Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility
2.1.1. Eligibility
2.1.2. Study Identification
2.2. Data Collection
2.3. Data Analysis
2.4. Methodologic Quality and Bias Assessment
3. Results
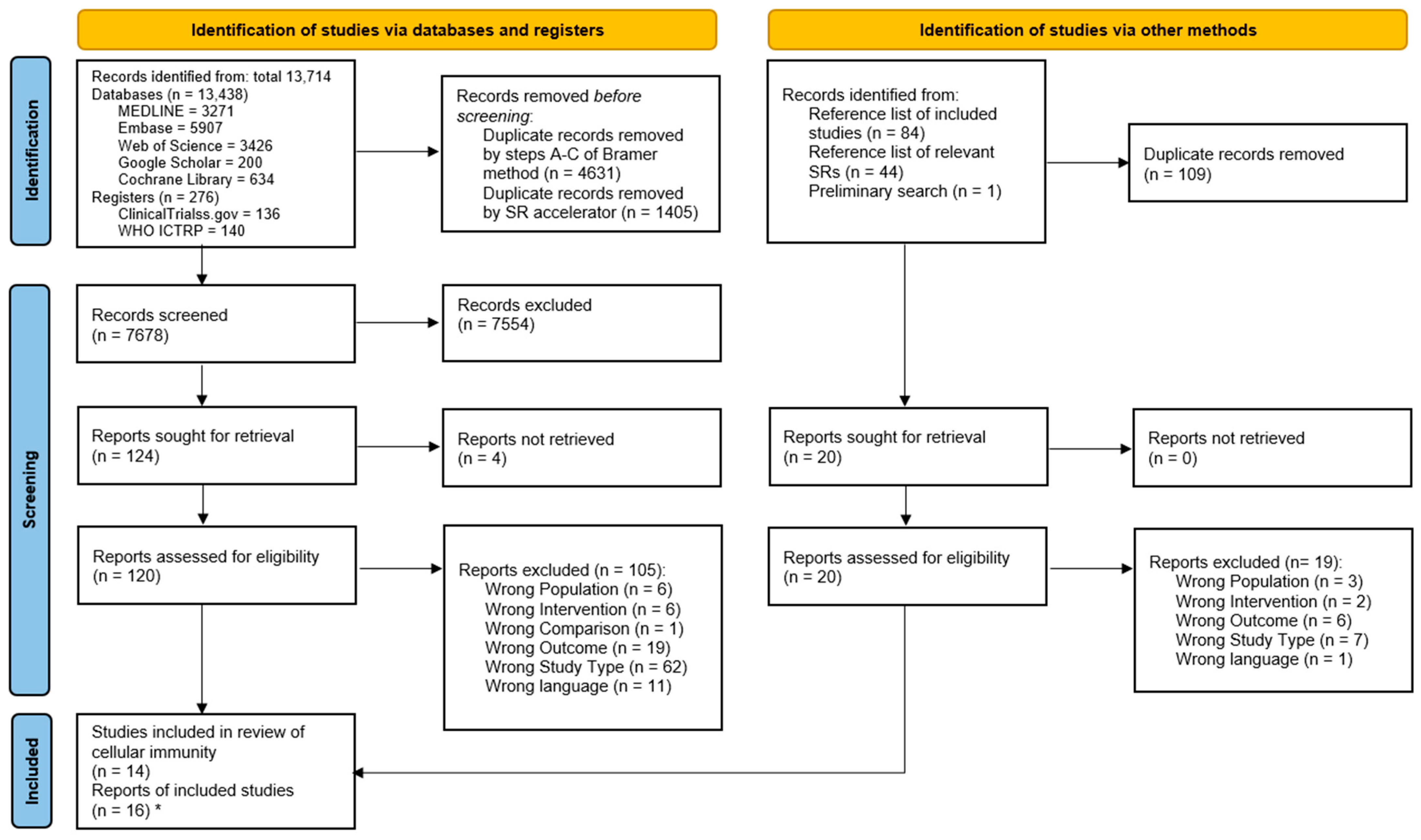
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Results of Analyses
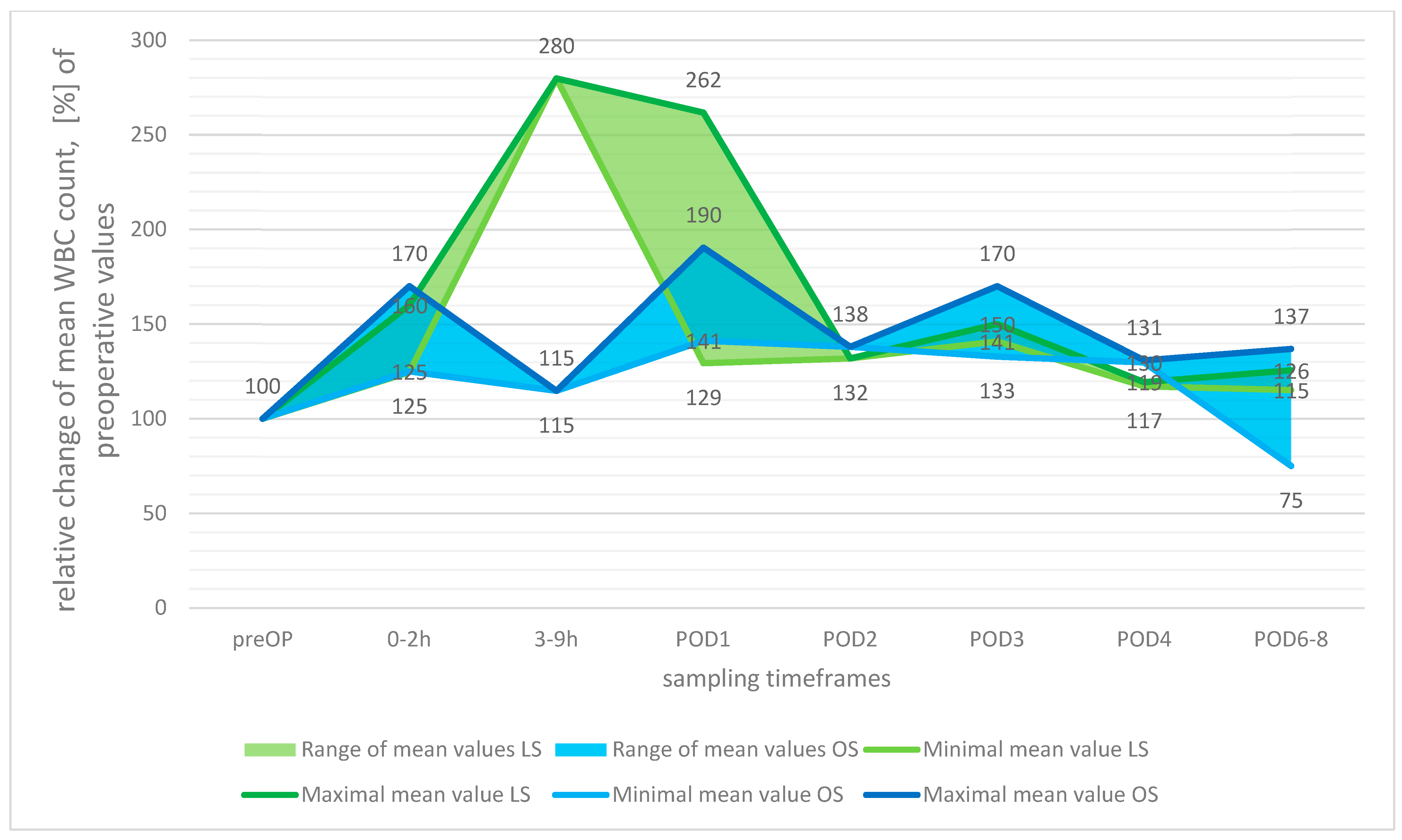
3.3.1. White Blood Cell Count
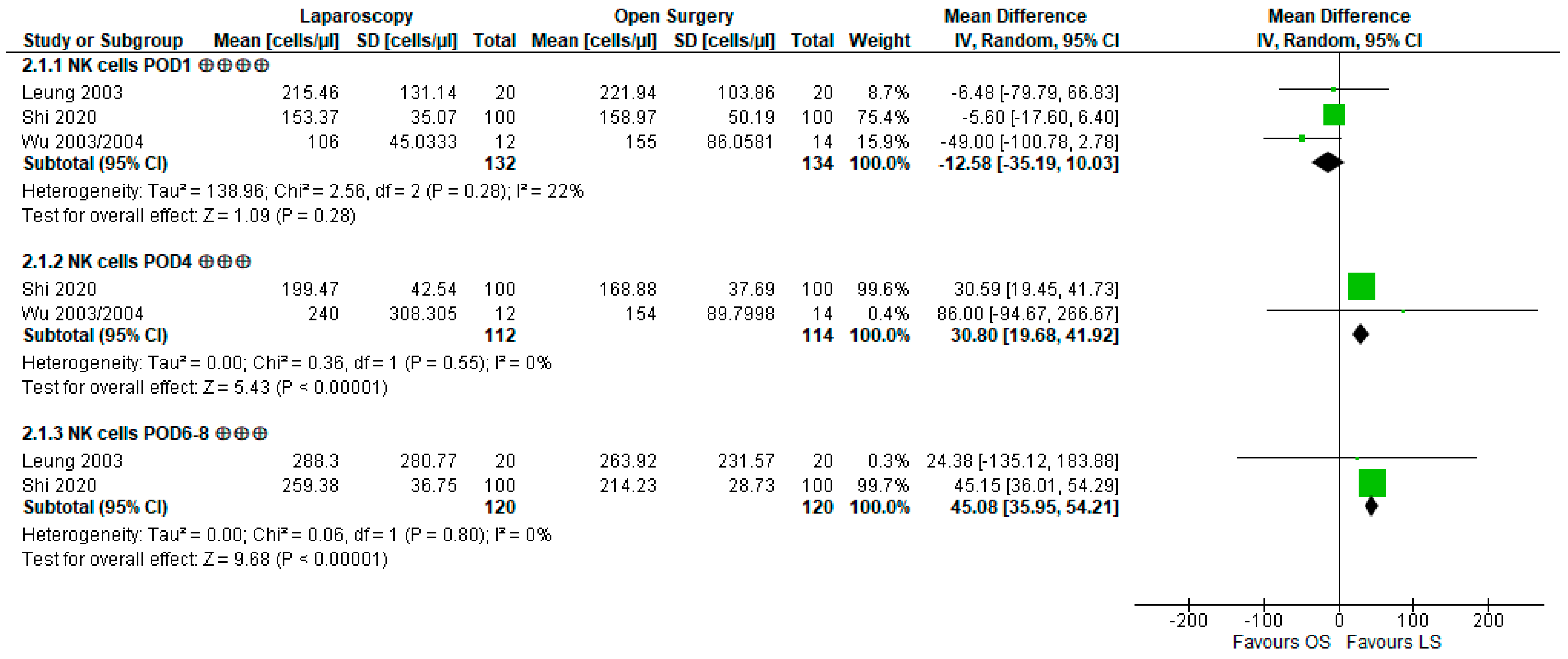
3.3.2. Natural Killer Cell Count and Lytic Activity
3.3.3. Lymphocytes and Subsets
3.3.4. CD4+/CD8+ T Lymphocyte Ratio
3.3.5. Monocytes and HLA-DR II Expression
3.4. Methodologic Quality of Included Studies
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhong, X.; Cao, Z.; Song, J.; Liu, Y.; Guo, Q. Relationship of Platelet Glycoprotein IIb/IIIa and CD62P With Hypercoagulable State After Oncologic Surgery. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620977906. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Tai, L.-H.; Falls, T.; de Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann. Surg. 2013, 258, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Thorson, C.M.; van Haren, R.M.; Ryan, M.L.; Curia, E.; Sleeman, D.; Levi, J.U.; Livingstone, A.S.; Proctor, K.G. Persistence of hypercoagulable state after resection of intra-abdominal malignancies. J. Am. Coll. Surg. 2013, 216, 580–589; Discussion 589–590. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C. The perioperative immune/inflammatory insult in cancer surgery: Time for intervention? Oncoimmunology 2013, 2, e27324. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wang, Z.; Jiang, B.; Di, J.; Su, X. Monitoring Pre- and Post-Operative Immune Alterations in Patients With Locoregional Colorectal Cancer Who Underwent Laparoscopy by Single-Cell Mass Cytometry. Front. Immunol. 2022, 13, 807539. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.V.; Peter, M.B.; Shenoy, H.G.; Horgan, K.; Hughes, T.A. Surgery induced immunosuppression. Surgeon 2011, 9, 38–43. [Google Scholar] [CrossRef]
- Shibutani, M.; Nakao, S.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Iseki, Y.; Hirakawa, K.; Ohira, M. Inflammation Caused by Surgical Stress Has a Negative Impact on the Long-term Survival Outcomes in Patients With Colorectal Cancer. Anticancer Res. 2020, 40, 3535–3542. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Choi, G.-S.; Park, J.S.; Kim, H.J.; Ryuk, J.-P.; Choi, W.-H. Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J. Korean Surg. Soc. 2012, 82, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Kim, R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Zhang, H.; Hamad, A.; Huang, H.; Tsung, A. Surgery-mediated tumor-promoting effects on the immune microenvironment. Semin. Cancer Biol. 2022, 86, 408–419. [Google Scholar] [CrossRef]
