Novel Multiparametric Magnetic Resonance Imaging-Based Deep Learning and Clinical Parameter Integration for the Prediction of Long-Term Biochemical Recurrence-Free Survival in Prostate Cancer after Radical Prostatectomy
Abstract
:Simple Summary
Abstract
1. Introduction
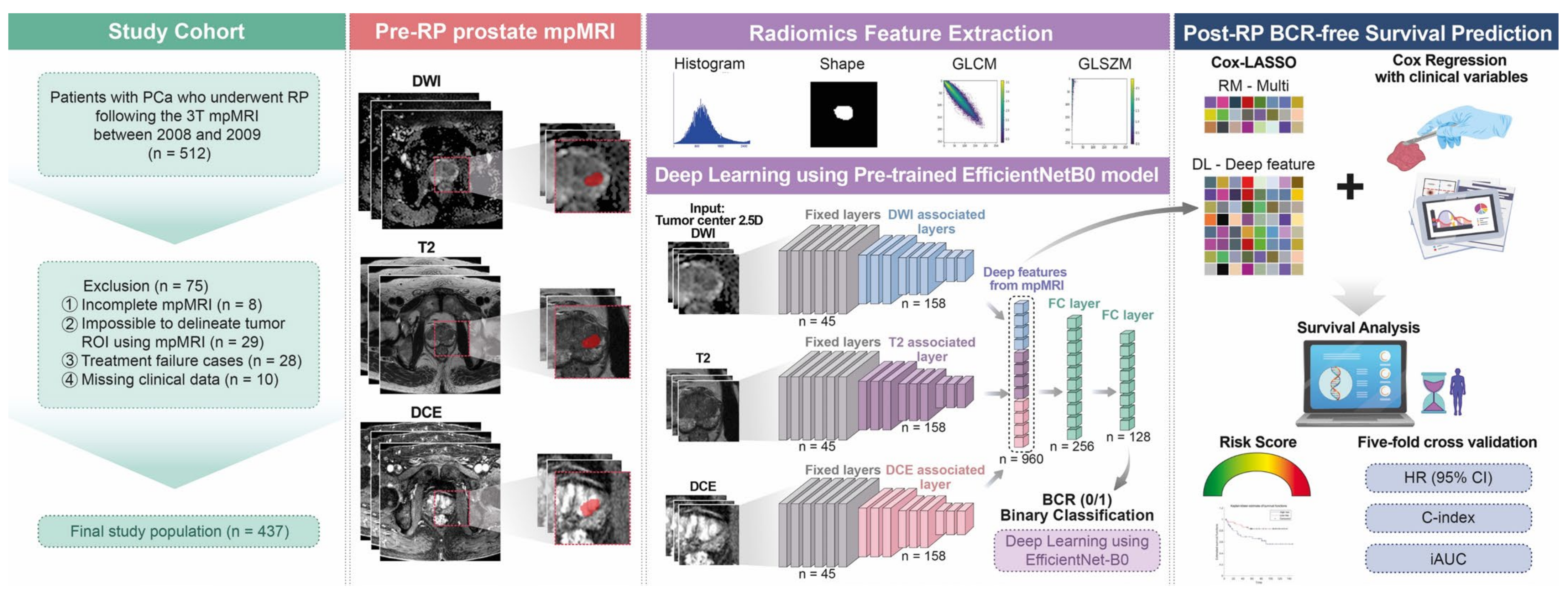
2. Materials and Methods
2.1. Patient Selection
2.2. mpMRI Acquisition Protocol and Interpretation
2.3. Clinical Variable-Based Risk Model Construction
2.4. Tumor ROI Delineation, Radiomics Feature Extraction, Selection, and Risk Model Generation
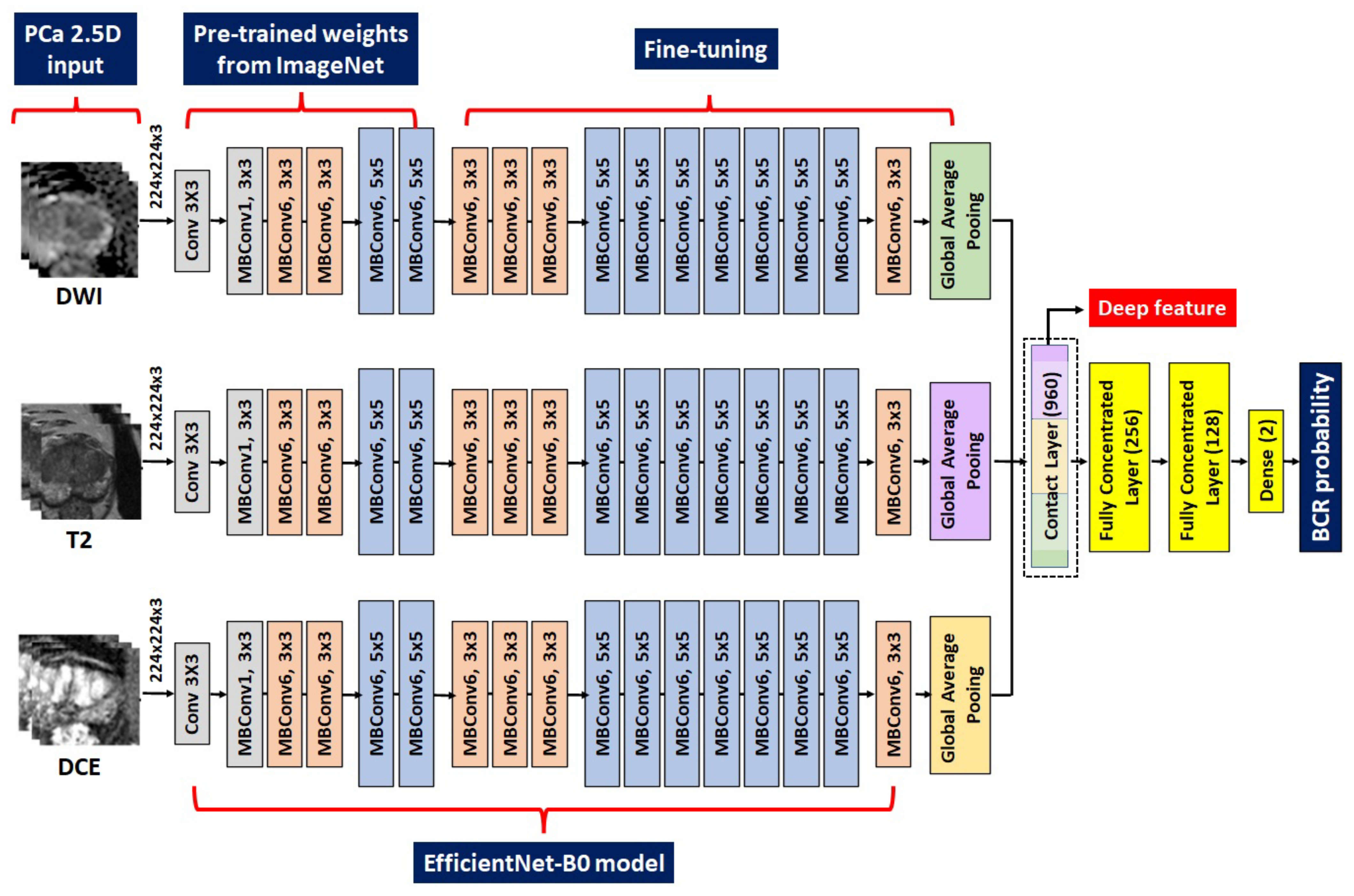
2.5. DL Procedures and Construction of the Related Risk Models
2.6. Statistical Analysis
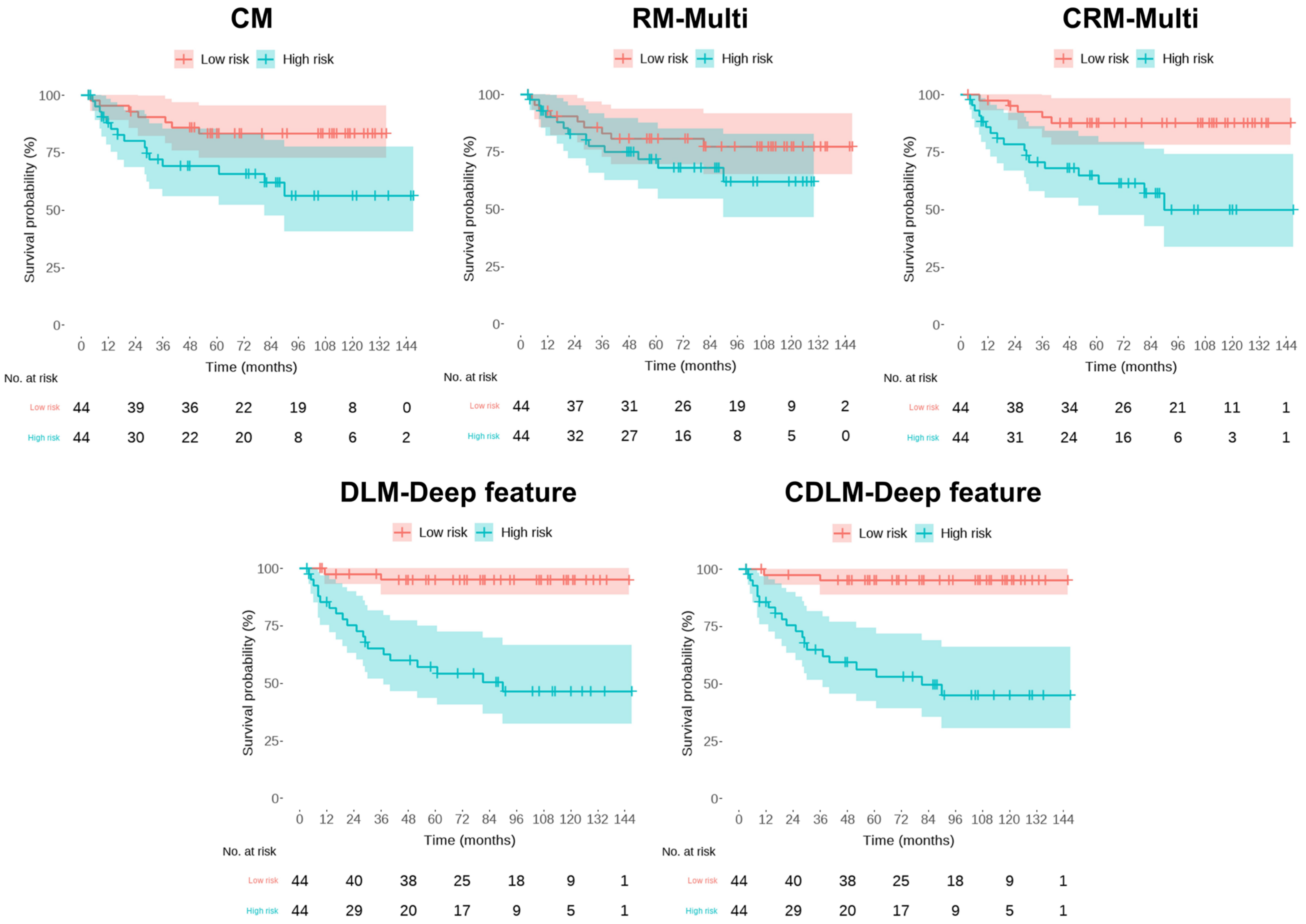
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Weiner, A.B.; Siebert, A.L.; Fenton, S.E.; Abida, W.; Agarwal, N.; Davis, I.D.; Dorff, T.B.; Gleave, M.; James, N.D.; Poon, D.M.C.; et al. First-line Systemic Treatment of Recurrent Prostate Cancer after Primary or Salvage Local Therapy: A Systematic Review of the Literature. Eur. Urol. Oncol. 2022, 5, 377–387. [Google Scholar] [CrossRef]
- Park, J.S.; Koo, K.C.; Choi, I.Y.; Lee, J.Y.; Hong, J.H.; Kim, C.S.; Lee, H.M.; Hong, S.K.; Byun, S.S.; Rha, K.H.; et al. Stratification based on adverse laboratory/pathological features for predicting overall survival in patients undergoing radical prostatectomy: A K-CaP registry-based analysis. Medicine 2019, 98, e17931. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Vartolomei, M.D.; Lucarelli, G.; Crocetto, F.; Barone, B.; Sciarra, A.; Del Giudice, F.; Muto, M.; Maggi, M.; et al. Prostate Cancer Radiogenomics—From Imaging to Molecular Characterization. Int. J. Mol. Sci. 2021, 22, 9971. [Google Scholar] [CrossRef]
- Shaikh, F.; Dupont-Roettger, D.; Dehmeshki, J.; Kubassova, O.; Quraishi, M.I. Advanced Imaging of Biochemical Recurrent Prostate Cancer with PET, MRI, and Radiomics. Front. Oncol. 2020, 10, 1359. [Google Scholar] [CrossRef]
- Santoro, A.A.; Di Gianfrancesco, L.; Racioppi, M.; Pinto, F.; Palermo, G.; Sacco, E.; Campetella, M.; Scarciglia, E.; Bientinesi, R.; Di Paola, V.; et al. Multiparametric magnetic resonance imaging of the prostate: Lights and shadows. Urologia 2021, 88, 280–286. [Google Scholar] [CrossRef]
- Chaddad, A.; Kucharczyk, M.J.; Cheddad, A.; Clarke, S.E.; Hassan, L.; Ding, S.; Rathore, S.; Zhang, M.; Katib, Y.; Bahoric, B.; et al. Magnetic Resonance Imaging Based Radiomic Models of Prostate Cancer: A Narrative Review. Cancers 2021, 13, 552. [Google Scholar] [CrossRef]
- Iacob, R.; Stoicescu, E.R.; Cerbu, S.; Manolescu, D.L.; Bardan, R.; Cumpanas, A. Could Biparametric MRI Replace Multiparametric MRI in the Management of Prostate Cancer? Life 2023, 13, 465. [Google Scholar] [CrossRef]
- Nematollahi, H.; Moslehi, M.; Aminolroayaei, F.; Maleki, M.; Shahbazi-Gahrouei, D. Diagnostic Performance Evaluation of Multiparametric Magnetic Resonance Imaging in the Detection of Prostate Cancer with Supervised Machine Learning Methods. Diagnostics 2023, 13, 806. [Google Scholar] [CrossRef]
- Rajwa, P.; Mori, K.; Huebner, N.A.; Martin, D.T.; Sprenkle, P.C.; Weinreb, J.C.; Ploussard, G.; Pradere, B.; Shariat, S.F.; Leapman, M.S. The Prognostic Association of Prostate MRI PI-RADS v2 Assessment Category and Risk of Biochemical Recurrence after Definitive Local Therapy for Prostate Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2021, 206, 507–516. [Google Scholar] [CrossRef]
