Appendiceal Mucinous Neoplasms: From Clinic to Pathology and Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
- –
- A systematic review providing a historical perspective on the evolution of the different classification systems of these tumors published since the 1990s up to the 2016 Peritoneal Surface Oncology Group International (PSOGI) consensus, the 2017 AJCC 8th edition, and the 2019 WHO classification.
- –
- A pathological review of our series to the adoption of the PSOGI and AJCC 8th edition classification criteria in order to evaluate which classification system best reflects the prognosis of our cohort of patients.
2. Review Sections
2.1. Clinical Aspects
2.2. Endoscopy and Imaging Modalities
2.3. Tumor Markers
2.4. Preoperatory Hystology
2.5. Peritoneal Dissemination
2.6. CRS + HIPEC
2.7. Systemic Chemotherapy (SCT)
2.8. Pathology
2.9. Other Histopathological Landmarks
2.9.1. Acellular Mucin
2.9.2. Signet Ring Cells
2.10. Prognostic Factors
| Stage of Disease | Type | Histological Nomenclature | Key Histologic Features | |
|---|---|---|---|---|
| Ronnett et al. [68] | Primary tumors | Benign lesions. | Villous adenoma | Adenomatous epithelium with villous architecture confined to the mucosa. |
| Cystadenoma | Adenomatous epithelium without villous architecture confined to the mucosa of a dilated appendix. | |||
| Dilated/ruptured adenoma. | Glands or strips of adenomatous epithelium within the wall or on the serosa of a dilated or ruptured appendix without a stromal response. Dissecting mucin or epithelium extending through the wall of the appendix. | |||
| Invasive lesions | Adenocarcinoma | Adenomatous epithelium invading the muscularis of the appendix accompanied by a stromal response. | ||
| Mucinous adenocarcinoma with SRC | Neoplasms with glandular and SRC differentiation, with or without neuroendocrine features that showed marked cytologic atypia and muscularis invasion. | |||
| Peritoneal implants | DPAM | Scant strips of simple proliferative epithelium with minimal to moderate cytologic atypia and no significant mitotic activity within abundant mucin. | ||
| PMCA I/D | Features of DPAM with focal areas of carcinoma +/− SRC. I- Arising from a well-differentiated mucinous adenocarcinoma. D- Arising from a villous adenoma with moderate to marked cytologic atypia and areas of poorly differentiated carcinoma in the wall and serosa of the appendix. | |||
| PMCA | Abundant proliferative epithelium, glands, nests, or individual cells including SRC, demonstrating marked cytologic atypia and mitotic activity. | |||
| Misdraji et al. [69] | Primary mucinous tumors | LAMN | Low-grade cytological atypia (nuclear enlargement, scarce nuclear stratification, and rare mitotic figures) and minimal architectural complexity (a uniform, flat, epithelial proliferation forming small papillary excrescences). No infiltrative invasion of the appendiceal wall. | |
| MACA | High cytological atypia (full thickness nuclear stratification, vesicular nuclei with prominent nucleoli and brisk mitotic figures) and infiltrative invasion of the appendicular wall. | |||
| Peritoneal implants. | LAMN with peritoneal dissemination. | Low-grade cytologic atypia with flat epithelium proliferation forming papillary excrescences, low cellularity. | ||
| MACA with peritoneal dissemination. | High-grade cytologic atypia, destructive invasion of the wall of the appendix, high cellularity, and abundant mitotic figures. | |||
| PSOGI classification [77] | Primary mucinous tumors. | Benign lesions. | Serrated polyp with or without dysplasia. | Tubular architecture with basal parts of the crypts showing serration, and dilatation. Muscularis mucosae intact. |
| Mucinous neoplasms. | LAMN | Pushing invasion with loss of the muscularis mucosae and fibrosis of the submucosa. Filiform villi, undulating and flat. Basally orientated nuclei with minimal atypia and rare mitotic figures. | ||
| HAMN | Pushing invasion with loss of the muscularis mucosae. Filiform villi, undulating, flat with pseudopapillae. Loss of nuclear polarity and frequent mitotic figures that may be atypical. | |||
| Mucinous adenocarcinoma | Infiltrating invasion (discohesive single cells or clusters of cells, small irregular glands within desmoplastic stroma). Variably sized glands and islands, and variable nuclear features and frequent mitotic figures that may be atypical. Can be well-, moderately-, and poorly differentiated. | |||