- DKG Deutsche Krebsgesellschaft. S3-Leitlinie Kolorektales Karzinom 2019; Leitlinienprogramm Onkologie: Berlin, Germany, 2019. [Google Scholar]
- WCRF International. Worldwide Cancer Data|World Cancer Research Fund International. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 7 March 2023).
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Kulkarni, N.; Arulampalam, T. Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: A meta-analysis. Tech. Coloproctol. 2020, 24, 1017–1024. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Nistala, K.R.Y.; Chong, C.S. Open versus laparoscopic Hartmann’s procedure: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 2421–2430. [Google Scholar] [CrossRef]
- Li, Y.S.; Meng, F.C.; Lin, J.K. Procedural and post-operative complications associated with laparoscopic versus open abdominal surgery for right-sided colonic cancer resection: A systematic review and meta-analysis. Medicine 2020, 99, e22431. [Google Scholar] [CrossRef]
- Dehlaghi Jadid, K.; Cao, Y.; Petersson, J.; Angenete, E.; Matthiessen, P. Long term oncological outcomes for laparoscopic versus open surgery for rectal cancer—A population-based nationwide noninferiority study. Color. Dis. 2022, 24, 1308–1317. [Google Scholar] [CrossRef]
- Kastner, C.; Reibetanz, J.; Germer, C.-T.; Wiegering, A. Evidenz in der minimal-invasiven onkologischen Chirurgie des Kolons und des Rektums. Chirurg 2021, 92, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, V.; Gerken, M.; Benz, S.; Völkel, V.; Draeger, T.; Fürst, A.; Klinkhammer-Schalke, M. Laparoscopic and open surgery in rectal cancer patients in Germany: Short and long-term results of a large 10-year population-based cohort. Surg. Endosc. 2020, 34, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, K.; Watanabe, J.; Suwa, Y.; Nakagawa, K.; Suwa, H.; Ozawa, M.; Ishibe, A.; Ota, M.; Kunisaki, C.; Endo, I. A multicenter, propensity score-matched cohort study about short-term and long-term outcomes after laparoscopic versus open surgery for locally advanced rectal cancer. Int. J. Color. Dis. 2021, 36, 1287–1295. [Google Scholar] [CrossRef]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Karantanos, T.; Theodoropoulos, G.E. Immune response after laparoscopic colectomy for cancer: A review. Gastroenterol. Rep. 2013, 1, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, J.; Zhang, S. Laparoscopic versus conventional open surgery for immune function in patients with colorectal cancer. Int. J. Color. Dis. 2011, 26, 1375–1385. [Google Scholar] [CrossRef]
