The Biological Role and Translational Implications of the Long Non-Coding RNA GAS5 in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Breast Cancer
1.2. Long Non-Coding RNAs
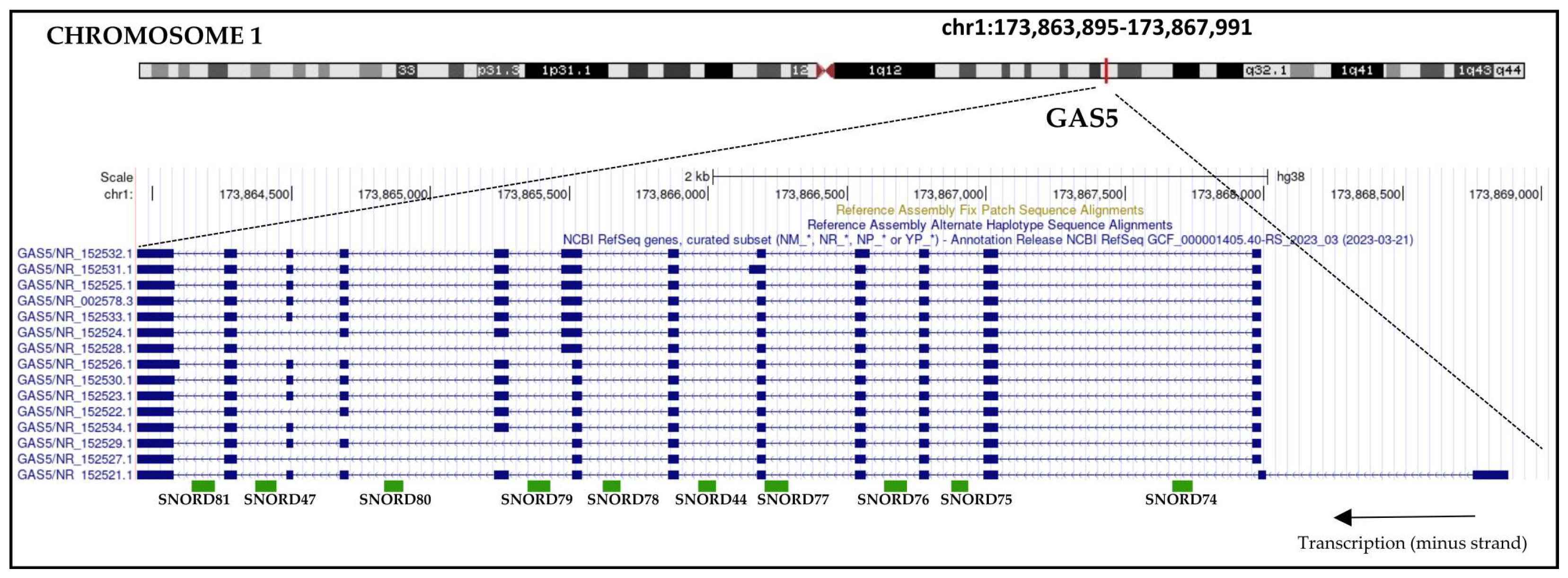
1.3. GAS5
2. GAS5 Expression and Functions in BC
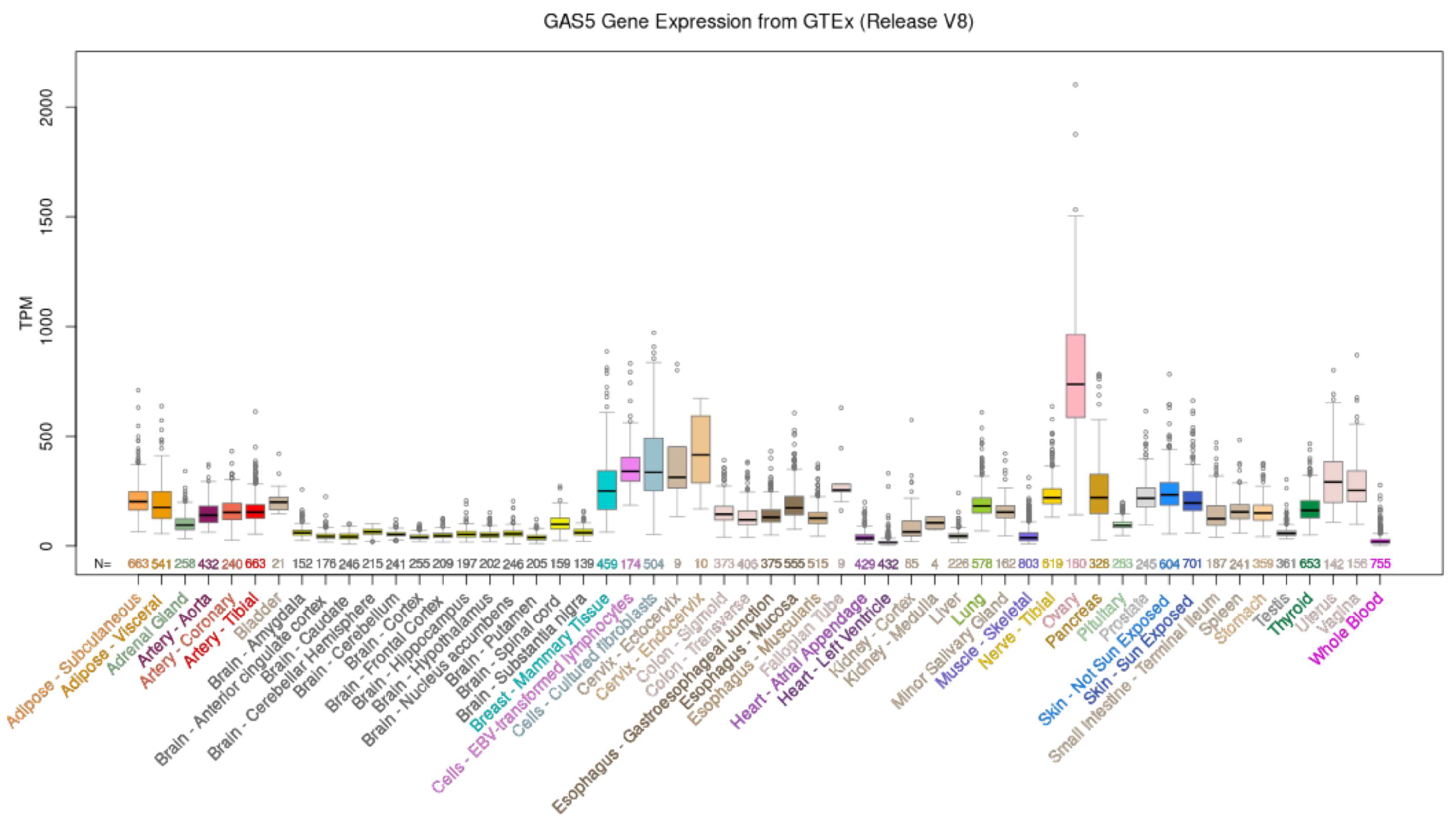
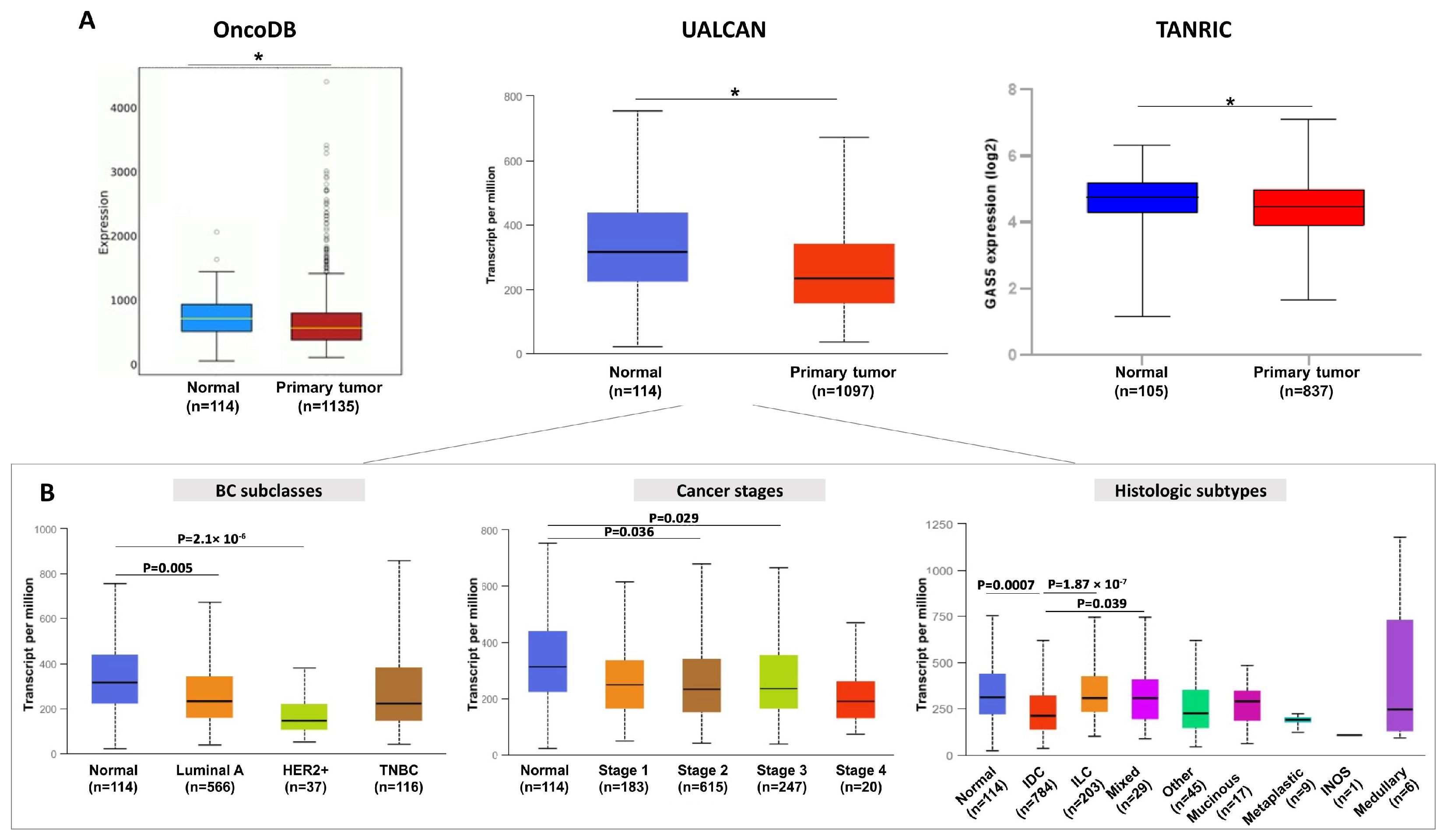
2.1. GAS5 Expression Is Dysregulated in BC Tissues
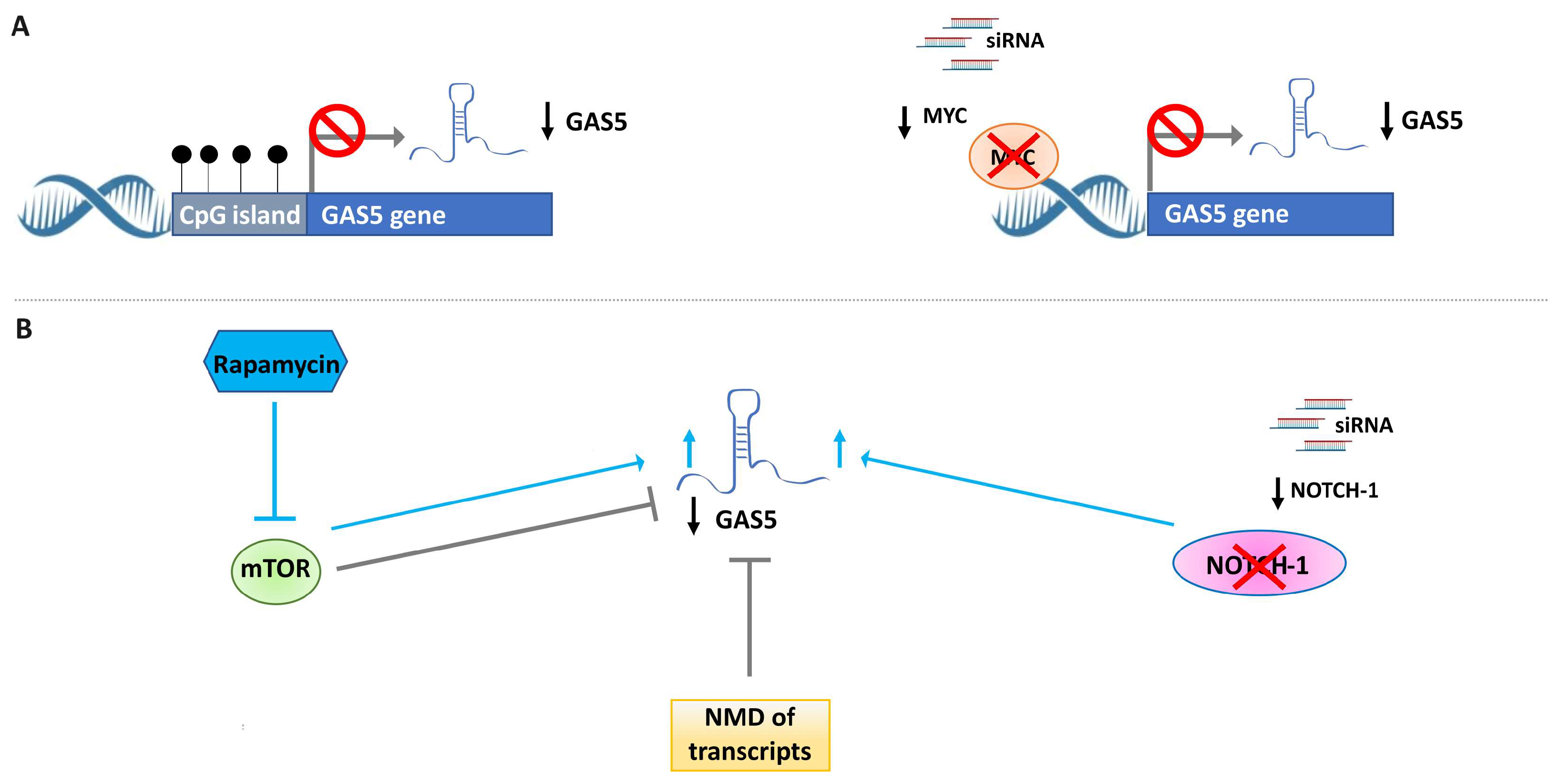
2.2. Different Mechanisms Are Involved in the Downregulation of GAS5 in BC
2.3. GAS5 Functions as ceRNA of miRNAs in BC
3. GAS5 as a Potential Biomarker and Therapeutic Target in BC
3.1. GAS5 Levels Modulate the Response to Apoptosis-Inducing Agents in BC Cells
3.2. GAS5 Levels Are Modulated by Different Anticancer Drugs
3.3. GAS5 Has Regulatory Functions in Drug Resistance of BC
3.4. GAS5 May Sensitize BC Cells to Radiation Therapy
3.5. GAS5 Levels in Liquid Biopsies from BC Patients
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Marchina, E.; Fontana, M.G.; Speziani, M.; Salvi, A.; Ricca, G.; Di Lorenzo, D.; Gervasi, M.; Caimi, L.; Barlati, S. BRCA1 and BRCA2 genetic test in high risk patients and families: Counselling and management. Oncol. Rep. 2010, 24, 1661–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeni, E.; Grossi, I.; Marchina, E.; Coniglio, A.; Incardona, P.; Cavalli, P.; Zorzi, F.; Chiodera, P.; Paties, C.; Crosatti, M.; et al. DNA methylation variations in familial female and male breast cancer. Oncol. Lett. 2021, 21, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, N.; Helbig, S.; Dörk, T. Hereditary breast cancer: Ever more pieces to the polygenic puzzle. Hered. Cancer Clin. Pract. 2013, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Boddicker, N.J.; Na, J.; Polley, E.C.; Hu, C.; Hart, S.N.; Gnanaolivu, R.D.; Larson, N.; Holtegaard, S.; Huang, H.; et al. Contralateral Breast Cancer Risk Among Carriers of Germline Pathogenic Variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J. Clin. Oncol. 