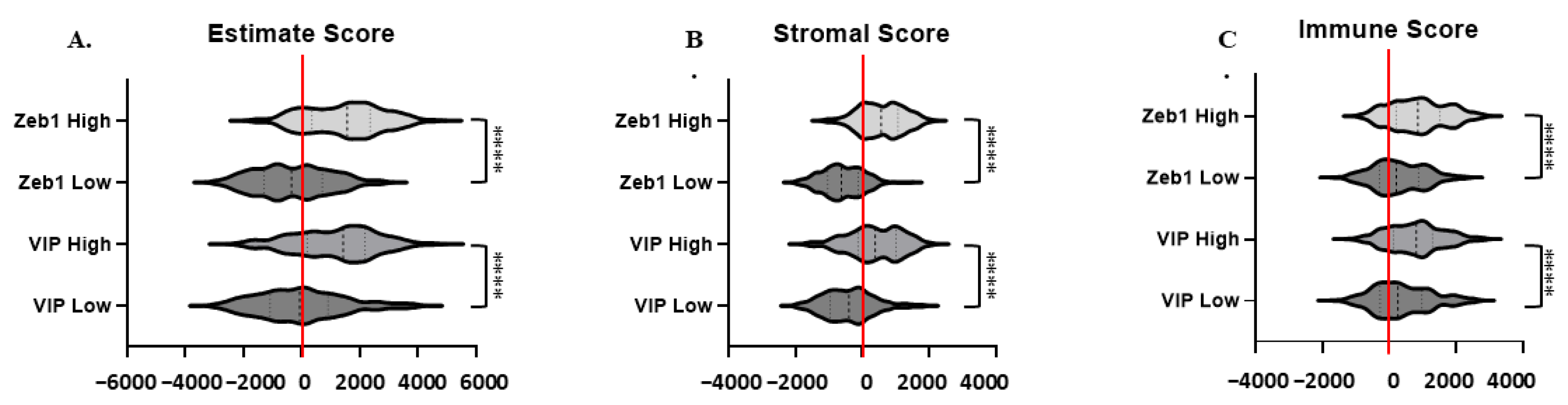
3.5. Estimate, Stromal, and Immune Scoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, M.; Pozo, D.; Ganea, D. The Significance of Vasoactive Intestinal Peptide in Immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef]
- Ng, S.Y.L.; Chow, B.K.C.; Kasamatsu, J.; Kasahara, M.; Lee, L.T.O. Agnathan VIP, PACAP and Their Receptors: Ancestral Origins of Today’s Highly Diversified Forms. PLoS ONE 2012, 7, e44691. [Google Scholar] [CrossRef] [Green Version]
- Harmar, A.J.; Arimura, A.; Gozes, I.; Journot, L.; Laburthe, M.; Pisegna, J.R.; Rawlings, S.R.; Robberecht, P.; Said, S.I.; Sreedharan, S.P.; et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998, 50, 265–270. [Google Scholar]
- Dickson, L.; Finlayson, K. VPAC and PAC receptors: From ligands to function. Pharmacol. Ther. 2009, 121, 294–316. [Google Scholar] [CrossRef]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. VIP/PACAP, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef] [Green Version]
- Fabricius, D.; Karacay, B.; Shutt, D.; Leverich, W.; Schafer, B.; Takle, E.; Thedens, D.; Khanna, G.; Raikwar, S.; Yang, B.; et al. Characterization of Intestinal and Pancreatic Dysfunction in VPAC1-Null Mutant Mouse. Pancreas 2011, 40, 861–871. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acids 2013, 45, 25–39. [Google Scholar] [CrossRef]
- Smalley, S.G.R.; Barrow, P.A.; Foster, N. Immunomodulation of innate immune responses by vasoactive intestinal peptide (VIP): Its therapeutic potential in inflammatory disease. Clin. Exp. Immunol. 2009, 157, 225–234. [Google Scholar] [CrossRef]
- Vassiliou, E.; Jiang, X.; Delgado, M.; Ganea, D. TH2 Lymphocytes Secrete Functional VIP upon Antigen Stimulation. Arch. Physiol. Biochem. 2001, 109, 365–368. [Google Scholar] [CrossRef]
- Reubi, J.C.; Läderach, U.; Waser, B.; Gebbers, J.O.; Robberecht, P.; Laissue, J.A. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 2000, 60, 3105–3112. [Google Scholar] [PubMed]
- Virgolini, I.; Raderer, M.; Kurtaran, A.; Angelberger, P.; Yang, Q.; Radosavljevic, M.; Leimer, M.; Kaserer, K.; Li, S.; Kornek, G.; et al. 123I-vasoactive intestinal peptide (VIP) receptor scanning: Update of imaging results in patients with adenocarcinomas and endocrine tumors of the gastrointestinal tract. Nucl. Med. Biol. 1996, 23, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I. Mack Forster Award Lecture Receptor nuclear medicine: Vasointestinal peptide and somatostatin receptor scintigraphy for diagnosis and treatment of tumour patients. Eur. J. Clin. Investig. 1997, 27, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Hamilton, G.; Brodowicz, T.; Haberl, I.; Fiebiger, W.C.; Scheithauer, W.; Virgolini, I.; Köstler, W.J.; Oberhuber, G.; Raderer, M. Serum levels of vasoactive intestinal peptide (VIP) in patients with adenocarcinomas of the gastrointestinal tract. Anticancer. Res. 2001, 21, 1183–1187. [Google Scholar]