- Gögenur, M.; Watt, S.K.; Gögenur, I. Bedre immunfunktion efter laparoskopisk end efter åben kolorektal cancer-kirurgi. Ugeskr. Laeger 2015, 177, V12140763. [Google Scholar]
- Sammour, T.; Kahokehr, A.; Chan, S.; Booth, R.J.; Hill, A.G. The humoral response after laparoscopic versus open colorectal surgery: A meta-analysis. J. Surg. Res. 2010, 164, 28–37. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Fürst, A.; Völkel, V.; Bohne, A.; Grundler, E.; Knüttel, H. Draft Search Strategy for: Influence of Laparoscopic Versus Open Surgical Resections on the Immune System in Patients with Colorectal Cancer: Protocol for a Systematic Review and Meta-analysis. Univ. Regensburg Publ. Server. 2021. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Bohne, A.; Grundler, E.; Knüttel, H.; Fürst, A.; Völkel, V. Data Archive of: Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis. Univ. Regensburg Publ. Server. 2023. [Google Scholar] [CrossRef]
- Bohne, A.; Grundler, E.; Knüttel, H.; Fürst, A.; Völkel, V. Data Archive (Search Documentation) of: Impact of Laparoscopic Versus Open Surgery on Immunity in Patients with Colorectal Cancer: A Systematic Review and Meta-Analysis. Univ. Regensburg Publ. Server. 2023. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A full systematic review was completed in 2 weeks using automation tools: A case study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 (updated February 2021); Higgins, J., Thomas, J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer, Version 4.6; WebPlotDigitizer: Pacifica, CA, USA, 2022. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- The Cochrane Collaboration. Review Manager; The Cochrane Collaboration: London, UK, 2020. [Google Scholar]
- Higgins, J.; Savovic, J. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2), Template for Completion, Version of 22 August 2019; Higgins, J.P.T., Savović, J., Page, M.J., Sterne, J.A.C., on Behalf of the RoB2 Development Group, Eds.; The Cochrane Collaboration: London, UK, 2019; Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 14 December 2022).