- Telecan, T.; Andras, I.; Crisan, N.; Giurgiu, L.; Cata, E.D.; Caraiani, C.; Lebovici, A.; Boca, B.; Balint, Z.; Diosan, L.; et al. More than Meets the Eye: Using Textural Analysis and Artificial Intelligence as Decision Support Tools in Prostate Cancer Diagnosis-A Systematic Review. J. Pers. Med. 2022, 12, 983. [Google Scholar] [CrossRef]
- Midiri, F.; Vernuccio, F.; Purpura, P.; Alongi, P.; Bartolotta, T.V. Multiparametric MRI and Radiomics in Prostate Cancer: A Review of the Current Literature. Diagnostics 2021, 11, 1829. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Musi, G.; Del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in prostate cancer: An up-to-date review. Ther. Adv. Urol. 2022, 14, 17562872221109020. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Bettermann, A.S.; Bamberg, F.; Benndorf, M.; Mix, M.; Nicolay, N.H.; Fechter, T.; Holscher, T.; Grosu, R.; Chiti, A.; et al. Radiomics in prostate cancer imaging for a personalized treatment approach—Current aspects of methodology and a systematic review on validated studies. Theranostics 2021, 11, 8027–8042. [Google Scholar] [CrossRef]
- Cho, H.H.; Kim, C.K.; Park, H. Overview of radiomics in prostate imaging and future directions. Br. J. Radiol. 2022, 95, 20210539. [Google Scholar] [CrossRef]
- Cutaia, G.; La Tona, G.; Comelli, A.; Vernuccio, F.; Agnello, F.; Gagliardo, C.; Salvaggio, L.; Quartuccio, N.; Sturiale, L.; Stefano, A.; et al. Radiomics and Prostate MRI: Current Role and Future Applications. J. Imaging 2021, 7, 34. [Google Scholar] [CrossRef]
- Bourbonne, V.; Fournier, G.; Vallieres, M.; Lucia, F.; Doucet, L.; Tissot, V.; Cuvelier, G.; Hue, S.; Le Penn Du, H.; Perdriel, L.; et al. External Validation of an MRI-Derived Radiomics Model to Predict Biochemical Recurrence after Surgery for High-Risk Prostate Cancer. Cancers 2020, 12, 814. [Google Scholar] [CrossRef] [Green Version]
- Kendrick, J.; Francis, R.; Hassan, G.M.; Rowshanfarzad, P.; Jeraj, R.; Kasisi, C.; Rusanov, B.; Ebert, M. Radiomics for Identification and Prediction in Metastatic Prostate Cancer: A Review of Studies. Front. Oncol. 2021, 11, 771787. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, E.; Mercatelli, L.; Marzi, C.; Pachetti, E.; Baccini, M.; Barucci, A.; Colantonio, S.; Gherardini, L.; Lattavo, L.; Pascali, M.A.; et al. Machine and Deep Learning Prediction of Prostate Cancer Aggressiveness Using Multiparametric MRI. Front. Oncol. 2021, 11, 802964. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Laudicella, R.; Balma, M.; Nicolotti, D.G.; Buschiazzo, A.; Grimaldi, S.; Lorenzon, L.; Bianchi, A.; Peano, S.; Bartolotta, T.V.; et al. Radiomics and artificial intelligence in prostate cancer: New tools for molecular hybrid imaging and theragnostics. Eur. Radiol. Exp. 2022, 6, 27. [Google Scholar] [CrossRef]
- Michaely, H.J.; Aringhieri, G.; Cioni, D.; Neri, E. Current Value of Biparametric Prostate MRI with Machine-Learning or Deep-Learning in the Detection, Grading, and Characterization of Prostate Cancer: A Systematic Review. Diagnostics 2022, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Belue, M.J.; Turkbey, B. Tasks for artificial intelligence in prostate MRI. Eur. Radiol. Exp. 2022, 6, 33. [Google Scholar] [CrossRef]
- Twilt, J.J.; van Leeuwen, K.G.; Huisman, H.J.; Futterer, J.J.; de Rooij, M. Artificial Intelligence Based Algorithms for Prostate Cancer Classification and Detection on Magnetic Resonance Imaging: A Narrative Review. Diagnostics 2021, 11, 959. [Google Scholar] [CrossRef]