- Ravindranathan, S.; Passang, T.; Li, J.-M.; Wang, S.; Dhamsania, R.; Ware, M.B.; Zaidi, M.Y.; Zhu, J.; Cardenas, M.; Liu, Y.; et al. Targeting vasoactive intestinal peptide-mediated signaling enhances response to immune checkpoint therapy in pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 6418. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR Review 1. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Laburthe, M.; Rousset, M.; Chevalier, G.; Boissard, C.; Dupont, C.; Zweibaum, A.; Rosselin, G. Vasoactive intestinal peptide control of cyclic adenosine 3′:5′-monophosphate levels in seven human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1980, 40, 2529–2533. [Google Scholar]
- Laburthe, M.; Rousset, M.; Boissard, C.; Chevalier, G.; Zweibaum, A.; Rosselin, G. Vasoactive intestinal peptide: A potent stimulator of adenosine 3′:5′-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc. Natl. Acad. Sci. USA 1978, 75, 2772–2775. [Google Scholar] [CrossRef] [Green Version]
- Moody, T.W.; Hill, J.M.; Jensen, R.T. VIP as a trophic factor in the CNS and cancer cells. Peptides 2003, 24, 163–177. [Google Scholar] [CrossRef]
- Moody, T.W.; Zia, F.; Draoui, M.; Brenneman, D.E.; Fridkin, M.; Davidson, A.; Gozes, I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc. Natl. Acad. Sci. USA 1993, 90, 4345–4349. [Google Scholar] [CrossRef] [Green Version]
- Moody, T.W.; Leyton, J.; Chan, D.; Brenneman, D.C.; Fridkin, M.; Gelber, E.; Levy, A.; Gozes, I. VIP receptor antagonists and chemotherapeutic drugs inhibit the growth of breast cancer cells. Breast Cancer Res. Treat. 2001, 68, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Valdehita, A.; Bajo, A.M.; Schally, A.V.; Varga, J.L.; Carmena, M.J.; Prieto, J.C. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol. Cell. Endocrinol. 2009, 302, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.S.; Chouchane, A.I.; Wang, E.; Kulik, G.; Marincola, F.M.; Chouchane, L. Cytoprotective effect of neuropeptides on cancer stem cells: Vasoactive intestinal peptide-induced antiapoptotic signaling. Cell Death Dis. 2017, 8, e2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas n.d. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 1 January 2020).
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Nanostring Tumor Signaling 360 n.d. Available online: https://nanostring.com/support-documents/tumor-signaling-360-panel/ (accessed on 1 January 2020).