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.3; Higgins, J.P.T., Thomas, J., Chan-dler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- McMaster University and Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2022. [Google Scholar]
- Tang, C.L.; Eu, K.W.; Tai, B.C.; Soh, J.G.; MacHin, D.; Seow-Choen, F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br. J. Surg. 2001, 88, 801–807. [Google Scholar] [CrossRef]
- Laforgia, R.; D’Elia, G.; Lattarulo, S. Cellular and humoral inflammatory response after laparoscopic and conven-tional colorectal surgery: Preliminary report. Ann. Ital. Chir. 2016, 87, 337–342. [Google Scholar] [PubMed]
- Duque, P.; Garutti, I.; Terradillos, E. Modulation of CCL2 Expression by Laparoscopic Versus Open Surgery for Colorectal Cancer Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2019, 29, 101–108. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kabeshima, Y.; Watanabe, M.; Yamamoto, S.; Kitajima, M. Randomized controlled trial of laparoscopic versus open colectomy for advanced colorectal cancer. Surg. Endosc. 2003, 17, 636–640. [Google Scholar] [CrossRef]
- Hewitt, P.M.; Ip, S.M.; Kwok, S.P.; Somers, S.S.; Li, K.; Leung, K.L.; Lau, W.Y.; Li, A.K. Laparoscopic-assisted vs. open surgery for colorectal cancer: Comparative study of immune effects. Dis. Colon Rectum 1998, 41, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Kvarnström, A.; Sokolov, A.; Swartling, T.; Kurlberg, G.; Mollnes, T.E.; Bengtsson, A. Alternative pathway activation of complement in laparoscopic and open rectal surgery. Scand. J. Immunol. 2012, 76, 49–53. [Google Scholar] [CrossRef]
- Kvarnström, A.; Swartling, T.; Kurlberg, G.; Bengtson, J.-P.; Bengtsson, A. Pro-inflammatory cytokine release in rectal surgery: Comparison between laparoscopic and open surgical techniques. Arch. Immunol. Ther. Exp. 2013, 61, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.L.; Tsang, K.S.; Ng, M.H.L.; Leung, K.J.; Lai, P.B.S.; Lee, J.F.Y.; Lau, W.Y. Lymphocyte subsets and natural killer cell cytotoxicity after laparoscopically assisted resection of rectosigmoid carcinoma. Surg. Endosc. 2003, 17, 1305–1310. [Google Scholar] [CrossRef]
- Ordemann, J.; Jacobi, C.A.; Schwenk, W.; Stösslein, R.; Müller, J.M. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg. Endosc. 2001, 15, 600–608. [Google Scholar] [CrossRef]
- Shi, L.; Guo, H.; Zheng, Z.; Liu, J.; Jiang, Y.; Su, Y. Laparoscopic Surgery Versus Open Surgery for Colorectal Cancer: Impacts on Natural Killer Cells. Cancer Control 2020, 27, 1073274820906811. [Google Scholar] [CrossRef]
