Regulation and Functions of α6-Integrin (CD49f) in Cancer Biology
Abstract
:Simple Summary
Abstract
1. Introduction
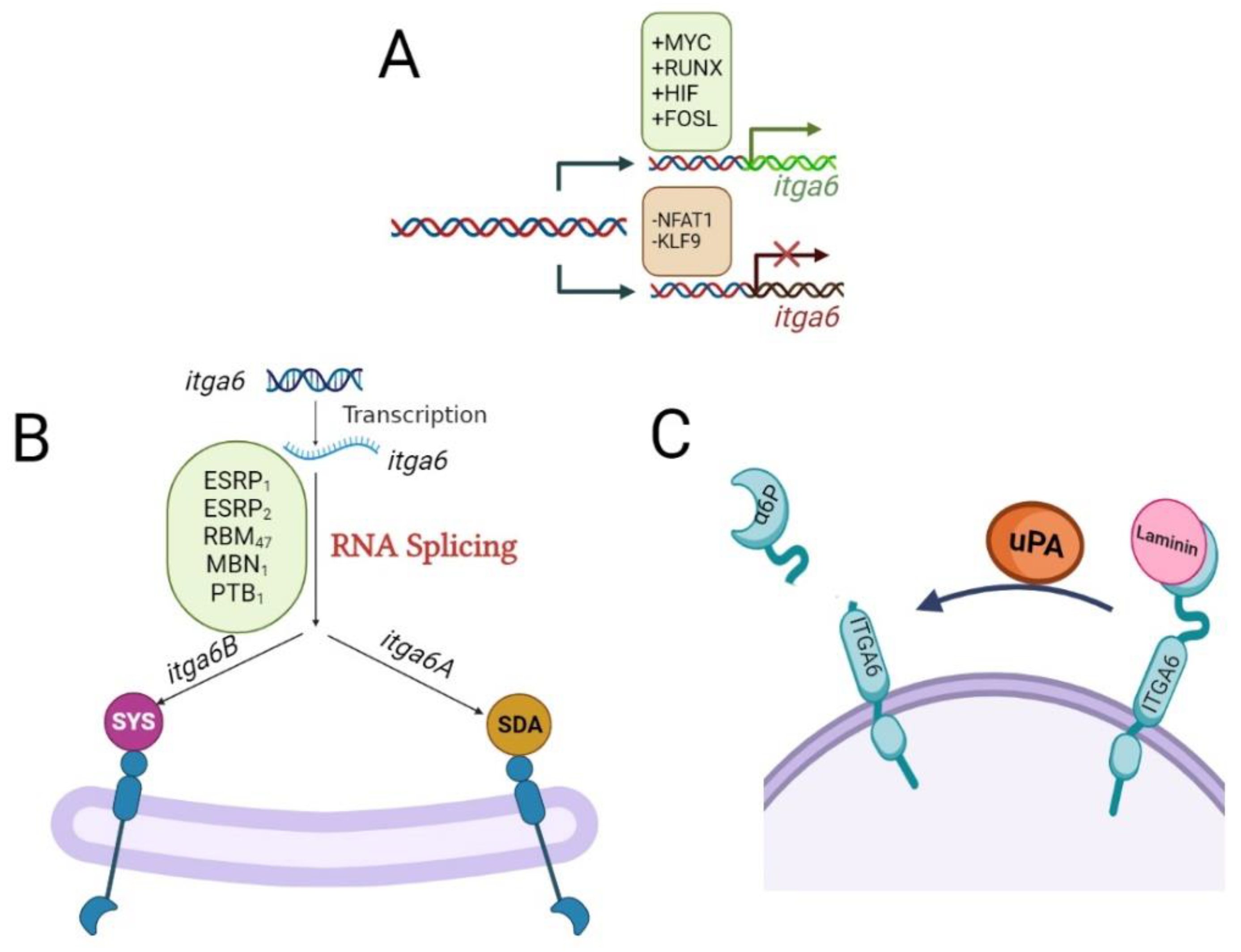
2. Molecular Mechanisms Regulating the Expression of ITGA6
2.1. Transcriptional Regulation of itga6 by Transcription Factor
2.2. Alternative Splicing Regulation of itga6
2.3. Regulation of itga6 by MicroRNAs
2.4. N6-Methyladenosine Modification of itga6 mRNA
3. Overview of Integrin Signaling
3.1. ITGA6 Integrin Signaling
3.2. Crosstalk between ITGA6 and Other Signaling Pathways in Cancer
4. ITGA6 as Biomarker of Cancer Stem Cells
4.1. ITGA6-Driven Signals of Stemness
4.2. ITGA6 and Epithelial-Mesenchymal Transition (EMT)
4.3. Changes in Matrix Composition/Structure and ITGA6 Expression
5. Involvement of ITGA6 in Cancer Metastasis
5.1. ITGA6 Role in Hypoxia
5.2. ITGA6 and Angiogenesis
5.3. ITGA6 in Extracellular Vesicles
5.4. ITGA6 and Metastasis to the Central Nervous System (CNS) of Acute Lymphoblastic Leukemia (ALL) Patients
6. ITGA6 as Potential Diagnostic Biomarker
7. ITGA6 and Cancer Drug Resistance
7.1. ITGA6 and Minimum Residual Disease
7.2. ITGA6 Role in Autophagy
8. Potential Therapeutics of Targeting ITGA6
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, M.Z. Integrin signaling in epithelial cells. Cancer Lett. 2007, 247, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, F.M. Role of integrins in regulating epidermal adhesion, growth and differentiation. Embo J. 2002, 21, 3919–3926. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, Y.; Chen, G.; Yang, X.; Hu, J.; Zhang, X.; Feng, G.; Wang, H. Integrin α6-Targeted Molecular Imaging of Central Nervous System Leukemia in Mice. Front. Bioeng. Biotechnol. 2022, 10, 812277. [Google Scholar] [CrossRef]
- You, G.R.; Chang, J.T.; Li, Y.L.; Chen, Y.J.; Huang, Y.C.; Fan, K.H.; Chen, Y.C.; Kang, C.J.; Cheng, A.J. Molecular Interplays between Cell Invasion and Radioresistance That Lead to Poor Prognosis in Head-Neck Cancer. Front. Oncol. 2021, 11, 681717. [Google Scholar] [CrossRef]
- Zhou, Z.; Qu, J.; He, L.; Peng, H.; Chen, P.; Zhou, Y. α6-Integrin alternative splicing: Distinct cytoplasmic variants in stem cell fate specification and niche interaction. Stem Cell Res. Ther. 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Ying, X.; Que, B.; Wang, X.; Chao, Y.; Zhang, H.; Yuan, Z.; Qi, D.; Lin, S.; Min, W.; et al. N6-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine 2019, 47, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Marsico, G.; Russo, L.; Quondamatteo, F.; Pandit, A. Glycosylation and integrin regulation in cancer. Trends Cancer 2018, 4, 537–552. [Google Scholar] [CrossRef]
- Shishido, S.; Bönig, H.; Kim, Y.M. Role of integrin alpha4 in drug resistance of leukemia. Front. Oncol. 2014, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Göttgens, E.L.; Span, P.N.; Zegers, M.M. Roles and Regulation of Epithelial Splicing Regulatory Proteins 1 and 2 in Epithelial-Mesenchymal Transition. Int. Rev. Cell. Mol. Biol. 2016, 327, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Li, J.Q.; Zhang, L.L.; Wang, H.; Wang, F.H.; Tao, Y.W.; Wang, Y.Q.; Guo, Q.R.; Li, J.J.; Liu, Y.; et al. The Biological Functions and Clinical Applications of Integrins in Cancers. Front. Pharmacol. 2020, 11, 579068. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Blazejewski, S.M.; Bennison, S.A.; Ha, N.T.; Liu, X.N.; Smith, T.H.; Dougherty, K.J.; Toyo-Oka, K. Rpsa Signaling Regulates Cortical Neuronal Morphogenesis via Its Ligand, PEDF, and Plasma Membrane Interaction Partner, Itga6. Cereb. Cortex 2022, 32, 770–795. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Kim, H.N.; Hsieh, Y.T.; Ruan, Y.; Ogana, H.A.; Lee, S.; Pham, J.; Geng, H.; Park, E.; Klemm, L.; et al. Integrin a6 mediates the drug resistance of acute lymphoblastic B-cell leukemia. Blood 2020, 136, 210–223. [Google Scholar] [CrossRef]
- Grenier, J.M.P.; Testut, C.; Fauriat, C.; Mancini, S.J.C.; Aurrand-Lions, M. Adhesion Molecules Involved in Stem Cell Niche Retention During Normal Haematopoiesis and in Acute Myeloid Leukaemia. Front. Immunol. 2021, 12, 756231. [Google Scholar] [CrossRef]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A.; Jurisica, I.; Dick, J.E. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef]