- Naik, N.; Tokas, T.; Shetty, D.K.; Hameed, B.M.Z.; Shastri, S.; Shah, M.J.; Ibrahim, S.; Rai, B.P.; Chlosta, P.; Somani, B.K. Role of Deep Learning in Prostate Cancer Management: Past, Present and Future Based on a Comprehensive Literature Review. J. Clin. Med. 2022, 11, 3575. [Google Scholar] [CrossRef]
- Alhasan, A.S. Clinical Applications of Artificial Intelligence, Machine Learning, and Deep Learning in the Imaging of Gliomas: A Systematic Review. Cureus 2021, 13, e19580. [Google Scholar] [CrossRef]
- Erickson, B.J. Basic Artificial Intelligence Techniques: Machine Learning and Deep Learning. Radiol. Clin. 2021, 59, 933–940. [Google Scholar] [CrossRef]
- Checcucci, E.; Autorino, R.; Cacciamani, G.E.; Amparore, D.; De Cillis, S.; Piana, A.; Piazzolla, P.; Vezzetti, E.; Fiori, C.; Veneziano, D.; et al. Artificial intelligence and neural networks in urology: Current clinical applications. Minerva Urol. Nefrol. 2020, 72, 49–57. [Google Scholar] [CrossRef]
- Montagnon, E.; Cerny, M.; Cadrin-Chenevert, A.; Hamilton, V.; Derennes, T.; Ilinca, A.; Vandenbroucke-Menu, F.; Turcotte, S.; Kadoury, S.; Tang, A. Deep learning workflow in radiology: A primer. Insights Imaging 2020, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, L.L.; Bellon, P.S.; Del Barrio, A.P.; Fernandez-Miranda, P.M.; Gonzalez, D.R.; Vega, J.A.; Mandly, A.A.G.; Blanco, J.A.P. A primer on deep learning and convolutional neural networks for clinicians. Insights Imaging 2021, 12, 117. [Google Scholar] [CrossRef]
- Shah, U.; Biswas, M.R.; Alzubaidi, M.S.; Ali, H.; Alam, T.; Househ, M.; Shah, Z. Recent Developments in Artificial Intelligence-Based Techniques for Prostate Cancer Detection: A Scoping Review. Stud. Health Technol. Inform. 2022, 289, 268–271. [Google Scholar]
- Yan, Y.; Shao, L.; Liu, Z.; He, W.; Yang, G.; Liu, J.; Xia, H.; Zhang, Y.; Chen, H.; Liu, C.; et al. Deep Learning with Quantitative Features of Magnetic Resonance Images to Predict Biochemical Recurrence of Radical Prostatectomy: A Multi-Center Study. Cancers 2021, 13, 3098. [Google Scholar] [CrossRef]
- Hiremath, A.; Shiradkar, R.; Fu, P.; Mahran, A.; Rastinehad, A.R.; Tewari, A.; Tirumani, S.H.; Purysko, A.; Ponsky, L.; Madabhushi, A. An integrated nomogram combining deep learning, Prostate Imaging-Reporting and Data System (PI-RADS) scoring, and clinical variables for identification of clinically significant prostate cancer on biparametric MRI: A retrospective multicentre study. Lancet Digit. Health 2021, 3, e445–e454. [Google Scholar] [CrossRef]
- Corradini, D.; Brizi, L.; Gaudiano, C.; Bianchi, L.; Marcelli, E.; Golfieri, R.; Schiavina, R.; Testa, C.; Remondini, D. Challenges in the Use of Artificial Intelligence for Prostate Cancer Diagnosis from Multiparametric Imaging Data. Cancers 2021, 13, 3944. [Google Scholar] [CrossRef]
- Simmons, M.N.; Stephenson, A.J.; Klein, E.A. Natural history of biochemical recurrence after radical prostatectomy: Risk assessment for secondary therapy. Eur. Urol. 2007, 51, 1175–1184. [Google Scholar] [CrossRef]
- Sakellakis, M.; Jacqueline Flores, L.; Ramachandran, S. Patterns of indolence in prostate cancer (Review). Exp. Ther. Med. 2022, 23, 351. [Google Scholar] [CrossRef]
- Kang, D.I.; Chung, J.I.; Ha, H.K.; Min, K.; Yoon, J.; Kim, W.; Seo, W.I.; Kang, P.; Jung, S.J.; Kim, I.Y. Korean prostate cancer patients have worse disease characteristics than their American counterparts. Asian Pac. J. Cancer Prev. 2013, 14, 6913–6917. [Google Scholar] [CrossRef] [Green Version]