- Veenhof, A.A.F.A.; Sietses, C.; von Blomberg, B.M.E.; van Hoogstraten, I.M.W.; vd Pas, M.H.G.M.; Meijerink, W.J.H.J.; vd Peet, D.L.; vd Tol, M.P.; Bonjer, H.J.; Cuesta, M.A. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: A randomized trial. Int. J. Color. Dis. 2011, 26, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Veenhof, A.A.F.A.; Vlug, M.S.; van der Pas, M.H.G.M.; Sietses, C.; van der Peet, D.L.; de Lange-de Klerk, E.S.M.; Bonjer, H.J.; Bemelman, W.A.; Cuesta, M.A. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: A randomized trial. Ann. Surg. 2012, 255, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Jiang, Z.; Zhao, K.; Li, G.; Liu, F.; Pan, H.; Li, J. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J. Gastrointest. Surg. 2012, 16, 1379–1388. [Google Scholar] [CrossRef]
- Wu, F.P.K.; Sietses, C.; von Blomberg, B.M.E.; van Leeuwen, P.A.M.; Meijer, S.; Cuesta, M.A. Systemic and peritoneal inflammatory response after laparoscopic or conventional colon resection in cancer patients: A prospective, randomized trial. Dis. Colon Rectum 2003, 46, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.P.K.; Hoekman, K.; Sietses, C.; von Blomberg, B.M.E.; Meijer, S.; Bonjer, H.J.; Cuesta, M.A. Systemic and peritoneal angiogenic response after laparoscopic or conventional colon resection in cancer patients: A prospective, randomized trial. Dis. Colon Rectum 2004, 47, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, J.; Song, Y.; Zhou, J.; Sun, F.; Wang, J.; Duan, Y.; Hu, Y.; Liu, Y.; Wang, X.; et al. Laparoscopic surgery contributes more to nutritional and immunologic recovery than fast-track care in colorectal cancer. World J. Surg. Oncol. 2015, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Watt, D.G.; Horgan, P.G.; McMillan, D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: A systematic review. Surgery 2015, 157, 362–380. [Google Scholar] [CrossRef]
- Market, M.; Tennakoon, G.; Auer, R.C. Postoperative Natural Killer Cell Dysfunction: The Prime Suspect in the Case of Metastasis Following Curative Cancer Surgery. Int. J. Mol. Sci. 2021, 22, 1378. [Google Scholar] [CrossRef]
- Heffernan, D.S.; Monaghan, S.F.; Thakkar, R.K.; Machan, J.T.; Cioffi, W.G.; Ayala, A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit. Care 2012, 16, R12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Huang, R.; Jiang, T.; Huang, K.; Cao, J.; Qiu, Z. Laparoscopic and open resection for colorectal cancer: An evaluation of cellular immunity. BMC Gastroenterol 