- Landowski, T.H.; Gard, J.; Pond, E.; Pond, G.D.; Nagle, R.B.; Geffre, C.P.; Cress, A.E. Targeting Integrin alpha 6 Stimulates Curative-Type Bone Metastasis Lesions in a Xenograft Model. Mol. Cancer Ther. 2014, 13, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Golbert, D.C.F.; Santana-Van-Vliet, E.; Ribeiro-Alves, M.; da Fonseca, M.M.B.; Lepletier, A.; Mendes-da-Cruz, D.A.; Loss, G.; Cotta-de-Almeida, V.; Vasconcelos, A.T.R.; Savino, W. Small interference ITGA6 gene targeting in the human thymic epithelium differentially regulates the expression of immunological synapse-related genes. Cell Adhes. Migr. 2018, 12, 152–167. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.Q.; Jiang, C.H.; Li, R.F. Integrin and gene network analysis reveals that ITGA5 and ITGB1 are prognostic in non-small-cell lung cancer. Oncotargets Ther. 2016, 9, 2317–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, N.; Kaneda, K.; Saito, Y.; Ichihara, E.; Morishita, K. The Increased Expression of Integrin alpha 6 (ITGA6) Enhances Drug Resistance in EVI1(high) Leukemia. PLoS ONE 2012, 7, e30706. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.H.; Li, H.; Wang, M.Z.; Ju, S.G.; Li, F.Y.; Chen, P.F.; Lu, H.B.; Han, X.W.; Ren, J.Z. PSMC2/ITGA6 axis plays critical role in the development and progression of hepatocellular carcinoma. Cell Death Discov. 2021, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, J.; Su, Q.; Zhang, W.M.; Jiang, Y.X.; Fan, G.; Qian, C.; Li, B.Z.; Zhuang, W.Z. Downregulation of ITGA6 confers to the invasion of multiple myeloma and promotes progression to plasma cell leukaemia. Br. J. Cancer 2021, 124, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Kareddula, A.; Medina, D.J.; Petrosky, W.; Dolfi, S.; Tereshchenko, I.; Walton, K.; Aviv, H.; Sadimin, E.; Tabakin, A.L.; Singer, E.A.; et al. The role of chromodomain helicase DNA binding protein 1 (CHD1) in promoting an invasive prostate cancer phenotype. Ther. Adv. Urol. 2021, 13, 17562872211022462. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Z.; Huang, L.T.; Wang, J.H. Parthenolide leads to proteomic differences in thyroid cancer cells and promotes apoptosis. BMC Complement. Med. Ther. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- De Arcangelis, A.; Hamade, H.; Alpy, F.; Normand, S.; Bruyère, E.; Lefebvre, O.; Méchine-Neuville, A.; Siebert, S.; Pfister, V.; Lepage, P. Hemidesmosome integrity protects the colon against colitis and colorectal cancer. Gut 2017, 66, 1748–1760. [Google Scholar] [CrossRef]
- de Melker, A.A.; Sonnenberg, A. Integrins: Alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays 1999, 21, 499–509. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrin alpha 6 beta 4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker. Cancers 2020, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Pulkkinen, L.; Kimonis, V.E.; Xu, Y.; Spanou, E.N.; McLean, W.I.; Uitto, J. Homozygous α6 integrin mutation in junctional epidermolysis bullosa with congenital duodenal atresia. Hum. Mol. Genet. 1997, 6, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Peacock, D.L.; Schwab, L.P.; Seagroves, T.N. ITGA6 (CD49F) is directly regulated by hypoxia-inducible factors. Cancer Res. 2014, 74, 3885. [Google Scholar] [CrossRef]
- Groulx, J.F.; Boudjadi, S.; Beaulieu, J.F. MYC Regulates alpha 6 Integrin Subunit Expression and Splicing Under Its Pro-Proliferative ITGA6A Form in Colorectal Cancer Cells. Cancers 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozaka, Y.; Seki, N.; Tanaka, T.; Asai, S.; Moriya, S.; Idichi, T.; Wada, M.; Tanoue, K.; Kawasaki, Y.; Mataki, Y.; et al. Molecular Pathogenesis and Regulation of the miR-29-3p-Family: Involvement of ITGA6 and ITGB1 in Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Z.Q.; Yuan, Y.W.; Zhang, P.P.; Li, Y.Q.; Wang, Y.Q.; Tang, X.R.; Wen, X.; Hong, X.H.; Lei, Y.; et al. NFAT1 Hypermethylation Promotes Epithelial-Mesenchymal Transition and Metastasis in Nasopharyngeal Carcinoma by Activating ITGA6 Transcription. Neoplasia 2019, 21, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Hannigan, M.M.; Ingles, N.N.; Webb, B.M.; Weber-Bonk, K.L.; Yu, P.; Bebek, G.; Singh, S.; Sizemore, S.T.; Varadan, V.; et al. The transcriptional repressor BCL11A promotes breast cancer metastasis. J. Biol. Chem. 2020, 295, 11707–11719. [Google Scholar] [CrossRef]
- Tani, T.T.; Mercurio, A.M. PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J. Biol. Chem. 2001, 276, 36535–36542. [Google Scholar] [CrossRef] [Green Version]
- Villa-Diaz, L.G.; Kim, J.K.; Laperle, A.; Palecek, S.P.; Krebsbach, P.H. Inhibition of Focal Adhesion Kinase Signaling by Integrin α6β1 Supports Human Pluripotent Stem Cell Self-Renewal. Stem Cells 2016, 34, 1753–1764. [Google Scholar] [CrossRef]
- Krebsbach, P.H.; Villa-Diaz, L.G. The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017, 26, 1090–1099. [Google Scholar] [CrossRef]
- Goel, H.L.; Gritsko, T.; Pursell, B.; Chang, C.; Shultz, L.D.; Greiner, D.L.; Norum, J.H.; Toftgard, R.; Shaw, L.M.; Mercurio, A.M. Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 2014, 7, 747–761. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.H.; Yang, Z.L.; Zhou, E.X.; Miao, X.Y.; Zou, Q.; Li, J.H.; Liang, L.F.; Zeng, G.X.; Chen, S.L. Overexpression of Thy1 and ITGA6 is associated with invasion, metastasis and poor prognosis in human gallbladder carcinoma. Oncol. Lett. 2016, 12, 5136–5144. [Google Scholar] [CrossRef] [Green Version]