2023, 41, 1703–1713. [Google Scholar] [CrossRef]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological types of breast cancer: How special are they? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, I.O.; Galea, M.; Broughton, N.; Locker, A.; Blamey, R.W.; Elston, C.W. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992, 20, 479–489. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 17, 430–447. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Jungreis, I.; Lagarde, J.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Armstrong, J.; Barnes, I.; et al. GENCODE 2021. Nucleic Acids Res. 2021, 49, 917. [Google Scholar] [CrossRef] [PubMed]
- Hon, C.-C.; Ramilowski, J.A.; Harshbarger, J.; Bertin, N.; Rackham, O.J.L.; Gough, J.; Denisenko, E.; Schmeier, S.; Poulsen, T.M.; Severin, J.; et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 2017, 543, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long non-coding RNAs: Mechanism of action and functional utility. Non-Coding RNA Res. 2016, 1, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; King, R.M.; Philipson, L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Feng, C.; Qin, Y.; Xiao, J.; Zhang, Z.; Ma, L. LncBook 2.0: Integrating human long non-coding RNAs with multi-omics annotations. Nucleic Acids Res. 2023, 51, D186–D191. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B. GAS5-mediated regulation of cell signaling. Mol. Med. Rep. 2020, 22, 3049–3056. [Google Scholar] [CrossRef]