2010, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Sadahiro, R.; Knight, B.; James, F.; Hannon, E.; Charity, J.; Daniels, I.R.; Burrage, J.; Knox, O.; Crawford, B.; Smart, N.J.; et al. Major surgery induces acute changes in measured DNA methylation associated with immune response pathways. Sci. Rep. 2020, 10, 5743. [Google Scholar] [CrossRef] [Green Version]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef] [Green Version]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, aan3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Bartal, I.; Melamed, R.; Greenfeld, K.; Atzil, S.; Glasner, A.; Domankevich, V.; Naor, R.; Beilin, B.; Yardeni, I.Z.; Ben-Eliyahu, S. Immune perturbations in patients along the perioperative period: Alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav. Immun. 2010, 24, 376–386. [Google Scholar] [CrossRef]
- Dąbrowska, A.M.; Słotwiński, R. The immune response to surgery and infection. Cent. Eur. J. Immunol. 2014, 39, 532–537. [Google Scholar] [CrossRef]
- Tai, L.-H.; de Souza, C.T.; Bélanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Pahl, J.; Cerwenka, A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 2017, 222, 11–20. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, M.; Li, K.; Li, J.; Cai, Z.; Hu, B. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol 2020, 20, 31. [Google Scholar] [CrossRef] [Green Version]
- Welsch, T.; Müller, S.A.; Ulrich, A.; Kischlat, A.; Hinz, U.; Kienle, P.; Büchler, M.W.; Schmidt, J.; Schmied, B.M. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int. J. Color. Dis. 2007, 22, 1499–1507. [Google Scholar] [CrossRef]
- Su’a, B.U.; Mikaere, H.L.; Rahiri, J.L.; Bissett, I.B.; Hill, A.G. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br. J. Surg. 2017, 104, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Paik, K.Y.; Lee, I.K.; Lee, Y.S.; Sung, N.Y.; Kwon, T.S. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res. Treat. 2014, 46, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Lee, C.S.; Han, S.R.; Park, S.M.; Lee, Y.S.; Lee, I.K. Differences in the prognostic impact of post-operative systemic inflammation and infection in colorectal cancer patients: Using white blood cell counts and procalcitonin levels. Surg. Oncol. 2020, 35, 374–381. [Google Scholar] [CrossRef]