- Bigoni-Ordóñez, G.D.; Czarnowski, D.; Parsons, T.; Madlambayan, G.J.; Villa-Diaz, L.G. Integrin α6 (CD49F), the microenvironment and cancer stem cells. Curr. Stem Cell Res. Ther. 2019, 14, 428–436. [Google Scholar] [CrossRef]
- Xie, S.L.; Yu, X.; Li, Y.R.; Ma, H.Y.; Fan, S.; Chen, W.X.; Pan, G.K.; Wang, W.W.; Zhang, H.Q.; Li, J.S.; et al. Upregulation of lncRNA ADAMTS9-AS2 Promotes Salivary Adenoid Cystic Carcinoma Metastasis via PI3K/Akt and MEK/Erk Signaling. Mol. Ther. 2018, 26, 2766–2778. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.C.; Liu, Z.; Yang, Y.J.; Guo, M.; Zhang, J.Q.; Zheng, J.F. KDM5B promotes self-renewal of hepatocellular carcinoma cells through the microRNA-448-mediated YTHDF3/ITGA6 axis. J. Cell. Mol. Med. 2021, 25, 5949–5962. [Google Scholar] [CrossRef]
- Cataldo, A.; Romero-Cordoba, S.; Plantamura, I.; Cosentino, G.; Hidalgo-Miranda, A.; Tagliabue, E.; Iorio, M.V. MiR-302b as a Combinatorial Therapeutic Approach to Improve Cisplatin Chemotherapy Efficacy in Human Triple-Negative Breast Cancer. Cancers 2020, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhang, X.; Zhao, X. Long non-coding RNA OIP5-AS1 suppresses microRNA-92a to augment proliferation and metastasis of ovarian cancer cells through upregulating ITGA6. J. Ovarian Res. 2022, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Laudato, S.; Patil, N.; Abba, M.L.; Leupold, J.H.; Benner, A.; Gaiser, T.; Marx, A.; Allgayer, H. P53-induced miR-30e-5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int. J. Cancer 2017, 141, 1879–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Yang, P.; Zhong, C.; Shen, X.; Shi, X.; Li, X. The circ-PITX1 promotes non-small cell lung cancer development via the miR-30e-5p/ITGA6 axis. Cell Cycle 2022, 21, 304–321. [Google Scholar] [CrossRef]
- Li, B.; Jiang, J.; Assaraf, Y.G.; Xiao, H.; Chen, Z.S.; Huang, C. Surmounting cancer drug resistance: New insights from the perspective of N6-methyladenosine RNA modification. Drug Resist. Updat. 2020, 53, 100720. [Google Scholar] [CrossRef]
- Yi, W.F.; Yu, Y.; Li, Y.F.; Yang, J.; Gao, S.Y.; Xu, L.F. The tumor-suppressive effects of alpha-ketoglutarate-dependent dioxygenase FTO via N6-methyladenosine RNA methylation on bladder cancer patients. Bioengineered 2021, 12, 5323–5333. [Google Scholar] [CrossRef]
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin α2 in cell adhesion and disease. Genes Dis. 2019, 6, 16–24. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as attractive targets for cancer therapeutics. Acta Pharm. Sin. B 2021, 11, 2726–2737. [Google Scholar] [CrossRef]
- McDonald, P.C.; Dedhar, S. New Perspectives on the Role of Integrin-Linked Kinase (ILK) Signaling in Cancer Metastasis. Cancers 2022, 14, 3209. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Vite, L.; Weber-Bonk, K.L.; Abdul-Karim, F.W.; Booth, C.N.; Keri, R.A. Focal Adhesion Kinase Provides a Collateral Vulnerability That Can Be Leveraged to Improve mTORC1 Inhibitor Efficacy. Cancers 2022, 14, 3374. [Google Scholar] [CrossRef]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoudjit, F.; Vuori, K. Integrin signaling in cancer cell survival and chemoresistance. Chemother. Res. Pract. 2012, 2012, 283181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Zhou, R.; Zhao, Y.X.; Wu, G. Integrin alpha 6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci. Rep. 2016, 6, 33376. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Kariya, Y.; Kariya, Y.; Gu, J. Roles of Integrin α6β4 Glycosylation in Cancer. Cancers 2017, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- An, J.S.; Moon, J.H.; Kim, C.; No, J.K.; Eun, Y.G.; Lim, Y.C. Integrin alpha 6 as a stemness driver is a novel promising target for HPV (+) head and neck squamous cell carcinoma. Exp. Cell Res. 2021, 407, 112815. [Google Scholar] [CrossRef]
- Yao, H.; Price, T.T.; Cantelli, G.; Ngo, B.; Warner, M.J.; Olivere, L.; Ridge, S.M.; Jablonski, E.M.; Therrien, J.; Tannheimer, S.; et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 2018, 560, 55–60. [Google Scholar] [CrossRef]
- Kim, H.N.; Ruan, Y.; Ogana, H.; Kim, Y.M. Cadherins, Selectins, and Integrins in CAM-DR in Leukemia. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Fujita, M.; Ieguchi, K.; Davari, P.; Yamaji, S.; Taniguchi, Y.; Sekiguchi, K.; Takada, Y.K.; Takada, Y. Cross-talk between integrin α6β4 and insulin-like growth factor-1 receptor (IGF1R) through direct α6β4 binding to IGF1 and subsequent α6β4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions. J. Biol. Chem. 2012, 287, 12491–12500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lottini, T.; Duranti, C.; Iorio, J.; Martinelli, M.; Colasurdo, R.; D’Alessandro, F.N.; Buonamici, M.; Coppola, S.; Devescovi, V.; La Vaccara, V.; et al. Combination Therapy with a Bispecific Antibody Targeting the hERG1/β1 Integrin Complex and Gemcitabine in Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 2013. [Google Scholar] [CrossRef]