| Mucinous adenocarcinoma with SRC. | Infiltrating invasion. Poorly differentiated, with <50% of SRC. | |||
| SRC carcinoma. | Infiltrating invasion. Poorly differentiated, with >50% of SRC. | |||
| Peritoneal implants | No epithelial component. | Mucin without epithelial cells. | Acellular mucin. Abundant mucin without evidence of neoplastic epithelium. Extensive sampling required to discard presence of neoplastic epithelium. | |
| Epithelial component | LG-MCP | Abundant mucin with low cellularity (<20% tumor volume composed of neoplastic epithelium). Low-grade cytological features with low proliferative activity. | ||
| HG-MCP | Abundant cellularity (>20% tumor volume composed of neoplastic epithelium). High-grade cytological features with high proliferative activity (can be mixed with areas of low-grade cytological features). Infiltrative invasion into subjacent tissues. Must lack SRC. | |||
| HG-MCP with SRC | Abundant cellularity (>20% tumor volume composed of neoplastic epithelium). High-grade cytological features with high proliferative activity. Infiltrative invasion into subjacent tissues. SRC component present. | |||
| AJCC 8th edition [80] | Primary lesions. | Benign lesions | Adenoma | LAMN confined to the mucosa with intact muscularis mucosae. |
| Premalignant lesions | High-grade dysplasia | Neoplastic cells confined to crypts that do not invade the lamina propria. | ||
| Intramucosal adenocarcinoma | Neoplastic cells invade the lamina propria with or without extension into but not through the muscularis mucosae. pTis. | |||
| Mucinous appendiceal neoplasms | LAMN | Neoplastic cells extend through the wall of the appendix with a pushing front, without features of invasion. Tis (LAMN)- LAMN confined by the muscularis propria, acellular mucin, or mucinous epithelium may extend into de muscularis propria. pT3- involvement of the subserosa. pT4a- involvement of the visceral peritoneum (with acellular mucin or mucinous epithelium). pT4b- direct involvement of adjacent organs or structures. | ||
| HAMN | Tumors with architectural features of LAMN with areas of high-grade dysplasia. pT staging follows that of mucinous adenocarcinoma. | |||
| Mucinous adenocarcinoma. | Neoplastic epithelium displays infiltrative and destructive growth into the wall of the appendix, beyond the muscularis mucosae. Associated with desmoplastic reaction. pT1- involvement of the submucosa through the muscularis mucosa. pT2- involvement of the muscularis propria. pT3- involvement of the subserosa or mesoappendix. pT4a- involvement of the visceral peritoneum (with acellular mucin or mucinous epithelium) pT4b- direct involvement of adjacent organs or structures. | |||
| Peritoneal implants. | EIVA | M1a | Intraperitoneal acellular mucin without neoplastic epithelium in the disseminated peritoneal mucinous deposits. | |
| M1bG1 | Intraperitoneal dissemination containing tumor cells with low-grade cytologic atypia without SRC. Low cellularity (<20%). No infiltrative invasion of the peritoneum; may be involved with pushing front without desmoplastic reaction. Perineural or lymphovascular invasion rarely observed. | |||
| EIVB | M1bG2 | Intraperitoneal dissemination containing tumor cells with mixture of low- and high-grade cytologic atypia without SRC. High cellularity (>20%). Infiltrative invasion of the peritoneum and into adjacent organs. Perineural or lymphovascular invasion may be present. | ||
| M1bG3 | Intraperitoneal dissemination with tumor cells displaying adverse histological features. High cellularity (>20%). Infiltrative invasion of the peritoneum, adjacent organs. Perineural or lymphovascular invasion may be present. |
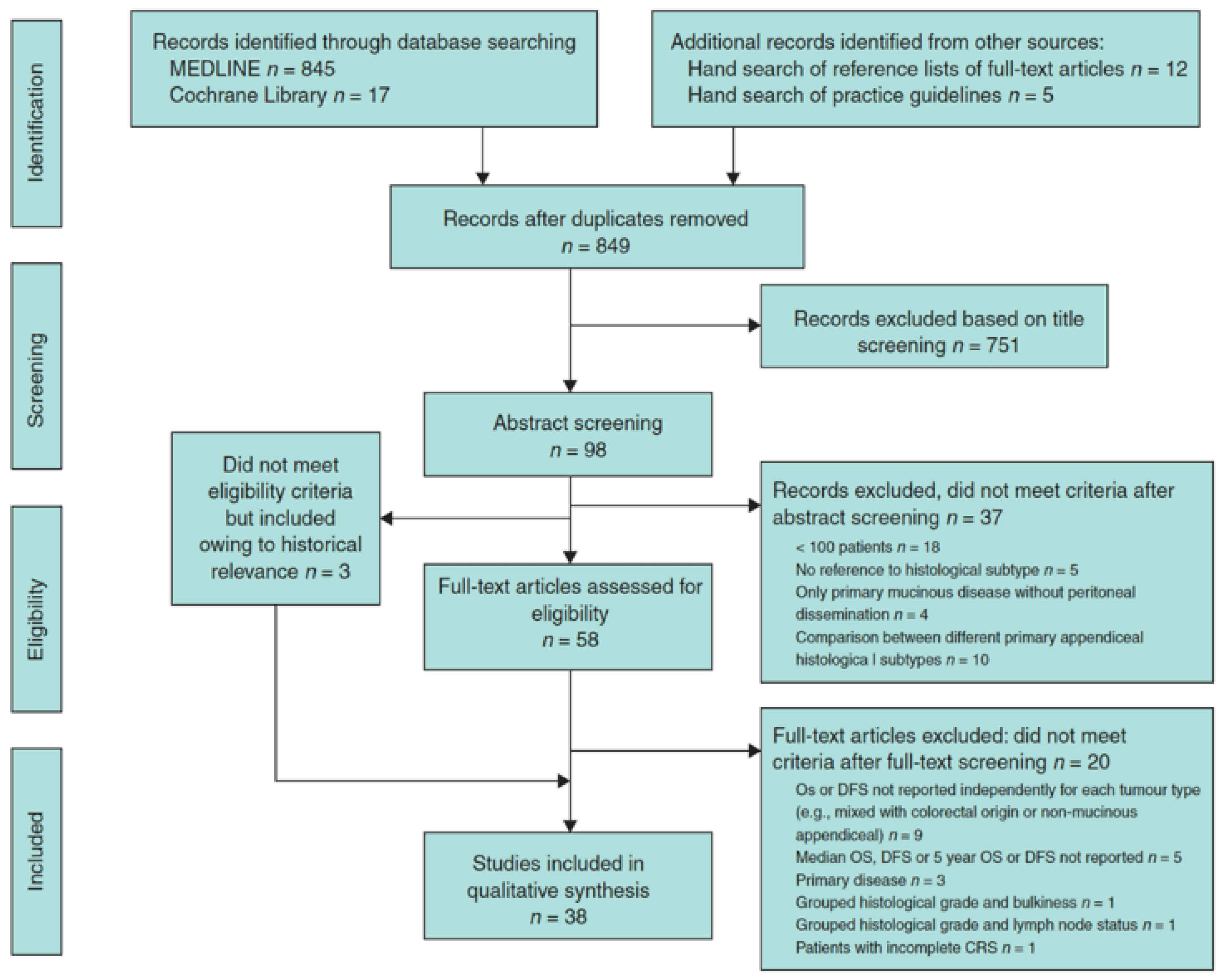
3. Systematic Review on Mucinous Tumors of the Appendix with Peritoneal Dissemination [93]
- Target population: patients with PD from a mucinous tumor of the appendix treated with CRS + HIPEC.
- The studies had to report OS and DFS results based on any pathologic classification. In addition, the results had to be shown as median and/or 5-year OS or DFS rate for each histologic category of peritoneal implants. At the least, the survival results of two different histological categories had to be compared in univariable or multivariable analysis.