- Griffith, B.D.; Turcotte, S.; Lazarus, J.; Lima, F.; Bell, S.; Delrosario, L.; McGue, J.; Krishnan, S.; Oneka, M.D.; Nathan, H.; et al. MHC Class II Expression Influences the Composition and Distribution of Immune Cells in the Metastatic Colorectal Cancer Microenvironment. Cancers 2022, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, E.; Enchev, E.; Minkov, G.; Halacheva, K.; Yovtchev, Y. Poor Outcome Could Be Predicted by Lower Monocyte Human Leukocyte Antigen-DR Expression in Patients with Complicated Intra-Abdominal Infections: A Review. Surg. Infect. 2020, 21, 77–80. [Google Scholar] [CrossRef]
- Egen, J.G.; Ouyang, W.; Wu, L.C. Human Anti-tumor Immunity: Insights from Immunotherapy Clinical Trials. Immunity 2020, 52, 36–54. [Google Scholar] [CrossRef]
- Zbar, A.P.; Guillou, P.J.; Bland, K.; Syrigos, K.N. Immunology for Surgeons; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-1-85233-482-6. [Google Scholar]
- Dang, Y.; Shi, X.; Xu, W.; Zuo, M. The Effect of Anesthesia on the Immune System in Colorectal Cancer Patients. J. Gastroenterol. Hepatol. 2018, 2018, 7940603. [Google Scholar] [CrossRef] [Green Version]
- Delogu, G.; Moretti, S.; Antonucci, A.; Marcellini, S.; Masciangelo, R.; Famularo, G.; Signore, L.; de Simone, C. Apoptosis and surgical trauma: Dysregulated expression of death and survival factors on peripheral lymphocytes. Arch. Surg. 2000, 135, 1141–1147. [Google Scholar] [CrossRef]
- Santiago-Sánchez, G.S.; Hodge, J.W.; Fabian, K.P. Tipping the scales: Immunotherapeutic strategies that disrupt immunosuppression and promote immune activation. Front. Immunol. 2022, 13, 993624. [Google Scholar] [CrossRef] [PubMed]
- Milašienė, V.; Stratilatovas, E.; Norkienė, V. The importance of T-lymphocyte subsets on overall survival of colorectal and gastric cancer patients. Medicina 2007, 43, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, Y.; Chen, N.; Wu, W.; Wang, Z.; Lin, F.; Guo, C.; Liu, C.; Li, W.; Liu, L. High postoperative monocyte indicates inferior Clinicopathological characteristics and worse prognosis in lung adenocarcinoma or squamous cell carcinoma after lobectomy. BMC Cancer 2018, 18, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, T.C. Minimizing the Three Stages of Publication Bias. JAMA 1990, 263, 1392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xiao, G.; Huang, M.; Long, H. Effect of laparoscopic radical operation on systemic immunity in patients with colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2005, 8, 407–409. [Google Scholar] [PubMed]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [Green Version]