- Yoon, S.-O.; Shin, S.; Lipscomb, E.A. A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: The α6β4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res. 2006, 66, 2732–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski-Chauvel, A.; Gouaze-Andersson, V.; Baricault, L.; Martin, E.; Delmas, C.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. Alpha6-integrin regulates FGFR1 expression through the ZEB1/YAP1 transcription complex in glioblastoma stem cells resulting in enhanced proliferation and stemness. Cancers 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedano Prieto, D.M.; Cheng, Y.; Chang, C.C.; Yu, J.; Takada, Y.K.; Takada, Y. Direct integrin binding to insulin-like growth factor-2 through the C-domain is required for insulin-like growth factor receptor type 1 (IGF1R) signaling. PLoS ONE 2017, 12, e0184285. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Zhang, J.; Yarden, Y.; Fu, L. The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduct. Target. Ther. 2021, 6, 109. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Okoye, I.; Esmaily, M.; Ghasemi Chaleshtari, M.; Masjedi, A.; Azizi, G.; Irandoust, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sci. 2019, 237, 116952. [Google Scholar] [CrossRef]
- Senft, D.; Jeremias, I. A rare subgroup of leukemia stem cells harbors relapse-inducing potential in acute lymphoblastic leukemia. Exp. Hematol. 2019, 69, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907. [Google Scholar] [CrossRef]
- Zhang, K.; Myllymaki, S.M.; Gao, P.; Devarajan, R.; Kytola, V.; Nykter, M.; Wei, G.H.; Manninen, A. Oncogenic K-Ras upregulates ITGA6 expression via FOSL1 to induce anoikis resistance and synergizes with alpha V-Class integrins to promote EMT. Oncogene 2017, 36, 5681–5694. [Google Scholar] [CrossRef] [Green Version]
- Toledo-Guzmán, M.E.; Bigoni-Ordóñez, G.D.; Ibáñez Hernández, M.; Ortiz-Sánchez, E. Cancer stem cell impact on clinical oncology. World J. Stem Cells 2018, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; de-Maya-Girones, J.D.; Calabuig-Farinas, S.; Lucas, R.; Martinez, A.; Pardo-Sanchez, J.M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M.; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanzani, E.; Pedrosa, L.; Bourmeau, G.; Anezo, O.; Noguera-Castells, A.; Esteve-Codina, A.; Passoni, L.; Matteoli, M.; de la Iglesia, N.; Seano, G.; et al. Dual Role of Integrin Alpha-6 in Glioblastoma: Supporting Stemness in Proneural Stem-Like Cells While Inducing Radioresistance in Mesenchymal Stem-Like Cells. Cancers 2021, 13, 3055. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Ohkuma, M.; Sakashita, H.; Matsuzaki, S.; Tanaka, F.; Mimori, K.; Kamohara, Y.; Inoue, H.; Mori, M. CD133+ CD44+ population efficiently enriches colon cancer initiating cells. Ann. Surg. Oncol. 2008, 15, 2927–2933. [Google Scholar] [CrossRef]
- Haraguchi, N.; Ishii, H.; Mimori, K.; Ohta, K.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Mizushima, T.; Yamamoto, H. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. Int. J. Oncol. 2013, 43, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Wazir, U.; Orakzai, M.; Martin, T.A.; Jiang, W.G.; Mokbel, K. Correlation of TERT and Stem Cell Markers in the Context of Human Breast Cancer. Cancer Genom. Proteom. 2019, 16, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Ying, M.Y.; Tilghman, J.; Wei, Y.Y.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Ji, H.K.; Laterra, J. Kruppel-like Factor-9 (KLF9) Inhibits Glioblastoma Stemness through Global Transcription Repression and Integrin alpha 6 Inhibition. J. Biol. Chem. 2014, 289, 32742–32756. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Nicolau, N.; de la Torre, R.M.; Farinas, O.; Savio, A.; Vilarrodona, A.; Casaroli-Marano, R.P. Extrinsic modulation of integrin alpha 6 and progenitor cell behavior in mesenchymal stem cells. Stem Cell Res. 2020, 47, 101899. [Google Scholar] [CrossRef]
- Almotiri, A.; Alzahrani, H.; Menendez-Gonzalez, J.B.; Abdelfattah, A.; Alotaibi, B.; Saleh, L.; Greene, A.; Georgiou, M.; Gibbs, A.; Alsayari, A.; et al. Zeb1 modulates hematopoietic stem cell fates required for suppressing acute myeloid leukemia. J. Clin. Investig. 2021, 131, e129115. [Google Scholar] [CrossRef]
- Tokumo, K.; Masuda, T.; Nakashima, T.; Namba, M.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; et al. Association between Plasminogen Activator Inhibitor-1 and Osimertinib Tolerance in EGFR-Mutated Lung Cancer via Epithelial-Mesenchymal Transition. Cancers 2023, 15, 1092. [Google Scholar] [CrossRef]