- Carithers, L.J.; Moore, H.M. The Genotype-Tissue Expression (GTEx) Project. Biopreservation Biobanking 2015, 13, 307–308. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Liu, C.-J.; Hu, F.-F.; Xie, G.-Y.; Miao, Y.-R.; Li, X.-W.; Zeng, Y.; Guo, A.-Y. GSCA: An integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief. Bioinform. 2023, 24, bbac558. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [Green Version]
- The Human Protein Atlas, n.d. Available online: https://www.proteinatlas.org/ (accessed on 1 January 2020).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Fernandez-Martin, A.; Ganea, D.; Delgado, M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 2006, 107, 3632–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-M.; Petersen, C.T.; Li, J.-X.; Panjwani, R.; Chandra, D.J.; Giver, C.R.; Blazar, B.R.; Waller, E.K. Modulation of Immune Checkpoints and Graft-versus-Leukemia in Allogeneic Transplants by Antagonizing Vasoactive Intestinal Peptide Signaling. Cancer Res. 2016, 76, 6802–6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, C.T.; Hassan, M.; Morris, A.B.; Jeffery, J.; Lee, K.; Jagirdar, N.; Staton, A.D.; Raikar, S.S.; Spencer, H.T.; Sulchek, T.; et al. Improving ex vivo T cell expansion from DLBCL patients for T cell therapies via antagonism of PI3K δ and VIP. Blood 2017, 130, 3195. [Google Scholar]
- Petersen, C.T.; Hassan, M.; Morris, A.B.; Jeffery, J.; Lee, K.; Jagirdar, N.; Staton, A.D.; Raikar, S.S.; Spencer, H.T.; Sulchek, T.; et al. Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kδ inhibitors and VIP antagonists. Blood Adv. 2018, 2, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Sánchez-Franco, F.; Palacios, N.; Sánchez, I.; Cacicedo, L. IGF-I and vasoactive intestinal peptide (VIP) regulate cAMP-response element-binding protein (CREB)-dependent transcription via the mitogen-activated protein kinase (MAPK) pathway in pituitary cells: Requirement of Rap1. J. Mol. Endocrinol. 2005, 34, 699–712. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Patel, B.A.; Keenan, C.M.; Dicay, M.; Chapman, K.; McCafferty, D.-M.; Savidge, T.C.; Beck, P.L.; MacNaughton, W.K.; Sharkey, K.A. Inhibiting Inducible Nitric Oxide Synthase in Enteric Glia Restores Electrogenic Ion Transport in Mice With Colitis. Gastroenterology 2015, 149, 445–455.e3. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.; Gonzalez-Rey, E. Vasoactive Intestinal Peptide Induces Cell Cycle Arrest and Regulatory Functions in Human T Cells at Multiple Levels. Mol. Cell. Biol. 2010, 30, 2537–2551. [Google Scholar] [CrossRef] [Green Version]
- Muraro, N.; Pírez, N.; Ceriani, M. The circadian system: Plasticity at many levels. Neuroscience 2013, 247, 280–293. [Google Scholar] [CrossRef]
- Caramel, J.; Ligier, M.; Puisieux, A. Pleiotropic Roles for ZEB1 in Cancer. Cancer Res. 2018, 78, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, T.; Carbone, A.; Mazzarelli, F.; Cuttano, R.; Dama, E.; Nittoli, T.; Albanesi, J.; Barisciano, G.; Forte, N.; Palumbo, O.; et al. Loss of circadian gene Timeless induces EMT and tumor progression in colorectal cancer via Zeb1-dependent mechanism. Cell Death Differ. 2022, 29, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, I.H.; Waller, E.K.; Dhamsania, R.K.; Chandrasekaran, S. Gene Expression Analysis Links Autocrine Vasoactive Intestinal Peptide and ZEB1 in Gastrointestinal Cancers. Cancers 2023, 15, 3284. https://doi.org/10.3390/cancers15133284
Rao IH, Waller EK, Dhamsania RK, Chandrasekaran S. Gene Expression Analysis Links Autocrine Vasoactive Intestinal Peptide and ZEB1 in Gastrointestinal Cancers. Cancers. 2023; 15(13):3284. https://doi.org/10.3390/cancers15133284
Chicago/Turabian StyleRao, Ishani H., Edmund K. Waller, Rohan K. Dhamsania, and Sanjay Chandrasekaran. 2023. "Gene Expression Analysis Links Autocrine Vasoactive Intestinal Peptide and ZEB1 in Gastrointestinal Cancers" Cancers 15, no. 13: 3284. https://doi.org/10.3390/cancers15133284