3.1. Results
3.2. Conclusion
4. Which Classification System Defines the Best Prognosis of Mucinous Neoplasms of the Appendix with Peritoneal Dissemination: TNM or PSOGI [99]?
4.1. Pathological Evaluation
4.2. Results
4.3. Discussion
4.4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.L.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene 2023, 42, 530–540. [Google Scholar] [CrossRef]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.H.J.T.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef]
- Chicago Consensus Working Group. The Chicago Consensus on peritoneal surface malignancies: Management of appendiceal neoplasms. Cancer 2020, 126, 2525–2533. [Google Scholar] [CrossRef]
- Rokitansky, C.F. A Manual of Pathology Anatomy; English translation of the Vienna Edition; Blancard and Lea: Philadelphia, PA, USA, 1855. [Google Scholar]
- Appendiceal Mucinous Lesions—UpToDate. Available online: https://www.uptodate.com/contents/appendiceal-mucinous-lesions/print (accessed on 12 March 2023).
- Carr, N.J.; Bibeau, F.; Bradley, R.F. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology 2017, 71, 847–858. [Google Scholar] [CrossRef]
- Moran, B.J.; Cecil, T.D. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg. Oncol. Clin. N. Am. 2003, 12, 585–603. [Google Scholar] [CrossRef]
- Wert, R. Klinische and Anastomische Untersuchungen Zur Lehre von der Bauchgeschwullsten and der Laparotomie. Arch. Für Gynäkologie 1884, 84, 100–118. [Google Scholar] [CrossRef]
- Frankel, E. Uher das sogenanute pseudomyxoma peritonei. Med. Wochenschr. 1901, 48, 965–970. [Google Scholar]
- Rizvi, S.A.; Syed, W.; Shergill, R. Approach to pseudomyxoma peritonei. World J. Gastrointest. Surg. 2018, 10, 49–56. [Google Scholar] [CrossRef]
- Esquivel, J.; Sugarbaker, P.H. Clinical presentation of the Pseudomyxoma peritonei syndrome. Br. J. Surg. 2000, 87, 1414–1418. [Google Scholar] [CrossRef]
- Smeenk, R.M.; Velthuysen, M.L.F.; Verwaal, V.J.; Zoetmulder, F.N. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur. J. Surg. Oncol. 2008, 34, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.J.; Cahan, M.; Cohen, P.; Lambert, L.A. Increased risk of mucinous neoplasm of the appendix in adults undergoing interval appendectomy. JAMA Surg. 2013, 148, 703–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mällinen, J. Risk of Appendiceal Neoplasm in Periappendicular Abscess in Patients Treated with Interval Appendectomy vs. Follow-Up with Magnetic Resonance Imaging: 1-Year Outcomes of the Peri-Appendicitis Acuta Randomized Clinical Trial. JAMA Surg. 2019, 154, 200–207. [Google Scholar] [CrossRef]
- Peltrini, R. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: A systematic review and meta-analysis. Surg. J. R. Coll. Surg. Edinb. Irel. 2021, 19, e549–e558. [Google Scholar] [CrossRef]
- Alajääski, J. The association between appendicitis severity and patient age with appendiceal neoplasm histology—A population-based study. Int. J. Colorectal. Dis. 2022, 37, 1173–1180. [Google Scholar] [CrossRef]
- Benedix, F.; Reimer, A.; Gastinger, I.; Mroczkowski, P.; Lippert, H.; Kube, R. Primary appendiceal carcinoma epidemiology, surgery and survival: Results of a German multi-center study. Eur. J. Surg. Oncol. 2010, 36, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, P.; Jelinek, J.S.; Chang, D.; Koslowe, P.; Sugarbaker, P.H. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J. Am. Coll. Surg. 1995, 181, 530–538. [Google Scholar] [PubMed]
- Pickhardt, P.J.; Levy, A.D.; Rohrmann, C.A.; Kende, A.I. Primary neoplasms of the appendix manifesting as acute appendicitis: CT findings with pathologic comparison. Radiology 2002, 224, 775–781. [Google Scholar] [CrossRef]
- Milovanov, V.; Sardi, A.; Aydin, N.; Nieroda, C.; Sittig, M.; Gushchin, V. External Validation of the Simplified Preoperative Assessment for Low-Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2017, 24, 1783–1786. [Google Scholar] [CrossRef]
- Bree, E.; Koops, W.; Kröger, R.; Ruth, S.; Witkamp, A.J.; Zoetmulder, F.A.N. Peritoneal carcinomatosis from colorectal or appendiceal origin: Correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J. Surg. Oncol. 2004, 86, 64–73. [Google Scholar] [CrossRef]