- Okuyama, K.; Suzuki, K.; Yanamoto, S. Relationship between Tumor Budding and Partial Epithelial-Mesenchymal Transition in Head and Neck Cancer. Cancers 2023, 15, 1111. [Google Scholar] [CrossRef]
- Li, T.; Wan, Y.C.; Su, Z.Y.; Li, J.Y.; Han, M.N.; Zhou, C.Y. Mesenchymal Stem Cell-Derived Exosomal microRNA-3940-5p Inhibits Colorectal Cancer Metastasis by Targeting Integrin alpha 6. Dig. Dis. Sci. 2021, 66, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Morgner, J.; Ghatak, S.; Jakobi, T.; Dieterich, C.; Aumailley, M.; Wickström, S.A. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat. Commun. 2015, 6, 8198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laperle, A.; Hsiao, C.; Lampe, M.; Mortier, J.; Saha, K.; Palecek, S.P.; Masters, K.S. α-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 2015, 5, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ido, H.; Harada, K.; Yagi, Y.; Sekiguchi, K. Probing the integrin-binding site within the globular domain of laminin-511 with the function-blocking monoclonal antibody 4C7. Matrix Biol. 2006, 25, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escobar, B.; De Felipe, B.; Sanchez-Alcazar, J.A.; Sasaki, T.; Copp, A.J.; Ybot-Gonzalez, P. Laminin and integrin expression in the ventral ectodermal ridge of the mouse embryo: Implications for regulation of BMP signalling. Dev. Dyn. 2012, 241, 1808–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Goel, H.L.; Gao, H.; Pursell, B.; Shultz, L.D.; Greiner, D.L.; Ingerpuu, S.; Patarroyo, M.; Cao, S.; Lim, E. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes Dev. 2015, 29, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Nagpal, A.; Needham, K.; Lane, D.J.R.; Ayton, S.; Redvers, R.P.; John, M.; Selistre-de-Araujo, H.S.; Denoyer, D.; Pouliot, N. Integrin αvβ3 Is a Master Regulator of Resistance to TKI-Induced Ferroptosis in HER2-Positive Breast Cancer. Cancers 2023, 15, 1216. [Google Scholar] [CrossRef]
- Chen, L.; Bao, L.; Niu, Y.L.; Wang, J.E.; Kumar, A.; Xing, C.; Wang, Y.F.; Luo, W.B. LncIHAT Is Induced by Hypoxia-Inducible Factor 1 and Promotes Breast Cancer Progression. Mol. Cancer Res. 2021, 19, 678–687. [Google Scholar] [CrossRef]
- Lin, T.; Cheng, H.; Liu, D.; Wen, L.; Kang, J.; Xu, L.; Shan, C.; Chen, Z.; Li, H.; Lai, M.; et al. A Novel Six Autophagy-Related Genes Signature Associated with Outcomes and Immune Microenvironment in Lower-Grade Glioma. Front. Genet. 2021, 12, 698284. [Google Scholar] [CrossRef] [PubMed]
- Ramovs, V.; te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Hsiao, H.F.; Yeh, Y.C.; Chen, T.W.; Li, T.K. Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J. Exp. Clin. Cancer Res. 2018, 37, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Wen, Y.; Jin, R.; Chen, H. New attempts for central nervous infiltration of pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev. 2019, 38, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.D.; Keewan, E.; Matlawska-Wasowska, K. Metabolic Reprogramming and Cell Adhesion in Acute Leukemia Adaptation to the CNS Niche. Front. Cell. Dev. Biol. 2021, 9, 767510. [Google Scholar] [CrossRef]
- Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Armstrong, L.; Hughes, O.; Yung, S.; Hyslop, L.; Stewart, R.; Wappler, I.; Peters, H.; Walter, T.; Stojkovic, P.; Evans, J. The role of PI3K/AKT, MAPK/ERK and NFκβ signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006, 15, 1894–1913. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Yang, X.; Chen, Y.; Jin, X.; Li, L.; Guo, Y.; Li, X.; Xie, Y.; Zhang, Y.; Wang, H. EVI1 induces autophagy to promote drug resistance via regulation of ATG7 expression in leukemia cells. Carcinogenesis 2021, 41, 961–971. [Google Scholar] [CrossRef]
- Schwab, L.P.; Peacock, D.L.; Majumdar, D.; Ingels, J.F.; Jensen, L.C.; Smith, K.D.; Cushing, R.C.; Seagroves, T.N. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012, 14, R6. [Google Scholar] [CrossRef]
- Biagioni, A.; Tavakol, S.; Ahmadirad, N.; Zahmatkeshan, M.; Magnelli, L.; Mandegary, A.; Samareh Fekri, H.; Asadi, M.H.; Mohammadinejad, R.; Ahn, K.S. Small nucleolar RNA host genes promoting epithelial–mesenchymal transition lead cancer progression and metastasis. IUBMB Life 2021, 73, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell. Dev. Biol. 2020, 8, 588066. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Tan, X.D.; Liu, P.; Yang, Y.F.; Huang, Y.P.; Liu, X.L.; Meng, X.L.; Yu, B.Q.; Wu, M.W.; Jin, H.Y. ITGA6 and RPSA synergistically promote pancreatic cancer invasion and metastasis via PI3K and MAPK signaling pathways. Exp. Cell Res. 2019, 379, 30–47. [Google Scholar] [CrossRef]