- Jeong, I.G.; Dajani, D.; Verghese, M.; Hwang, J.; Cho, Y.M.; Hong, J.H.; Kim, C.S.; Ahn, H.; Ro, J.Y. Differences in the aggressiveness of prostate cancer among Korean, Caucasian, and African American men: A retrospective cohort study of radical prostatectomy. Urol. Oncol. 2016, 34, 3.e9–3.e14. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, H.J.; Jeon, S.S.; Kwak, C.; Sung, G.T.; Kwon, T.G.; Park, J.Y.; Paick, S.H. Establishment of Korean prostate cancer database by the Korean Urological Oncology Society. Investig. Clin. Urol. 2017, 58, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Nakai, Y.; Miyake, M.; Anai, S.; Inoue, T.; Fujii, T.; Konishi, N.; Fujimoto, K. Trends in risk classification and primary therapy of Japanese patients with prostate cancer in Nara urological research and treatment group (NURTG)—Comparison between 2004–2006, 2007–2009, and 2010–2012. BMC Cancer 2017, 17, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, W.I.; Kang, P.M.; Kang, D.I.; Yoon, J.H.; Kim, W.; Chung, J.I. Cancer of the Prostate Risk Assessment (CAPRA) Preoperative Score Versus Postoperative Score (CAPRA-S): Ability to predict cancer progression and decision-making regarding adjuvant therapy after radical prostatectomy. J. Korean Med. Sci. 2014, 29, 1212–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.B.; Yang, T.; Hu, J.M.; Zhu, W.H.; Jiang, H.W.; Ding, Q. Prognostic factors in Chinese patients with prostate cancer receiving primary androgen deprivation therapy: Validation of Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score and impacts of pre-existing obesity and diabetes mellitus. Int. J. Clin. Oncol. 2018, 23, 591–598. [Google Scholar] [CrossRef]
- Tilki, D.; Mandel, P.; Schlomm, T.; Chun, F.K.; Tennstedt, P.; Pehrke, D.; Haese, A.; Huland, H.; Graefen, M.; Salomon, G. External validation of the CAPRA-S score to predict biochemical recurrence, metastasis and mortality after radical prostatectomy in a European cohort. J. Urol. 2015, 193, 1970–1975. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Lee, H.M.; Cho, S.W. Prostate cancer: Role of pretreatment multiparametric 3-T MRI in predicting biochemical recurrence after radical prostatectomy. AJR Am. J. Roentgenol. 2014, 202, W459–W465. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar]
- Bang, S.; Yu, J.; Chung, J.H.; Song, W.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Lee, H.M.; et al. Usefulness of MRI targeted prostate biopsy for detecting clinically significant prostate cancer in men with low prostate-specific antigen levels. Sci. Rep. 2021, 11, 21951. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.M.; Montagnon, E.; Yamashita, R.; Pan, I.; Cadrin-Chenevert, A.; Perdigon Romero, F.; Chartrand, G.; Kadoury, S.; Tang, A. Deep Learning: An Update for Radiologists. Radiographics 2021, 41, 1427–1445. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Xie, H.; Yang, Z.; Zhou, H. Boosted EfficientNet: Detection of Lymph Node Metastases in Breast Cancer Using Convolutional Neural Networks. Cancers 2021, 13, 661. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the 36th International Conference on Machine Learning (ICML), Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. Available online: http://proceedings.mlr.press/v97/tan19a.html (accessed on 1 May 2023).