- Low, R.N.; Sebrechts, C.P.; Barone, R.M.; Muller, W. Diffusion-weighted MRI of peritoneal tumors: Comparison with conventional MRI and surgical and histopathologic findings—A feasibility study. AJR Am. J. Roentgenol. 2009, 193, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Barone, R.M.; Lucero, J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015, 22, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Menassel, B.; Duclos, A.; Passot, G.; Dohan, A.; Payet, C.; Isaac, S. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur. J. Surg. Oncol. 2016, 42, 558–566. [Google Scholar] [CrossRef]
- Tan, G.H.C.; Kwek, J.W.; Hosseini, R.; Chanyaputhipong, J.; Tham, C.K.; Soo, K.C. Proposed radiological criteria for pre-operative determination of resectability in peritoneal-based malignancies. J. Med. Imaging. Radiat. Oncol. 2016, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dineen, S.P.; Royal, R.E.; Hughes, M.S.; Sagebiel, T.; Bhosale, P.; Overman, M. A Simplified Preoperative Assessment Predicts Complete Cytoreduction and Outcomes in Patients with Low-Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2015, 22, 3640–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouquot, M.; Dohan, A.; Gayat, E.; Barat, M.; Glehen, O.; Pocard, M. Prediction of Resectability in Pseudomyxoma Peritonei with a New CT Score. Ann. Surg. Oncol. 2018, 25, 694–701. [Google Scholar] [CrossRef]
- Sabesan, A.; Felder, S.; Feuerlein, S.; Lam, C.; McGettigan, M.; Powers, B.D.; Dessureault, S.; Dineen, S.P. Preoperative Radiographic Assessment Predicts Incomplete Cytoreduction in Patients with Low Grade Mucinous Adenocarcinoma of the Appendix. Ann. Surg. Oncol. 2020, 27, 165–170. [Google Scholar] [CrossRef]
- Chandramohan, A.; Thrower, A.; Smith, S.A.; Shah, N.; Moran, B. “PAUSE”: A method for communicating radiological extent of peritoneal malignancy. Clin. Radiol. 2017, 72, 972–980. [Google Scholar] [CrossRef]
- van Ruth, S.; Hart, A.M.; Bonfrer, J.M.G.; Verwaal, V.J.; Zoetmulder, F.N. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2002, 9, 961–967. [Google Scholar] [CrossRef]
- Taflampas, P.; Dayal, S.; Chandrakumaran, K.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal Pseudomyxoma Peritonei: Analysis of 519 patients. Eur. J. Surg. Oncol. 2014, 40, 515–520. [Google Scholar] [CrossRef]
- Kusamura, S.; Hutanu, I.; Baratti, D.; Deraco, M. Circulating tumor markers: Predictors of incomplete cytoreduction and powerful determinants of outcome in pseudomyxoma peritonei. J. Surg. Oncol. 2013, 108, 1–8. [Google Scholar] [CrossRef]
- Kozman, M.A.; Fisher, O.M.; Rebolledo, B.-A.J.; Valle, S.J.; Alzahrani, N.; Liauw, W.; Morris, D.L. CA 19-9 to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with epithelial appendiceal mucinous neoplasms and peritoneal dissemination undergoing cytoreduction surgery and intraperitoneal chemotherapy: A retrospective cohort study. Eur. J. Surg. Oncol. 2017, 43, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Carmignani, C.P.; Hampton, R.; Sugarbaker, C.E.; Chang, D.; Sugarbaker, P.H. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J. Surg. Oncol. 2004, 87, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Sefre, F.; Chandrakumaran, K.; Banerjee, S.; Sexton, R.; Thomas, J.M.; Moran, B. Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2005, 7, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Martinetti, A.; Seregni, E.; Laterza, B.; Oliva, D.G.; Deraco, M. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2007, 14, 2300–2308. [Google Scholar] [CrossRef]
- Delhorme, J.-B.; Villeneuve, L.; Bouché, O.; Averous, G.; Dohan, A.; Gornet, J.-M.; You, B.; Bibeau, F.; Dartigues, P.; Eveno, C.; et al. Appendiceal tumors and pseudomyxoma peritonei: French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (RENAPE, RENAPATH, SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR). Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2022, 54, 30–39. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann. Surg. 1994, 219, 109–111. [Google Scholar] [CrossRef]
- Kusamura, S.; Baratti, D.; Zaffaroni, N.; Villa, R.; Laterza, B.; Balestra, M.R. Pathophysiology and biology of peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2010, 2, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Yonemura, Y.; Endou, Y.; Nojima, N.; Kawamura, T.; Fujitia, H.; Kaji, M.; Ajisaka, H.; Bandou, E.; Sasaki, T.; Yamaguchi, T.; et al. A possible role of cytokines in the formation of peritoneal dissemination. Int. J. Oncol. 1997, 11, 349–358. [Google Scholar] [CrossRef]