- Xu, H.; Pumiglia, K.; LaFlamme, S.E. Laminin-511 and alpha 6 integrins regulate the expression of CXCR4 to promote endothelial morphogenesis. J. Cell Sci. 2020, 133, jcs246595. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, J.; Xu, A.; Bao, J.; Cho, W.C.; Zhu, J.; Shen, J. Integrin α6 overexpression promotes lymphangiogenesis and lymphatic metastasis via activating the NF-κB signaling pathway in lung adenocarcinoma. Cell. Oncol. 2022, 45, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Saltarella, I.; Altamura, C.; Campanale, C.; Laghetti, P.; Vacca, A.; Frassanito, M.A.; Desaphy, J.F. Anti-Angiogenic Activity of Drugs in Multiple Myeloma. Cancers 2023, 15, 1990. [Google Scholar] [CrossRef]
- Huizer, K.; Sacchetti, A.; Swagemakers, S.; van der Spek, P.J.; Dik, W.; Mustafa, D.A.; Kros, J.M. Circulating angiogenic cells in glioblastoma: Toward defining crucial functional differences in CAC-induced neoplastic versus reactive neovascularization. Neuro-Oncol. Adv. 2020, 2, vdaa040. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Yang, S.; Ding, G.; Luo, M. Anti-vascular endothelial growth factor in glioblastoma: A systematic review and meta-analysis. Neurol. Sci. 2018, 39, 2021–2031. [Google Scholar] [CrossRef]
- Dar, A.; Kollet, O.; Lapidot, T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp. Hematol. 2006, 34, 967–975. [Google Scholar] [CrossRef]
- Gaudichon, J.; Jakobczyk, H.; Debaize, L.; Cousin, E.; Galibert, M.D.; Troadec, M.B.; Gandemer, V. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: Reconciling biological concepts and clinical issues. Blood Rev. 2019, 36, 40–56. [Google Scholar] [CrossRef]
- Asada, T.; Nakahata, S.; Fauzi, Y.R.; Ichikawa, T.; Inoue, K.; Shibata, N.; Fujii, Y.; Imamura, N.; Hiyoshi, M.; Nanashima, A.; et al. Integrin alpha 6A (ITGA6A)-type Splice Variant in Extracellular Vesicles Has a Potential as a Novel Marker of the Early Recurrence of Pancreatic Cancer. Anticancer Res. 2022, 42, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Tan, Z.B.; Liu, Y.P.; Shi, F.Y.; She, J.J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res. Ther. 2022, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Zhang, K.D.; Zhao, Z.Y.; Qin, Z.; Tang, H.C. Prognosis-related autophagy genes in female lung adenocarcinoma. Medicine 2022, 101, e28500. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.B.; Weng, F.Y.; Wang, L.; Tong, X.W.; Yao, Y.N.; Li, H.F. Extracellular vesicle-mediated delivery of miR-127-3p inhibits the proliferation and invasion of choriocarcinoma cells by targeting ITGA6. Exp. Cell Res. 2022, 414, 113098. [Google Scholar] [CrossRef] [PubMed]
- Khademi, R.; Mohammadi, Z.; Khademi, R.; Saghazadeh, A.; Rezaei, N. Nanotechnology-based diagnostics and therapeutics in acute lymphoblastic leukemia: A systematic review of preclinical studies. Nanoscale Adv. 2023, 5, 571–595. [Google Scholar] [CrossRef] [PubMed]
- Modvig, S.; Jeyakumar, J.; Marquart, H.V.; Christensen, C. Integrins and the Metastasis-like Dissemination of Acute Lymphoblastic Leukemia to the Central Nervous System. Cancers 2023, 15, 2504. [Google Scholar] [CrossRef]
- Lenk, L.; Alsadeq, A.; Schewe, D.M. Involvement of the central nervous system in acute lymphoblastic leukemia: Opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020, 39, 173–187. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, E.; Yang, D.; Rolf, N.; Reid, G.S.D.; Lim, C.J.; Vercauteren, S.M. Clinical and Laboratory Features Associated with Flow Cytometric CD49f Expression in Pediatric B Cell Acute Lymphoblastic Leukemia. Blood 2019, 134 (Suppl. 1), 5209. [Google Scholar] [CrossRef]
- Scharff, B.F.S.S.; Modvig, S.; Thastrup, M.; Levinsen, M.; Degn, M.; Ryder, L.P.; Schmiegelow, K.; Christensen, C.R.L.; Marquart, H.V. A Comprehensive Study of Human Integrins in Pediatric Lymphoblastic Leukemia Supports a Role of CD49f (Integrin α6) in the Localization to Bone Marrow but Not Spinal Fluid. Blood 2019, 134 (Suppl. 1), 5205. [Google Scholar] [CrossRef]
- Scharff, B.; Modvig, S.; Marquart, H.V.; Christensen, C. Integrin-Mediated Adhesion and Chemoresistance of Acute Lymphoblastic Leukemia Cells Residing in the Bone Marrow or the Central Nervous System. Front. Oncol. 2020, 10, 775. [Google Scholar] [CrossRef]