- Goustin, A.; Thepsuwan, P.; Kosir, M.; Lipovich, L. The Growth-Arrest-Specific (GAS)-5 Long Non-Coding RNA: A Fascinating lncRNA Widely Expressed in Cancers. Non-Coding RNA 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Xie, Z.; Li, J.; Lin, J.; Zheng, G.; Liu, W.; Tang, S.; Cen, S.; Ye, G.; Li, Z.; et al. Gas5 protects against osteoporosis by targeting upf1/smad7 axis in osteoblast differentiation. Elife 2020, 9, e59079. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Parag, S.; Patel, R.; Lui, A.; Murr, M.; Cai, J.; Patel, N.A. Stabilization of lncRNA GAS5 by a Small Molecule and Its Implications in Diabetic Adipocytes. Cell Chem. Biol. 2019, 26, 319–330.e6. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Bullock, M.; Calin, G. The Clinical Relevance of Long Non-Coding RNAs in Cancer. Cancers 2015, 7, 2169–2182. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 902. [Google Scholar] [CrossRef]
- Faranda, T.; Grossi, I.; Manganelli, M.; Marchina, E.; Baiocchi, G.; Portolani, N.; Crosatti, M.; De Petro, G.; Salvi, A. Differential expression profiling of long non-coding RNA GAS5 and miR-126-3p in human cancer cells in response to sorafenib. Sci. Rep. 2019, 9, 9118. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Li, C.; Lan, T.; Wu, L.; Yuan, Y.; Liu, Q.; Liu, Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol. Med. Rep. 2016, 13, 1541–1550. [Google Scholar] [CrossRef] [Green Version]
- Pickard, M.R.; Williams, G.T. Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes 2015, 6, 484–499. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Cho, M.; Wang, X. OncoDB: An interactive online database for analysis of gene expression and viral infection in cancer. Nucleic Acids Res. 2022, 50, D1334–D1339. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Roebuck, P.; Diao, L.; Liu, L.; Yuan, Y.; Weinstein, J.N.; Liang, H. TANRIC: An interactive open platform to explore the function of IncRNAs in cancer. Cancer Res. 2015, 75, 3728–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshi, A.; Sharifi, F.S.; Khorramian Ghahfarokhi, M.; Faghih, Z.; Doosti, A.; Ostovari, S.; Mahmoudi Maymand, E.; Ghahramani Seno, M.M. Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol. Ther. Nucleic Acids 2018, 12, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhai, L.; Wang, H.; Liu, C.; Zhang, J.; Chen, W.; Wei, Q. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget 2016, 7, 27778–27786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, L.; Ju, H.; Yang, Z.; Ge, Q.; Zhang, Z.; Liu, F.; Yang, L.; Gong, H.; Shi, C.; Qu, L.; et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 2021, 3, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qian, L.; Tang, X.; Chen, Y.; Zhao, Z.; Zhang, C. Long non-coding RNA growth arrest-specific 5 (GAS5) acts as a tumor suppressor by promoting autophagy in breast cancer. Mol. Med. Rep. 2020, 22, 2460–2468. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Wang, Z.; Wang, P.; Gao, X.; Wang, Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. Pharmacother. 2018, 104, 451–457. [Google Scholar] [CrossRef]
- Zheng, S.; Li, M.; Miao, K.; Xu, H. lncRNA GAS5-promoted apoptosis in triple-negative breast cancer by targeting miR-378a-5p/SUFU signaling. J. Cell. Biochem. 2020, 121, 2225–2235. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, L.; Yuan, H.; Huang, X.W.; Xiang, T.; Dai, S. Up-regulated lncRNA GAS5 promotes chemosensitivity and apoptosis of triple-negative breast cancer cells. Cell Cycle 2019, 18, 1965–1975. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xie, R.; Jiang, J.; Zhai, L.; Yang, C.H.; Zhang, J.; Wang, X.; Chen, D.X.; Niu, H.T.; Chen, L. 5-Aza-dC suppresses melanoma progression by inhibiting GAS5 hypermethylation. Oncol. Rep. 2022, 48, 123. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Hong, L.; Wang, Q.; Huang, J.; Jiang, L. Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J. Cell. Physiol. 2019, 234, 23571–23580. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, H.B.J.; Fumagalli, S.; Dennis, P.B.; Reinhard, C.; Pearson, R.B.; Thomas, G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997, 16, 3693–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a Multi-Small-Nucleolar-RNA (snoRNA) Host Gene and a Member of the 5′-Terminal Oligopyrimidine Gene Family Reveals Common Features of snoRNA Host Genes. Mol. Cell. Biol. 1998, 18, 6897–6909. [Google Scholar] [CrossRef] [Green Version]
- Mourtada-Maarabouni, M.; Hasan, A.M.; Farzaneh, F.; Williams, G.T. Inhibition of Human T-Cell Proliferation by Mammalian Target of Rapamycin (mTOR) Antagonists Requires Noncoding RNA Growth-Arrest-Specific Transcript 5 (GAS5). Mol. Pharmacol. 2010, 78, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yacqub-Usman, K.; Pickard, M.R.; Williams, G.T. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 2015, 75, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Pickard, M.R.; Williams, G.T. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: Implications for chemotherapy. Breast Cancer Res. Treat. 2014, 145, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Wang, B. Notch-1 promotes breast cancer cells proliferation by regulating LncRNA GAS5. Int. J. Clin. Exp. Med. 2015, 8, 14464–14471. [Google Scholar]
- Tokgun, P.E.; Tokgun, O.; Kurt, S.; Tomatir, A.G.; Akca, H. MYC-driven regulation of long non-coding RNA profiles in breast cancer cells. Gene 2019, 714, 143955. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Pan, T.; Jiang, D.; Jin, L.; Geng, Y.; Feng, X.; Shen, A.; Zhang, L. The lncRNA-GAS5/miR-221-3p/DKK2 Axis Modulates ABCB1-Mediated Adriamycin Resistance of Breast Cancer via the Wnt/β-Catenin Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 19, 1434–1448. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.B.; Wei, Y.H. LncRNA GAS5 affects epithelial-mesenchymal transition and invasion of breast cancer cells by regulating miR-216b. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4873–4881. [Google Scholar] [PubMed]