- Jayne, D.G. The molecular biology of peritoneal carcinomatosis from gastrointestinal cancer. Ann. Acad. Med. Singap. 2003, 32, 219–225. [Google Scholar]
- Cortés-Guiral, D. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Primer. 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.G.; O’Leary, R.; Gill, A.; Hick, A.; Guillou, P.J. A three-dimensional in-vitro model for the study of peritoneal tumour metastasis. Clin. Exp. Metastasis 1999, 17, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Li, M.; Li, J.C. Recent advances in the research of lymphatic stomata. Anat. Rec. 2010, 293, 754–761. [Google Scholar] [CrossRef]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Tsukiyama, G.; Endou, Y.; Miura, M. The natural history of free cancer cells in the peritoneal cavity. In Advances in Peritoneal Surface Oncology; Gonzalez-Moreno, S., Ed.; Springer: Berlin, Germany, 2007; pp. 11–23. [Google Scholar]
- Sacchi, G.; Paolo, N.; Venezia, F.; Rossi, A.; Nicolai, G.A.; Garosi, G. Possible role of milky spots in mesothelial transplantation. Int. J. Artif. Organs. 2007, 30, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Chandramohan, A.; Moran, B. Pseudomyxoma peritonei: Natural history and treatment. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2017, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei after Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Floriano, I.; Silvinato, A.; Reis, J.C.; Cafalli, C.; Bernardo, W.M. Efficacy and safety in the use of intraperitoneal hyperthermia chemotherapy and peritoneal cytoreductive surgery for pseudomyxoma peritonei from appendiceal neoplasm: A systematic review. Clin. Sao Paulo Braz. 2022, 77, 100039. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Bakkers, C.; van Erning, F.N.; Burger, J.W.A.; Nienhuijs, S.W.; Simkens, G.A.A.M.; Creemers, G.-J.M.; Hemmer, P.H.J.; Punt, C.J.A.; Lemmens, V.E.P.P.; et al. Adjuvant Systemic Chemotherapy vs. Active Surveillance Following up-front Resection of Isolated Synchronous Colorectal Peritoneal Metastases. JAMA Oncol. 2020, 6, e202701. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A.; Blazer, D.G.; Kim, M.K.; Shen, P.; Stewart, J.H.; Guy, C.; Hsu, D.S. Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J. Am. Coll. Surg. 2012, 214, 599–606; discussion 606–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrantonio, F.; Perrone, F.; Mennitto, A.; Gleeson, E.M.; Milione, M.; Tamborini, E.; Busico, A.; Settanni, G.; Berenato, R.; Caporale, M.; et al. Toward the molecular dissection of peritoneal pseudomyxoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.A.; Compton, C.C.; Hanna, N.N.; Kosinski, L.A.; Washington, M.K.; Kakar, S.; Weiser, M.R.; Overman, M.J. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: Analysis of the National Cancer Data Base. Cancer 2016, 122, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Fields, A.C.; Meyerhardt, J.A.; Davids, J.S.; Shabat, G.; Bleday, R.; Goldberg, J.E.; Nash, G.M.; Melnitchouk, N. Systemic chemotherapy and survival in patients with metastatic low-grade appendiceal mucinous adenocarcinoma. J. Surg. Oncol. 2019, 120, 446–451. [Google Scholar] [CrossRef]
- Bijelic, L.; Kumar, A.S.; Stuart, O.A.; Sugarbaker, P.H. Systemic Chemotherapy Prior to Cytoreductive Surgery and HIPEC for Carcinomatosis from Appendix Cancer: Impact on Perioperative Outcomes and Short-Term Survival. Gastroenterol. Res. Pract. 2012, 2012, 163284. [Google Scholar] [CrossRef]
- Spiliotis, J.; Kopanakis, N.; Efstathiou, E.; Vassiliadou, D.; Argiriou, O.; Rogdakis, A.; Valavanis, C. Perioperative systemic chemotherapy for peritoneal mucinous appendiceal carcinomas treated with cytoreductive surgery & HIPEC. J. BUON Off. J. Balk. Union Oncol. 2017, 22, 783–789. [Google Scholar]
- Turner, K.M.; Hanna, N.N.; Zhu, Y.; Jain, A.; Kesmodel, S.B.; Switzer, R.A.; Taylor, L.M.; Richard Alexander, H. Assessment of neoadjuvant chemotherapy on operative parameters and outcome in patients with peritoneal dissemination from high-grade appendiceal cancer. Ann. Surg. Oncol. 2013, 20, 1068–1073. [Google Scholar] [CrossRef]
- Lieu, C.H.; Lambert, L.A.; Wolff, R.A.; Eng, C.; Zhang, N.; Wen, S.; Rafeeq, S.; Taggart, M.; Fournier, K.; Royal, R.; et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 652–658. [Google Scholar] [CrossRef]