- Gaynes, J.S.; Jonart, L.M.; Zamora, E.A.; Naumann, J.A.; Gossai, N.P.; Gordon, P.M. The central nervous system microenvironment influences the leukemia transcriptome and enhances leukemia chemo-resistance. Haematologica 2017, 102, e136–e139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhang, H.; Yuan, M.; Zhang, P.; Wang, Y.; Zheng, M.; Lv, Z.; Odhiambo, W.O.; Li, C.; Liu, C.; et al. Identification and characterization of a murine model of BCR-ABL1+ acute B-lymphoblastic leukemia with central nervous system metastasis. Oncol. Rep. 2019, 42, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.; Cardinali, J.L.; Mnayer, L.O.; DiGiuseppe, J.A. CD49f protein expression varies among genetic subgroups of B lymphoblastic leukemia and is distinctly low in KMT2A-rearranged cases. Cytom. B Clin. Cytom. 2021, 100, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, T.S.; Lee, H.W.; Kang, M.C.; Yoon, H.J.; Kim, J.H.; Park, J.H. Integrin alpha 6: A novel therapeutic target in esophageal squamous cell carcinoma. Int. J. Oncol. 2013, 43, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Chi, W.W.; Cao, H.; Cui, W.N.; Meng, W.X.; Guo, W.; Wang, B.S. Screening and clinical significance of tumor markers in head and neck squamous cell carcinoma through bioinformatics analysis. Mol. Med. Rep. 2019, 19, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.X.; Zeballos, C.; Bouamar, H.; Cserhati, M.; Cigarroa, F.G.; Sun, L.Z. RNA interference reveals tumor promoting roles of integrin alpha 6 (ITGA6) in hepatocellular carcinoma. Cancer Res. 2019, 79, 4642. [Google Scholar] [CrossRef]
- Feng, C.; Jin, X.X.; Han, Y.Y.; Guo, R.X.; Zou, J.J.; Li, Y.Z.; Wang, Y. Expression and Prognostic Analyses of ITGA3, ITGA5, and ITGA6 in Head and Neck Squamous Cell Carcinoma. Med. Sci. Monit. 2020, 26, e926800. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Hou, K.Z.; Jin, Y.; Bao, B.W.; Tang, S.Y.; Qi, J.F.; Yang, Y.; Che, X.F.; Liu, Y.P.; Hu, X.J.; et al. Lung adenocarcinoma-specific three-integrin signature contributes to poor outcomes by metastasis and immune escape pathways. J. Transl. Intern. Med. 2021, 9, 249–263. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, J.; Zhu, Y.; Shen, Y. Comprehensive Analysis of a Nine-Gene Signature Related to Tumor Microenvironment in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2021, 9, 700607. [Google Scholar] [CrossRef]
- Lin, B.Q.; Zhang, W.B.; Zhao, J.; Zhou, X.H.; Li, Y.J.; Deng, J.; Zhao, Q.; Fu, G.; Xie, C.M.; Xu, Y.K.; et al. An Optimized Integrin α6-Targeted Magnetic Resonance Probe for Molecular Imaging of Hepatocellular Carcinoma in Mice. J. Hepatocell. Carcinoma 2021, 8, 645–656. [Google Scholar] [CrossRef]
- Yang, W.H.; Lai, Z.Y.; Li, Y.; Mu, J.B.; Yang, M.D.; Xie, J.; Xu, J. Immune signature profiling identified prognostic factors for gastric cancer. Chin. J. Cancer Res. 2019, 31, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, S.; Liu, J.; Huang, Y.; Deng, W.; Shu, G.; Yin, G. Integrated Analysis of Prognostic and Immune Associated Integrin Family in Ovarian Cancer. Front. Genet. 2020, 11, 705. [Google Scholar] [CrossRef]
- Givant-Horwitz, V.; Davidson, B.; Van De Putte, G.; Dong, H.P.; Goldberg, I.; Amir, S.; Kristensen, G.B.; Reich, R. Expression of the 67 kDa laminin receptor and the α6 integrin subunit in serous ovarian carcinoma. Clin. Exp. Metastasis 2003, 20, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Pineda, J.C.; Toledo-Leyva, A.; Osorio-Trujillo, J.C.; Hernández-Ramírez, V.I.; Talamás-Rohana, P. The translational blocking of α5 and α6 integrin subunits affects migration and invasion, and increases sensitivity to carboplatin of SKOV-3 ovarian cancer cell line. Exp. Cell Res. 2017, 351, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Chen, R.F.; Wang, J.Y.; Yue, H.R.; Lu, X.; Li, J. The prognostic value of ITGA and ITGB superfamily members in patients with high grade serous ovarian cancer. Cancer Cell Int. 2020, 20, 257. [Google Scholar] [CrossRef]
- Wei, L.; Yin, F.; Chen, C.; Li, L. Expression of integrin α-6 is associated with multi drug resistance and prognosis in ovarian cancer. Oncol. Lett. 2019, 17, 3974–3980. [Google Scholar] [CrossRef] [Green Version]
- Elsharif, A.; Patterson, L.; Shnyder, S.; Sheldrake, H. The role of integrins in acute leukemias and potential as targets for therapy. Tumor Microenviron. 2018, 1, 63. [Google Scholar] [CrossRef]
- Hara, J.; Matsuda, Y.; Fujisaki, H.; Tokimasa, S.; Ohta, H.; Osagi, Y.; Takai, K. Expression of adhesion molecules in childhood B-lineage-cell neoplasms. Int. J. Hematol. 2000, 72, 69–73. [Google Scholar]
- Herring, E.; Kanaoka, S.; Tremblay, E.; Beaulieu, J.F. Droplet digital PCR for quantification of ITGA6 in a stool mRNA assay for the detection of colorectal cancers. World J. Gastroenterol. 2017, 23, 2891–2898. [Google Scholar] [CrossRef]
- Herring, E.; Kanaoka, S.; Tremblay, E.; Beaulieu, J.F. A Stool Multitarget mRNA Assay for the Detection of Colorectal Neoplasms. Color. Cancer Methods Protoc. 2018, 1765, 217–227. [Google Scholar]