- Cooperberg, M.R.; Pasta, D.J.; Elkin, E.P.; Litwin, M.S.; Latini, D.M.; Du Chane, J.; Carroll, P.R. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol. 2005, 173, 1938–1942. [Google Scholar] [CrossRef] [Green Version]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef]
- Cohen, M.S.; Hanley, R.S.; Kurteva, T.; Ruthazer, R.; Silverman, M.L.; Sorcini, A.; Hamawy, K.; Roth, R.A.; Tuerk, I.; Libertino, J.A. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: The Lahey Clinic Medical Center experience and an international meta-analysis. Eur. Urol. 2008, 54, 371–381. [Google Scholar] [CrossRef]
- Kuroiwa, K.; Shiraishi, T.; Naito, S.; Clinicopathological Research Group for Localized Prostate Cancer. Gleason score correlation between biopsy and prostatectomy specimens and prediction of high-grade Gleason patterns: Significance of central pathologic review. Urology 2011, 77, 407–411. [Google Scholar] [CrossRef]
- Epstein, J.I.; Feng, Z.; Trock, B.J.; Pierorazio, P.M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 2012, 61, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Sfoungaristos, S.; Perimenis, P. Clinical and pathological variables that predict changes in tumour grade after radical prostatectomy in patients with prostate cancer. Can. Urol. Assoc. J. 2013, 7, E93–E97. [Google Scholar] [CrossRef]
- Scattoni, V.; Maccagnano, C.; Capitanio, U.; Gallina, A.; Briganti, A.; Montorsi, F. Random biopsy: When, how many and where to take the cores? World J. Urol. 2014, 32, 859–869. [Google Scholar] [CrossRef]
- Van Praet, C.; Libbrecht, L.; D’Hondt, F.; Decaestecker, K.; Fonteyne, V.; Verschuere, S.; Rottey, S.; Praet, M.; De Visschere, P.; Lumen, N.; et al. Agreement of Gleason score on prostate biopsy and radical prostatectomy specimen: Is there improvement with increased number of biopsy cylinders and the 2005 revised Gleason scoring? Clin. Genitourin. Cancer 2014, 12, 160–166. [Google Scholar] [CrossRef]
- Schreiber, D.; Wong, A.T.; Rineer, J.; Weedon, J.; Schwartz, D. Prostate biopsy concordance in a large population-based sample: A Surveillance, Epidemiology and End Results study. J. Clin. Pathol. 2015, 68, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, K.; O’Callaghan, M.; Vincent, A.; Cohen, P.; Borg, M.; Roder, D.; Evans, S.; Millar, J.; Moretti, K. Extent and predictors of grade upgrading and downgrading in an Australian cohort according to the new prostate cancer grade groupings. Asian J. Urol. 2019, 6, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Calio, B.P.; Sidana, A.; Sugano, D.; Gaur, S.; Maruf, M.; Jain, A.L.; Merino, M.J.; Choyke, P.L.; Wood, B.J.; Pinto, P.A.; et al. Risk of Upgrading from Prostate Biopsy to Radical Prostatectomy Pathology-Does Saturation Biopsy of Index Lesion during Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsy Help? J. Urol. 2018, 199, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, S.; Mirtalebi, M.; Daneshpajouhnejad, P.; Barahimi, A.; Mazdak, H.; Izadpanahi, M.H.; Mohammadi, M.; Taheri, D. Discrepancies between Biopsy Gleason Score and Radical Prostatectomy Specimen Gleason Score: An Iranian Experience. Urol. J. 2019, 16, 56–61. [Google Scholar]
- Jang, W.S.; Koh, D.H.; Kim, J.; Lee, J.S.; Chung, D.Y.; Ham, W.S.; Rha, K.H.; Choi, Y.D. The prognostic impact of downgrading and upgrading from biopsy to radical prostatectomy among men with Gleason score 7 prostate cancer. Prostate 2019, 79, 1805–1810. [Google Scholar] [CrossRef]
- Malkiewicz, B.; Kielb, P.; Karwacki, J.; Czerwinska, R.; Dlugosz, P.; Leminski, A.; Nowak, L.; Krajewski, W.; Szydelko, T. Utility of Lymphadenectomy in Prostate Cancer: Where Do We Stand? J. Clin. Med. 2022, 11, 2343. [Google Scholar] [CrossRef]
- Cheung, D.C.; Fleshner, N.; Sengupta, S.; Woon, D. A narrative review of pelvic lymph node dissection in prostate cancer. Transl. Androl. Urol. 2020, 9, 3049–3055. [Google Scholar] [CrossRef]
- Lorent, M.; Maalmi, H.; Tessier, P.; Supiot, S.; Dantan, E.; Foucher, Y. Meta-analysis of predictive models to assess the clinical validity and utility for patient-centered medical decision making: Application to the CAncer of the Prostate Risk Assessment (CAPRA). BMC Med. Inform. Decis. Mak. 2019, 19, 2. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Shiradkar, R.; Leo, P.; Algohary, A.; Fu, P.; Tirumani, S.H.; Mahran, A.; Buzzy, C.; Obmann, V.C.; Mansoori, B.; et al. A novel imaging based Nomogram for predicting post-surgical biochemical recurrence and adverse pathology of prostate cancer from pre-operative bi-parametric MRI. EBioMedicine 2021, 63, 103163. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, H.H.; Lee, H.Y.; Park, H. Clinical impact of variability on CT radiomics and suggestions for suitable feature selection: A focus on lung cancer. Cancer Imaging 2019, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Kang, J.; Park, H.; Sohn, I.; Lee, S.H.; Lee, H.Y. Deciphering the tumor microenvironment through radiomics in non-small cell lung cancer: Correlation with immune profiles. PLoS ONE 2020, 15, e0231227. [Google Scholar] [CrossRef] [Green Version]