- Milovanov, V.; Sardi, A.; Ledakis, P.; Aydin, N.; Nieroda, C.; Sittig, M.; Nunez, M.; Gushchin, V. Systemic chemotherapy (SC) before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with peritoneal mucinous carcinomatosis of appendiceal origin (PMCA). Eur. J. Surg. Oncol. 2015, 41, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I.; Russell, G.; Randle, R.W.; Shen, P.; Stewart, J.H.; Levine, E.A. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): Overview of 481 cases. Ann. Surg. Oncol. 2015, 22, 1274–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Lu, D.; Xue, S.; Fan, X.; Zhai, X.; Wang, C.; Xu, H.; Pang, S. Preoperative systemic chemotherapy does not benefit for appendiceal pseudomyxoma peritonei. ANZ J. Surg. 2023, 93, 219–226. [Google Scholar] [CrossRef]
- Blackham, A.U.; Swett, K.; Eng, C.; Sirintrapun, J.; Bergman, S.; Geisinger, K.R.; Votanopoulos, K.; Stewart, J.H.; Shen, P.; Levine, E.A. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2014, 109, 740–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronnett, B.M.; Zahn, C.M.; Kurman, R.J.; Kass, M.E.; Sugarbaker, P.H.; Shmookler, B.M. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am. J. Surg. Pathol. 1995, 19, 1390–1408. [Google Scholar] [CrossRef]
- Misdraji, J.; Yantiss, R.K.; Graeme-Cook, F.M.; Balis, U.J.; Young, R.H. Appendiceal mucinous neoplasms: A clinicopathologic analysis of 107 cases. Am. J. Surg. Pathol. 2003, 27, 1089–1103. [Google Scholar] [CrossRef]
- Bradley, R.F.; Stewart, J.H.; Russell, G.B.; Levine, E.A.; Geisinger, K.R. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am. J. Surg. Pathol. 2006, 30, 551–559. [Google Scholar] [CrossRef]
- Carr, N.J.; Sobin, L.H. Adenocarcinoma of the Appendix. In WHO Classification of Tumors of the Digestive System; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; WHO: Lyon, French, 2010. [Google Scholar]
- Carr, N.J.; Finch, J.; Ilesley, I.C.; Chandrakumaran, K.; Mohamed, F.; Mirnezami, A.; Cecil, T.; Moran, B. Pathology and prognosis in pseudomyxoma peritonei: A review of 274 cases. J. Clin. Pathol. 2012, 65, 919–923. [Google Scholar] [CrossRef]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. (Eds.) . AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; ISBN 978-0-387-88442-4. [Google Scholar]
- Milovanov, V.; Sardi, A.; Studeman, K.; Nieroda, C.; Sittig, M.; Gushchin, V. The 7th Edition of the AJCC Staging Classification Correlates with Biologic Behavior of Mucinous Appendiceal Tumor with Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC). Ann. Surg. Oncol. 2016, 23, 1928–1933. [Google Scholar] [CrossRef]
- Overman, M.J.; Fournier, K.; Hu, C.-Y.; Eng, C.; Taggart, M.; Royal, R.; Mansfield, P.; Chang, G.J. Improving the AJCC/TNM staging for adenocarcinomas of the appendix: The prognostic impact of histological grade. Ann. Surg. 2013, 257, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Davison, J.M.; Choudry, H.A.; Pingpank, J.F.; Ahrendt, S.A.; Holtzman, M.P.; Zureikat, A.H.; Zeh, H.J.; Ramalingam, L.; Zhu, B.; Nikiforova, M.; et al. Clinicopathologic and molecular analysis of disseminated appendiceal mucinous neoplasms: Identification of factors predicting survival and proposed criteria for a three-tiered assessment of tumor grade. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Ltd. 2014, 27, 1521–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; González-Moreno, S.; Taflampas, P.; Chapman, S.; Moran, B.J.; Peritoneal Surface Oncology Group International. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am. J. Surg. Pathol. 2016, 40, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Natarajan, B.; Thomas, P.; Govindarajan, V.; Sharma, P.; Loggie, B. Proposed classification of pseudomyxoma peritonei: Influence of signet ring cells on survival. Am. Surg. 2013, 79, 1171–1176. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Milione, M.; Bruno, F.; Guaglio, M.; Deraco, M. Validation of the Recent PSOGI Pathological Classification of Pseudomyxoma Peritonei in a Single-Center Series of 265 Patients Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2018, 25, 404–413. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Klimstra, D.S.; Washington, M.K. Tumours of the appendix, WHO Classification of Tumours: Digestive System Tumours, 5th ed; International Agency for Research on Cancer: Lyon, France, 2019.