- Kaur, J.; Salehen, N.; Norazit, A.; Rahman, A.A.; Murad, N.A.A.; Rahman, N.M.A.N.A.; Ibrahim, K. Tumor Suppressive Effects of GAS5 in Cancer Cells. Non-Coding RNA 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Qian, T.; Li, S.; Xie, Y.; Tao, M. Metformin reverses tamoxifen resistance through the lncRNA GAS5-medicated mTOR pathway in breast cancer. Ann. Transl. Med. 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Esmatabadi, M.J.D.; Motamedrad, M.; Sadeghizadeh, M. Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine 2018, 42, 56–65. [Google Scholar] [CrossRef]
- Liu, K.; Gao, L.; Ma, X.; Huang, J.J.; Chen, J.; Zeng, L.; Ashby, C.R.; Zou, C.; Chen, Z.S. Long non-coding RNAs regulate drug resistance in cancer. Mol. Cancer 2020, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Filippova, E.A.; Fridman, M.V.; Burdennyy, A.M.; Loginov, V.I.; Pronina, I.V.; Lukina, S.S.; Dmitriev, A.A.; Braga, E.A. Long Noncoding RNA GAS5 in Breast Cancer: Epigenetic Mechanisms and Biological Functions. Int. J. Mol. Sci. 2021, 22, 6810. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Haussmann, J.; Corradini, S.; Nestle-Kraemling, C.; Bölke, E.; Njanang, F.J.D.; Tamaskovics, B.; Orth, K.; Ruckhaeberle, E.; Fehm, T.; Mohrmann, S.; et al. Recent advances in radiotherapy of breast cancer. Radiat. Oncol. 2020, 15, 71. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, L.; Yan, W.; Qiu, L.; Zhang, J.; Jia, X. LncRNA GAS5 Sensitizes Breast Cancer Cells to Ionizing Radiation by Inhibiting DNA Repair. Biomed. Res. Int. 2022, 2022, 1987519. [Google Scholar] [CrossRef]