- Priester, A.; Natarajan, S.; Khoshnoodi, P.; Margolis, D.J.; Raman, S.S.; Reiter, R.E.; Huang, J.; Grundfest, W.; Marks, L.S. Magnetic Resonance Imaging Underestimation of Prostate Cancer Geometry: Use of Patient Specific Molds to Correlate Images with Whole Mount Pathology. J. Urol. 2017, 197, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Pooli, A.; Johnson, D.C.; Shirk, J.; Markovic, D.; Sadun, T.Y.; Sisk, A.E., Jr.; Mohammadian Bajgiran, A.; Afshari Mirak, S.; Felker, E.R.; Hughes, A.K.; et al. Predicting Pathological Tumor Size in Prostate Cancer Based on Multiparametric Prostate Magnetic Resonance Imaging and Preoperative Findings. J. Urol. 2021, 205, 444–451. [Google Scholar] [CrossRef]
- Zhou, R.; Feng, Y.; Ye, J.; Han, Z.; Liang, Y.; Chen, Q.; Xu, X.; Huang, Y.; Jia, Z.; Zhong, W. Prediction of Biochemical Recurrence-Free Survival of Prostate Cancer Patients Leveraging Multiple Gene Expression Profiles in Tumor Microenvironment. Front. Oncol. 2021, 11, 632571. [Google Scholar] [CrossRef]
- Gevaert, T.; Van Eycke, Y.R.; Vanden Broeck, T.; Van Poppel, H.; Salmon, I.; Rorive, S.; Muilwijk, T.; Claessens, F.; De Ridder, D.; Joniau, S.; et al. The potential of tumour microenvironment markers to stratify the risk of recurrence in prostate cancer patients. PLoS ONE 2020, 15, e0244663. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Cheng, L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef]
- Stollmayer, R.; Budai, B.K.; Ronaszeki, A.; Zsombor, Z.; Kalina, I.; Hartmann, E.; Toth, G.; Szoldan, P.; Berczi, V.; Maurovich-Horvat, P.; et al. Focal Liver Lesion MRI Feature Identification Using Efficientnet and MONAI: A Feasibility Study. Cells 2022, 11, 1558. [Google Scholar] [CrossRef]
- Li, H.; Lee, C.H.; Chia, D.; Lin, Z.; Huang, W.; Tan, C.H. Machine Learning in Prostate MRI for Prostate Cancer: Current Status and Future Opportunities. Diagnostics 2022, 12, 289. [Google Scholar] [CrossRef]
- Shiradkar, R.; Ghose, S.; Mahran, A.; Li, L.; Hubbard, I.; Fu, P.; Tirumani, S.H.; Ponsky, L.; Purysko, A.; Madabhushi, A. Prostate Surface Distension and Tumor Texture Descriptors from Pre-Treatment MRI Are Associated with Biochemical Recurrence Following Radical Prostatectomy: Preliminary Findings. Front. Oncol. 2022, 12, 841801. [Google Scholar] [CrossRef]
- Shiradkar, R.; Ghose, S.; Jambor, I.; Taimen, P.; Ettala, O.; Purysko, A.S.; Madabhushi, A. Radiomic features from pretreatment biparametric MRI predict prostate cancer biochemical recurrence: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 1626–1636. [Google Scholar] [CrossRef]
- Bourbonne, V.; Vallieres, M.; Lucia, F.; Doucet, L.; Visvikis, D.; Tissot, V.; Pradier, O.; Hatt, M.; Schick, U. MRI-Derived Radiomics to Guide Post-operative Management for High-Risk Prostate Cancer. Front. Oncol. 2019, 9, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israeli, R.S.; Powell, C.T.; Corr, J.G.; Fair, W.R.; Heston, W.D. Expression of the prostate-specific membrane antigen. Cancer Res. 1994, 54, 1807–1811. [Google Scholar] [PubMed]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cysouw, M.C.F.; Jansen, B.H.E.; van de Brug, T.; Oprea-Lager, D.E.; Pfaehler, E.; de Vries, B.M.; van Moorselaar, R.J.A.; Hoekstra, O.S.; Vis, A.N.; Boellaard, R. Machine learning-based analysis of [(18)F]DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 340–349. [Google Scholar] [CrossRef]
- Leung, K.H.; Rowe, S.P.; Leal, J.P.; Ashrafinia, S.; Sadaghiani, M.S.; Chung, H.W.; Dalaie, P.; Tulbah, R.; Yin, Y.; VanDenBerg, R.; et al. Deep learning and radiomics framework for PSMA-RADS classification of prostate cancer on PSMA PET. EJNMMI Res. 2022, 12, 76. [Google Scholar] [CrossRef]
- Chan, T.H.; Haworth, A.; Wang, A.; Osanlouy, M.; Williams, S.; Mitchell, C.; Hofman, M.S.; Hicks, R.J.; Murphy, D.G.; Reynolds, H.M. Detecting localised prostate cancer using radiomic features in PSMA PET and multiparametric MRI for biologically targeted radiation therapy. EJNMMI Res. 2023, 13, 34. [Google Scholar] [CrossRef]
- Pozaruk, A.; Pawar, K.; Li, S.; Carey, A.; Cheng, J.; Sudarshan, V.P.; Cholewa, M.; Grummet, J.; Chen, Z.; Egan, G. Augmented deep learning model for improved quantitative accuracy of MR-based PET attenuation correction in PSMA PET-MRI prostate imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 9–20. [Google Scholar] [CrossRef]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Koeber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Zhang, N.; Liu, Y.; Li, N.; Du, P.; Yang, Y.; Liu, M.; Gong, K.; Yang, X.; et al. (68)Ga-PSMA-617 PET/CT: A promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1852–1861. [Google Scholar] [CrossRef]