- Gonzalez, R.S. WHO Classification. 2023. Available online: PathologyOutlines.com (accessed on 5 April 2023).
- Pai, R.K.; Beck, A.H.; Norton, J.A.; Longacre, T.A. Appendiceal mucinous neoplasms: Clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am. J. Surg. Pathol. 2009, 33, 1425–1439. [Google Scholar] [CrossRef]
- Sirintrapun, S.J.; Blackham, A.U.; Russell, G.; Votanopoulos, K.; Stewart, J.H.; Shen, P.; Levine, E.A.; Geisinger, K.R.; Bergman, S. Significance of signet ring cells in high-grade mucinous adenocarcinoma of the peritoneum from appendiceal origin. Hum. Pathol. 2014, 45, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, W.; Sardi, A.; Nieroda, C.; Sittig, M.; Milovanov, V.; Nunez, M.; Aydin, N.; Gushchin, V. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 4218–4225. [Google Scholar] [CrossRef]
- Munoz-Zuluaga, C.; Sardi, A.; King, M.C.; Nieroda, C.; Sittig, M.; MacDonald, R.; Gushchin, V. Outcomes in Peritoneal Dissemination from Signet Ring Cell Carcinoma of the Appendix Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 473–481. [Google Scholar] [CrossRef]
- Solomon, D.; DeNicola, N.; Feingold, D.; Liu, P.H.; Aycart, S.; Golas, B.J.; Sarpel, U.; Labow, D.M.; Magge, D.R. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J. Surg. Oncol. 2019, 119, 758–765. [Google Scholar] [CrossRef]
- Ihemelandu, C.; Sugarbaker, P.H. Clinicopathologic and Prognostic Features in Patients with Peritoneal Metastasis from Mucinous Adenocarcinoma, Adenocarcinoma with Signet Ring Cells, and Adenocarcinoid of the Appendix Treated with Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Legué, L.M.; van Erning, F.N.; Creemers, G.-J.; de Hingh, I.H.J.T.; Lemmens, V.E.P.P.; Huysentruyt, C.J. The prognostic relevance of histologic subtype in appendiceal adenocarcinoma. Eur. J. Surg. Oncol. 2020, 46, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Levinsky, N.C.; Morris, M.C.; Wima, K.; Sussman, J.J.; Ahmad, S.A.; Cloyd, J.M.; Kimbrough, C.; Fournier, K.; Lee, A.; Dineen, S.; et al. Should We Be Doing Cytoreductive Surgery with HIPEC for Signet Ring Cell Appendiceal Adenocarcinoma? A Study from the US HIPEC Collaborative. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Kim, M.; Kim, J.; Kim, C.W.; Ha, Y.J.; Kim, S.-Y.; Cho, D.-H.; Kim, J.C. Evaluation of the significance of pseudomyxoma peritonei patients based on the Peritoneal Surface Oncology Group International (PSOGI) classification. Asian J. Surg. 2021, 44, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Alzahrani, N.A.; Chua, T.C.; Morris, D.L. Histological Subtype Remains a Significant Prognostic Factor for Survival Outcomes in Patients with Appendiceal Mucinous Neoplasm with Peritoneal Dissemination. Dis. Colon Rectum 2017, 60, 360–367. [Google Scholar] [CrossRef]
- Martín-Román, L.; Lozano, P.; Vásquez, W.; Palencia, N.; Gómez, Y.; Fernández-Aceñero, M.J.; González-Bayón, L. Defining stage in mucinous tumours of the appendix with peritoneal dissemination: The importance of grading terminology: Systematic review. BJS Open 2021, 5, zrab059. [Google Scholar] [CrossRef]
- van Eden, W.J.; Kok, N.F.M.; Snaebjornsson, P.; Jóźwiak, K.; Woensdregt, K.; Bottenberg, P.D.; Boot, H.; Aalbers, A.G.J. Factors influencing long-term survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei originating from appendiceal neoplasms. BJS Open 2019, 3, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Reghunathan, M.; Kelly, K.J.; Valasek, M.A.; Lowy, A.M.; Baumgartner, J.M. Histologic Predictors of Recurrence in Mucinous Appendiceal Tumors with Peritoneal Dissemination after HIPEC. Ann. Surg. Oncol. 2018, 25, 702–708. [Google Scholar] [CrossRef]
- Choudry, H.A.; Pai, R.K.; Shuai, Y.; Ramalingam, L.; Jones, H.L.; Pingpank, J.F.; Ahrendt, S.S.; Holtzman, M.P.; Zureikat, A.H.; Zeh, H.J.; et al. Impact of Cellularity on Oncologic Outcomes Following Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion for Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2018, 25, 76–82. [Google Scholar] [CrossRef]