- Liang, W.; Lv, T.; Shi, X.; Liu, H.; Zhu, Q.; Zeng, J.; Yang, W.; Yin, J.; Song, Y. Circulating long noncoding RNA GAS5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medecine 2016, 95, e4608. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lv, Y.; Shao, C.; Chen, C.; Zhang, T.; Wei, Y.; Fan, H.; Lv, T.; Liu, H.; Song, Y. Tumor-derived exosomal lncRNA GAS5 as a biomarker for early-stage non-small-cell lung cancer diagnosis. J. Cell. Physiol. 2019, 234, 20721–20727. [Google Scholar] [CrossRef]
- Fayda, M.; Isin, M.; Tambas, M.; Guveli, M.; Meral, R.; Altun, M.; Sahin, D.; Ozkan, G.; Sanli, Y.; Isin, H.; et al. Do circulating long non-coding RNAs (lncRNAs) (LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy? Tumor Biol. 2016, 37, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Kresoja-Rakic, J.; Szpechcinski, A.; Kirschner, M.B.; Ronner, M.; Minatel, B.; Martinez, V.D.; Lam, W.L.; Weder, W.; Stahel, R.; Früh, M.; et al. miR-625-3p and lncRNA GAS5 in Liquid Biopsies for Predicting the Outcome of Malignant Pleural Mesothelioma Patients Treated with Neo-Adjuvant Chemotherapy and Surgery. Non-Coding RNA 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manganelli, M.; Grossi, I.; Ferracin, M.; Guerriero, P.; Negrini, M.; Ghidini, M.; Senti, C.; Ratti, M.; Pizzo, C.; Passalacqua, R.; et al. Longitudinal circulating levels of mir-23b-3p, mir-126-3p and lncrna gas5 in hcc patients treated with sorafenib. Biomedicines 2021, 9, 813. [Google Scholar] [CrossRef]
- Han, L.; Ma, P.; Liu, S.M.; Zhou, X. Circulating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effects. Tumor Biol. 2016, 37, 6847–6854. [Google Scholar] [CrossRef]
- Toraih, E.A.; El-Wazir, A.; Al Ageeli, E.; Hussein, M.H.; Eltoukhy, M.M.; Killackey, M.T.; Kandil, E.; Fawzy, M.S. Unleash multifunctional role of long noncoding RNAs biomarker panel in breast cancer: A predictor classification model. Epigenomics 2020, 12, 1215–1237. [Google Scholar] [CrossRef]