- Papp, L.; Spielvogel, C.P.; Grubmuller, B.; Grahovac, M.; Krajnc, D.; Ecsedi, B.; Sareshgi, R.A.M.; Mohamad, D.; Hamboeck, M.; Rausch, I.; et al. Supervised machine learning enables non-invasive lesion characterization in primary prostate cancer with [(68)Ga]Ga-PSMA-11 PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1795–1805. [Google Scholar] [CrossRef]
- Feliciani, G.; Celli, M.; Ferroni, F.; Menghi, E.; Azzali, I.; Caroli, P.; Matteucci, F.; Barone, D.; Paganelli, G.; Sarnelli, A. Radiomics Analysis on [(68)Ga]Ga-PSMA-11 PET and MRI-ADC for the Prediction of Prostate Cancer ISUP Grades: Preliminary Results of the BIOPSTAGE Trial. Cancers 2022, 14, 1888. [Google Scholar] [CrossRef]
- Meyer, A.R.; Joice, G.A.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Integration of PSMA-targeted PET imaging into the armamentarium for detecting clinically significant prostate cancer. Curr. Opin. Urol. 2018, 28, 493–498. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in prostate-specific membrane antigen PET of prostate cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Hofman, M.S.; Iravani, A.; Nzenza, T.; Murphy, D.G. Advances in Urologic Imaging: Prostate-Specific Membrane Antigen Ligand PET Imaging. Urol. Clin. 2018, 45, 503–524. [Google Scholar] [CrossRef]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Guglielmo, P.; Marturano, F.; Bettinelli, A.; Gregianin, M.; Paiusco, M.; Evangelista, L. Additional Value of PET Radiomic Features for the Initial Staging of Prostate Cancer: A Systematic Review from the Literature. Cancers 2021, 13, 6026. [Google Scholar] [CrossRef]


| Parameters | T2WI | DWI | DCE MRI |
|---|---|---|---|
| Imaging planes | Axial, sagittal, and coronal | Axial | Axial |
| Sequence | Turbo spin-echo | Echoplanar imaging | 3D fast-field echo |
| TR/TE (ms) | 3800–4700/80–100 | 4400–4800/63–75 | 7.4/3.9 |
| Flip angle (°) | NA | NA | 25 |
| Sense factor | 2 | 2 | 1 |
| B value (s/mm2) | NA | 0, 1000 | NA |
| Slice thickness (mm) | 3 | 3 | 4 |
| Slice gap (mm) | 0.3–1 | 1 | 0 |
| Matrix size | 512 × 304 | 124 × 120 | 224 × 179 |
| No. of excitations | 3 | 4 | 1 |
| FOV (mm) | 200 | 200 | 200 |
| Scan time | 3 min 48 s | 1 min 40 s | 5 min 58 s (every 3 s with 60 repetitions) |
| Clinical Parameters | Full Cohort (n = 437) | Non-BCR (n = 327, 74.8%) | BCR (n = 110, 25.2%) | p |
|---|---|---|---|---|
| FU duration, months, median (IQR) | 61 (24–120) | 78 (45–118) | 23 (9–52) | NA |
| 10-year BCR-free survival, n (%) | 76 (17) | 75 (17) | 1 (0) | |
| Age, years, median (IQR) | 66 (62–71) | 66 (62–71) | 66 (61–71) | 0.35 |
| Preoperative PSA, mg/dL, mean ± SD | 7.61 ± 6.45 | 6.35 ± 4.14 | 11.35 ± 9.81 | <0.001 |
| Pathologic stage, n (%) | ||||
| ≤T2 (pT1–pT2) | 312 (71) | 258 (79) | 54 (49) | |
| ≥T3 (pT3–pT4) | 125 (29) | 69 (21) | 56 (51) | |
| Adverse pathological features, n (%) | ||||
| Extracellular capsular extension | 121 (28) | 66 (20) | 55 (50) | <0.001 |
| Seminal vesicle invasion | 27 (6) | 5 (2) | 22 (20) | <0.001 |
| Positive surgical margin | 78 (18) | 30 (9) | 48 (44) | <0.001 |
| Pathologic GS ISUP score group, n (%) | <0.001 | |||
| ≤6 (Group 1) | 92 (21) | 87 (27) | 5 (5) | |
| 3 + 4 (Group 2) | 232 (53) | 183 (56) | 49 (45) | |
| 4 + 3 (Group 3) | 75 (17) | 42 (13) | 33 (30) | |
| 8 (Group 4) | 13 (03) | 6 (2) | 5 (6) | |
| ≥9 (Group 5) | 25 (06) | 9 (3) | 16 (15) | |
| Lymph node involvement, n (%) | ||||
| Yes | 3 (1) | 0 (0) | 3 (3) | 0.02 |
| No | 83 (19) | 48 (15) | 35 (32) | <0.001 |
| Not determined | 351 (80) | 279 (85) | 72 (65) | <0.001 |
| CM | HR (95% CI) | p | C-Index (95% CI) | * iAUC | ||||
|---|---|---|---|---|---|---|---|---|
| Train | Test | Train | Test | Train | Test | Train | Test | |
| 1-fold | 4.57 [2.98, 7.00] | 6.92 [2.96, 16.15] | <0.0001 | <0.0001 | 0.81 [0.76, 0.85] | 0.85 [0.77, 0.93] | 0.86 | 0.90 |
| 2-fold | 5.66 [3.69, 8.68] | 4.76 [2.04, 11.11] | <0.0001 | 0.0007 | 0.82 [0.77, 0.86] | 0.79 [0.67, 0.91] | 0.87 | 0.85 |
| 3-fold | 7.28 [4.74, 11.17] | 2.71 [1.16, 6.31] | <0.0001 | 0.0365 | 0.84 [0.80, 0.88] | 0.72 [0.60, 0.84] | 0.89 | 0.74 |
| 4-fold | 5.56 [3.63, 8.51] | 5.34 [2.23, 12.77] | <0.0001 | 0.0004 | 0.82 [0.78, 0.86] | 0.80 [0.70, 0.89] | 0.87 | 0.84 |
| 5-fold | 5.35 [3.49, 8.21] | 9.39 [3.91, 22.55] | <0.0001 | <0.0001 | 0.81 [0.77, 0.85] | 0.87 [0.80, 0.94] | 0.86 | 0.92 |
| Mean ± SD | 5.68 ± 0.99 [3.74, 7.62] | 5.82 ± 2.50 [0.93, 10.72] | 0.0000 ± 0.0000 | 0.0075 ± 0.0162 | 0.82 ± 0.01 [0.80, 0.84] | 0.81 ± 0.05 [0.69, 0.92] | 0.87 ± 0.01 | 0.85 ± 0.06 |
| RM-Multi | HR (95% CI) | p | C-Index(95% CI) | * iAUC | ||||
| 1-fold | 1.30 [0.85, 1.97] | 2.26 [0.97, 5.23] | 0.2697 | 0.0918 | 0.53 [0.47, 0.60] | 0.61 [0.49, 0.73] | 0.57 | 0.66 |