- Chen, Y.; Jiang, P.; Wen, J.; Wu, Z.; Li, J.; Chen, Y.; Wang, L.; Gan, D.; Chen, Y.; Yang, T.; et al. Integrated bioinformatics analysis of the crucial candidate genes and pathways associated with glucocorticoid resistance in acute lymphoblastic leukemia. Cancer Med. 2020, 9, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, C.; Fiskus, W.; Kadia, T.M.; DiNardo, C.D.; Mill, C.P.; Bhalla, K.N. EVI1 dysregulation: Impact on biology and therapy of myeloid malignancies. Blood Cancer J. 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, E.; Martinez-Soler, F.; Gimenez-Bonafe, P.; Vidal, N.; Tortosa, A. ITGA6 IS INVOLVED IN RADIORESISTANCE OF GLIOBLASTOMA STEM CELLS. Neuro-Oncology 2014, 16, ii106–ii107. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, X.Y.; Hou, Q.; Huang, M.; Zhang, H.F.; Jiang, Z.Z.; Yue, J.; Wu, S.X. Single-cell RNA-seq of esophageal squamous cell carcinoma cell line with fractionated irradiation reveals radioresistant gene expression patterns. BMC Genom. 2019, 20, 611. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.; Hsieh, Y.-T.; Kim, H.N.; Duchartre, Y.; Stephanie, S.; Muschen, M.; Wayner, E.; Heisterkamp, N.; Bonig, H.; Kim, Y.-M. Overcoming drug resistance of pre-B ALL cells by targeting integrin alpha6 associated cell-adhesion mediated drug resistance using a novel antibody, P5G10. Blood 2015, 126, 2525. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-C.; Gang, E.J.; Kim, H.N.; Abdel-Azim, N.; Chen, R.; Abdel-Azim, H.; Shung, K.K.; Kim, Y.-M. Integrin Antibody Decreases Deformability of Patient-Derived Pre-B Acute Lymphocytic Leukemia Cells as Measured by High-Frequency Acoustic Tweezers. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 589–595. [Google Scholar] [CrossRef]
- DiGiuseppe, J.A.; Fuller, S.G.; Borowitz, M.J. Overexpression of CD49f in precursor B-cell acute lymphoblastic leukemia: Potential usefulness in minimal residual disease detection. Cytom. B Clin. Cytom. 2009, 76, 150–155. [Google Scholar] [CrossRef]
- Shah Scharff, B.F.S.; Modvig, S.; Thastrup, M.; Levinsen, M.; Degn, M.; Ryder, L.P.; Schmiegelow, K.; Christensen, C.; Marquart, H.V. A comprehensive clinical study of integrins in acute lymphoblastic leukemia indicates a role of α6/CD49f in persistent minimal residual disease and α5 in the colonization of cerebrospinal fluid. Leuk. Lymphoma 2020, 61, 1714–1718. [Google Scholar] [CrossRef]
- Modvig, S.; Wernersson, R.; Øbro, N.F.; Olsen, L.R.; Christensen, C.; Rosthøj, S.; Degn, M.; Jürgensen, G.W.; Madsen, H.O.; Albertsen, B.K.; et al. High CD34 surface expression in BCP-ALL predicts poor induction therapy response and is associated with altered expression of genes related to cell migration and adhesion. Mol. Oncol. 2022, 16, 2015–2030. [Google Scholar] [CrossRef]
- Flotho, C.; Coustan-Smith, E.; Pei, D.; Iwamoto, S.; Song, G.; Cheng, C.; Pui, C.H.; Downing, J.R.; Campana, D. Genes contributing to minimal residual disease in childhood acute lymphoblastic leukemia: Prognostic significance of CASP8AP2. Blood 2006, 108, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.Y.; Weng, Y.J.; Ding, N.; Cheng, C.; Wang, L.L.; Zhou, Y.; Zhang, L.; Cui, Y.P.; Zhang, W.M. Autophagy-Related Three-Gene Prognostic Signature for Predicting Survival in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 650891. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, T.; Ferreira, E.; Jovanovic, K.; Veale, R.B.; Weiss, S.F. Knockdown of LRP/LR Induces Apoptosis in Breast and Oesophageal Cancer Cells. PLoS ONE 2015, 10, e0139584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ports, M.O.; Nagle, R.B.; Pond, G.D.; Cress, A.E. Extracellular engagement of alpha6 integrin inhibited urokinase-type plasminogen activator-mediated cleavage and delayed human prostate bone metastasis. Cancer Res. 2009, 69, 5007–5014. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.K.; Zhang, M.Q.; Wang, H.X.; Cai, J.; Chen, S.P.; Wang, Q.; Gong, J.; Leong, K.W.; Wang, J.; Zhang, X. Identification of an integrin α6-targeted peptide for nasopharyngeal carcinoma-specific nanotherapeutics. Adv. Ther. 2019, 2, 1900018. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, J.; Li, X.; Li, Y.; Feng, G. Integrin α6 targeted cancer imaging and therapy. Vis. Cancer Med. 2023, 4, 4. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khademi, R.; Malekzadeh, H.; Bahrami, S.; Saki, N.; Khademi, R.; Villa-Diaz, L.G. Regulation and Functions of α6-Integrin (CD49f) in Cancer Biology. Cancers 2023, 15, 3466. https://doi.org/10.3390/cancers15133466
Khademi R, Malekzadeh H, Bahrami S, Saki N, Khademi R, Villa-Diaz LG. Regulation and Functions of α6-Integrin (CD49f) in Cancer Biology. Cancers. 2023; 15(13):3466. https://doi.org/10.3390/cancers15133466
Chicago/Turabian StyleKhademi, Rahele, Hossein Malekzadeh, Sara Bahrami, Najmaldin Saki, Reyhane Khademi, and Luis G. Villa-Diaz. 2023. "Regulation and Functions of α6-Integrin (CD49f) in Cancer Biology" Cancers 15, no. 13: 3466. https://doi.org/10.3390/cancers15133466
APA StyleKhademi, R., Malekzadeh, H., Bahrami, S., Saki, N., Khademi, R., & Villa-Diaz, L. G. (2023). Regulation and Functions of α6-Integrin (CD49f) in Cancer Biology. Cancers, 15(13), 3466. https://doi.org/10.3390/cancers15133466