- Matzner, P.; Sorski, L.; Haldar, R.; Shaashua, L.; Benbenishty, A.; Lavon, H.; Azan, Y.; Sandbank, E.; Melamed, R.; Rosenne, E.; et al. Deleterious synergistic effects of distress and surgery on cancer metastasis: Abolishment through an integrated perioperative immune-stimulating stress-inflammatory-reducing intervention. Brain Behav. Immun. 2019, 80, 170–178. [Google Scholar] [CrossRef]


| First Author | Year of Publication | n | LS | OS | Inclusion of Stage IV | Colonic and/or Rectal Resections | Risk of Bias | Outcomes Evaluated |
|---|---|---|---|---|---|---|---|---|
| Duque [50] | 2019 | 37 | 18 | 19 | n.a. | Colorectal | Some concerns | WBC, Lym |
| Hasegawa [51] | 2003 | 50 | 24 | 26 | No | Colorectal | Some concerns | WBC, NKC Akt |
| Hewitt [52] | 1998 | 16 | 8 | 8 | No | Colorectal | Some concerns | CD4/8, HLA-DR II |
| Kvarnström [53,54] | 2012/2013 | 24 | 12 | 12 | n.a. | Rectal | High | WBC |
| Laforgia [49] | 2016 | 14 | 7 | 7 | Yes | Colorectal | High | CD4/8, WBC |
| Leung [55] | 2003 | 40 | 20 | 20 | No | Colorectal | Some concerns | NKC, NKC Akt, WBC, Lym, CD3+, CD4+, CD8+, BC |
| Ordemann [56] | 2001 | 40 | 20 | 20 | No | Colorectal | Low | CD4/8, WBC, HLA-DR II |
| Shi [57] | 2020 | 200 | 100 | 100 | No | Colorectal | Low | NKC, NKC Act |
| Tang [48] | 2001 | 161 | 80 | 81 | Yes | Colorectal | Some concerns | CD4/8, NKC |
| Veenhof [58] | 2011 | 40 | 22 | 18 | Yes | Rectal | Some concerns | WBC, HLA-DR II, Mono |
| Veenhof [59] | 2012 | 79 | 42 | 37 | n.a. | Colonic | Some concerns | HLA-DR II |
| Wang [60] | 2012 | 163 | 80 | 83 | No | Colonic | Low | CD4/8, CD3+, CD4+, CD8+ |
| Wu [61,62] | 2003/2004 | 26 | 12 | 14 | No | Colonic | Low | CD4/8, NKC, WBC, Lym, CD4+, CD8+, HLA-DR II, BC, Mono |
| Xu [63] | 2015 | 84 | 43 | 41 | No | Colorectal | Low | NKC, CD3+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohne, A.; Grundler, E.; Knüttel, H.; Fürst, A.; Völkel, V. Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3381. https://doi.org/10.3390/cancers15133381
Bohne A, Grundler E, Knüttel H, Fürst A, Völkel V. Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(13):3381. https://doi.org/10.3390/cancers15133381
Chicago/Turabian StyleBohne, Annika, Elena Grundler, Helge Knüttel, Alois Fürst, and Vinzenz Völkel. 2023. "Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 13: 3381. https://doi.org/10.3390/cancers15133381
APA StyleBohne, A., Grundler, E., Knüttel, H., Fürst, A., & Völkel, V. (2023). Influence of Laparoscopic Surgery on Cellular Immunity in Colorectal Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(13), 3381. https://doi.org/10.3390/cancers15133381