| 2-fold | 2.36 [1.55, 3.60] | 4.68 [2.02, 10.84] | 0.0001 | 0.0007 | 0.66 [0.61, 0.72] | 0.73 [0.62, 0.84] | 0.71 | 0.73 |
| 3-fold | 2.32 [1.52, 3.55] | 1.37 [0.59, 3.18] | 0.0001 | 0.5985 | 0.67 [0.61, 0.72] | 0.64 [0.52, 0.76] | 0.71 | 0.65 |
| 4-fold | 0.18 [1.53, 3.55] | 2.98 [1.28, 6.91] | 0.0001 | 0.0202 | 0.64 [0.58, 0.70] | 0.69 [0.57, 0.80] | 0.68 | 0.69 |
| 5-fold | 0.06 [1.47, 3.40] | 2.07 [0.89, 4.82] | 0.0003 | 0.1387 | 0.66 [0.60, 0.72] | 0.64 [0.55, 0.74] | 0.69 | 0.67 |
| Mean ± SD | 1.24 ± 1.11 [0.00, 3.42] | 2.67 ± 1.26 [0.20, 5.14] | 0.0541 ± 0.1205 | 0.1700 ± 0.500 | 0.63 ± 0.06 [0.52, 0.75] | 0.66 ± 0.04 [0.57, 0.76] | 0.67 ± 0.06 | 0.68 ± 0.03 |
| CRM-Multi | HR (95% CI) | p | C-Index(95% CI) | * iAUC | ||||
| 1-fold | 6.05 [3.93, 9.32] | 6.92 [2.96, 16.15] | <0.0001 | <0.0001 | 0.80 [0.76, 0.85] | 0.85 [0.77, 0.93] | 0.86 | 0.89 |
| 2-fold | 6.66 [4.33, 10.285] | 6.08 [2.62, 14.14] | <0.0001 | <0.0001 | 0.82 [0.78, 0.86] | 0.85 [0.76, 0.93] | 0.87 | 0.88 |
| 3-fold | 5.91 [3.84, 9.10] | 3.28 [1.41, 7.65] | <0.0001 | 0.0113 | 0.83 [0.79, 0.87] | 0.75 [0.64, 0.86] | 0.89 | 0.78 |
| 4-fold | 5.76 [3.76, 8.84] | 4.97 [2.13, 11.64] | <0.0001 | 0.0005 | 0.82 [0.78, 0.86] | 0.80 [0.72, 0.89] | 0.87 | 0.84 |
| 5-fold | 5.99 [3.90, 9.20] | 5.95 [2.53, 13.98] | <0.0001 | 0.0001 | 0.81 [0.76, 0.85] | 0.89 [0.83, 0.95] | 0.86 | 0.93 |
| Mean ± SD | 6.07 ± 0.35 [5.40, 6.75] | 5.44 ± 1.39 [2.71, 8.17] | 0.0000 ± 0.0000 | 0.0024 ± 0.0050 | 0.82 ± 0.01 [0.79, 0.84] | 0.83 ± 0.05 [0.72, 0.93] | 0.87 ± 0.01 | 0.87 ± 0.05 |
| DLM- Deep Feature | HR (95% CI) | p | C-Index (95% CI) | iAUC | ||||
| 1-fold | 7.67 [5.04, 11.67] | 6.41 [2.72, 15.13] | <0.0001 | <0.0001 | 0.89 [0.86, 0.92] | 0.80 [0.69, 0.92] | 0.91 | 0.83 |
| 2-fold | 6.77 [3.82, 11.99] | 2.52 [1.09, 5.83] | <0.0001 | 0.0527 | 0.77 [0.73, 0.82] | 0.72 [0.61, 0.83] | 0.84 | 0.74 |
| 3-fold | 8.95 [5.88, 13.63] | 7.27 [3.11, 16.96] | <0.0001 | <0.0001 | 0.89 [0.86, 0.91] | 0.83 [0.73, 0.93] | 0.91 | 0.86 |
| 4-fold | 4.72 [2.77, 8.04] | 2.80 [1.20, 6.54] | <0.0001 | 0.0310 | 0.75 [0.70, 0.81] | 0.68 [0.58, 0.79] | 0.83 | 0.71 |
| 5-fold | 3.19 [2.10, 4.86] | 2.85 [1.23, 6.61] | <0.0001 | 0.0256 | 0.71 [0.65, 0.76] | 0.67 [0.57, 0.78] | 0.70 | 0.70 |
| Mean ± SD | 6.26 ± 2.31 [1.74, 10.78] | 4.37 ± 2.28 [0.00, 8.83] | 0.0000 ± 0.0000 | 0.0219 ± 0.0224 | 0.80 ± 0.08 [0.64, 0.97] | 0.74 ± 0.06 [0.59, 0.88] | 0.84 ± 0.09 | 0.77 ± 0.06 |
| CDLM- Deep Feature | HR (95% CI) | p | C-Index(95% CI) | iAUC | ||||
| 1-fold | 8.43 [5.53, 12.84] | 12.95 [5.45, 30.78] | <0.0001 | <0.0001 | 0.91 [0.89, 0.94] | 1.00 [1.00, 1.00] | 0.94 | 1.00 |
| 2-fold | 6.02 [3.93, 9.22] | 3.78 [1.62, 8.82] | <0.0001 | 0.0042 | 0.82 [0.77, 0.86] | 0.85 [0.76, 0.93] | 0.87 | 0.90 |
| 3-fold | 8.65 [5.68, 13.16] | 7.65 [3.26, 17.92] | <0.0001 | <0.0001 | 0.92 [0.90, 0.95] | 0.87 [0.78, 0.96] | 0.95 | 0.90 |
| 4-fold | 6.07 [3.97, 9.28] | 7.08 [2.99, 16.77] | <0.0001 | <0.0001 | 0.82 [0.77, 0.86] | 0.85 [0.77, 0.92] | 0.87 | 0.89 |
| 5-fold | 4.77 [3.13, 7.27] | 7.13 [3.03, 16.76] | <0.0001 | <0.0001 | 0.83 [0.79, 0.87] | 0.89 [0.81, 0.98] | 0.88 | 0.93 |
| Mean ± SD | 6.79 ± 1.68 [3.49, 10.09] | 7.72 ± 3.30 [1.24, 14.19] | 0.0000 ± 0.0000 | 0.0008 ± 0.0019 | 0.86 ± 0.05 [0.76, 0.96] | 0.89 ± 0.06 [0.77, 1.00] | 0.90 ± 0.04 | 0.93 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.W.; Kim, E.; Na, I.; Kim, C.K.; Seo, S.I.; Park, H. Novel Multiparametric Magnetic Resonance Imaging-Based Deep Learning and Clinical Parameter Integration for the Prediction of Long-Term Biochemical Recurrence-Free Survival in Prostate Cancer after Radical Prostatectomy. Cancers 2023, 15, 3416. https://doi.org/10.3390/cancers15133416
Lee HW, Kim E, Na I, Kim CK, Seo SI, Park H. Novel Multiparametric Magnetic Resonance Imaging-Based Deep Learning and Clinical Parameter Integration for the Prediction of Long-Term Biochemical Recurrence-Free Survival in Prostate Cancer after Radical Prostatectomy. Cancers. 2023; 15(13):3416. https://doi.org/10.3390/cancers15133416
Chicago/Turabian StyleLee, Hye Won, Eunjin Kim, Inye Na, Chan Kyo Kim, Seong Il Seo, and Hyunjin Park. 2023. "Novel Multiparametric Magnetic Resonance Imaging-Based Deep Learning and Clinical Parameter Integration for the Prediction of Long-Term Biochemical Recurrence-Free Survival in Prostate Cancer after Radical Prostatectomy" Cancers 15, no. 13: 3416. https://doi.org/10.3390/cancers15133416
APA StyleLee, H. W., Kim, E., Na, I., Kim, C. K., Seo, S. I., & Park, H. (2023). Novel Multiparametric Magnetic Resonance Imaging-Based Deep Learning and Clinical Parameter Integration for the Prediction of Long-Term Biochemical Recurrence-Free Survival in Prostate Cancer after Radical Prostatectomy. Cancers, 15(13), 3416. https://doi.org/10.3390/cancers15133416