- Solomon, D. Surveillance of Low-Grade Appendiceal Mucinous Neoplasms with Peritoneal Metastases after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Are 5 Years Enough? A Multisite Experience. Ann. Surg. Oncol. 2020, 27, 147–153. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Martín-Román, L. Which classification system defines best prognosis of mucinous neoplasms of the appendix with peritoneal dissemination: TNM vs. PSOGI? J. Clin. Pathol. 2021, 76, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.K. Wittekind, Christian, Union for International Cancer Control TNM Classification of Malignant Tumours; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-26357-9. [Google Scholar]
- Carr, N.J.; McCarthy, W.F.; Sobin, L.H. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer 1995, 75, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Ronnett, B.M.; Yan, H.; Kurman, R.J.; Shmookler, B.M.; Wu, L.; Sugarbaker, P.H. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer 2001, 92, 85–91. [Google Scholar] [CrossRef]
- Govaerts, K.; Chandrakumaran, K.; Carr, N.J.; Cecil, T.D.; Dayal, S.; Mohamed, F.; Thrower, A.; Moran, B.J. Single centre guidelines for radiological follow-up based on 775 patients treated by cytoreductive surgery and HIPEC for appendiceal pseudomyxoma peritonei. Eur. J. Surg. Oncol. 2018, 44, 1371–1377. [Google Scholar] [CrossRef]
- Evans, T.; Aziz, O.; Chakrabarty, B.; Wilson, M.S.; Malcomson, L.; Lavelle, C.; O’Dwyer, S.T. Long-term outcomes for patients with peritoneal acellular mucinosis secondary to low grade appendiceal mucinous neoplasms. Eur. J. Surg. Oncol. 2021, 47, 188–193. [Google Scholar] [CrossRef]
- Wang, B.; Ma, R.; Rao, B.; Xu, H. Serum and ascites tumor markers in the diagnostic and prognostic prediction for appendiceal pseudomyxoma peritonei. BMC Cancer 2023, 23, 90. [Google Scholar] [CrossRef]
- Arjona-Sánchez, Á.; Martínez-López, A.; Valenzuela-Molina, F.; Rufián-Andújar, B.; Rufián-Peña, S.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Medina-Fernández, F.J.; Díaz-López, C.; et al. A Proposal for Modification of the PSOGI Classification According to the Ki-67 Proliferation Index in Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2022, 29, 126–136. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Martinez-López, A.; Moreno-Montilla, M.T.; Mulsow, J.; Lozano-Lominchar, P.; Martínez-Torres, B.; Rau, B.; Canbay, E.; Sommariva, A.; Milione, M.; et al. External multicentre validation of pseudomyxoma peritonei PSOGI-Ki67 classification. Eur. J. Surg. Oncol. 2023, S0748798323003773. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Misdraji, J.; van Velthuysen, M.-L.F.; Shia, J.; Taggart, M.W.; Yantiss, R.K.; Svrcek, M.; Carr, N. Acellular mucin in pseudomyxoma peritonei of appendiceal origin: What is adequate sampling for histopathology? J. Clin. Pathol. 2020, 73, 220–222. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Bayón, L.; Martín Román, L.; Lominchar, P.L. Appendiceal Mucinous Neoplasms: From Clinic to Pathology and Prognosis. Cancers 2023, 15, 3426. https://doi.org/10.3390/cancers15133426
González Bayón L, Martín Román L, Lominchar PL. Appendiceal Mucinous Neoplasms: From Clinic to Pathology and Prognosis. Cancers. 2023; 15(13):3426. https://doi.org/10.3390/cancers15133426
Chicago/Turabian StyleGonzález Bayón, Luis, Lorena Martín Román, and Pablo Lozano Lominchar. 2023. "Appendiceal Mucinous Neoplasms: From Clinic to Pathology and Prognosis" Cancers 15, no. 13: 3426. https://doi.org/10.3390/cancers15133426
APA StyleGonzález Bayón, L., Martín Román, L., & Lominchar, P. L. (2023). Appendiceal Mucinous Neoplasms: From Clinic to Pathology and Prognosis. Cancers, 15(13), 3426. https://doi.org/10.3390/cancers15133426