- Manganelli, M.; Grossi, I.; Corsi, J.; D’Agostino, V.G.; Jurikova, K.; Cusanelli, E.; Molfino, S.; Portolani, N.; Salvi, A.; De Petro, G. Expression of Cellular and Extracellular TERRA, TERC and TERT in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 6183. [Google Scholar] [CrossRef]
- Chen, X.; Xue, S.; Ren, G.; Li, Y.; Chao, W.; Chen, S.; Li, X. Level of LncRNA GAS5 and Hippocampal Volume are Associated with the Progression of Alzheimer’s Disease. Clin. Interv. Aging 2022, 17, 745–753. [Google Scholar] [CrossRef]
- Peng, T.; Ji, D.; Jiang, Y. Long non-coding RNA GAS5 suppresses rheumatoid arthritis progression via miR-128-3p/HDAC4 axis. Mol. Cell. Biochem. 2021, 476, 2491–2501. [Google Scholar] [CrossRef]
- Song, J.; Ahn, C.; Chun, C.H.; Jin, E.J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014, 32, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Hou, Y.; Li, Y.; Bian, Y.; Khan, M.; Li, F.; Huang, L.; Qiao, C. Long Non-coding RNA Gas5 Is Associated With Preeclampsia and Regulates Biological Behaviors of Trophoblast via MicroRNA-21. Front. Genet. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grossi, I.; Marchina, E.; De Petro, G.; Salvi, A. The Biological Role and Translational Implications of the Long Non-Coding RNA GAS5 in Breast Cancer. Cancers 2023, 15, 3318. https://doi.org/10.3390/cancers15133318
Grossi I, Marchina E, De Petro G, Salvi A. The Biological Role and Translational Implications of the Long Non-Coding RNA GAS5 in Breast Cancer. Cancers. 2023; 15(13):3318. https://doi.org/10.3390/cancers15133318
Chicago/Turabian StyleGrossi, Ilaria, Eleonora Marchina, Giuseppina De Petro, and Alessandro Salvi. 2023. "The Biological Role and Translational Implications of the Long Non-Coding RNA GAS5 in Breast Cancer" Cancers 15, no. 13: 3318. https://doi.org/10.3390/cancers15133318






