Surgery in Recurrent Ovarian Cancer: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
- -
- Languages: English and Spanish
- -
- Date: the last 5 years
- -
- Type of study: randomized controlled trial
2.4. Statistical Analysis
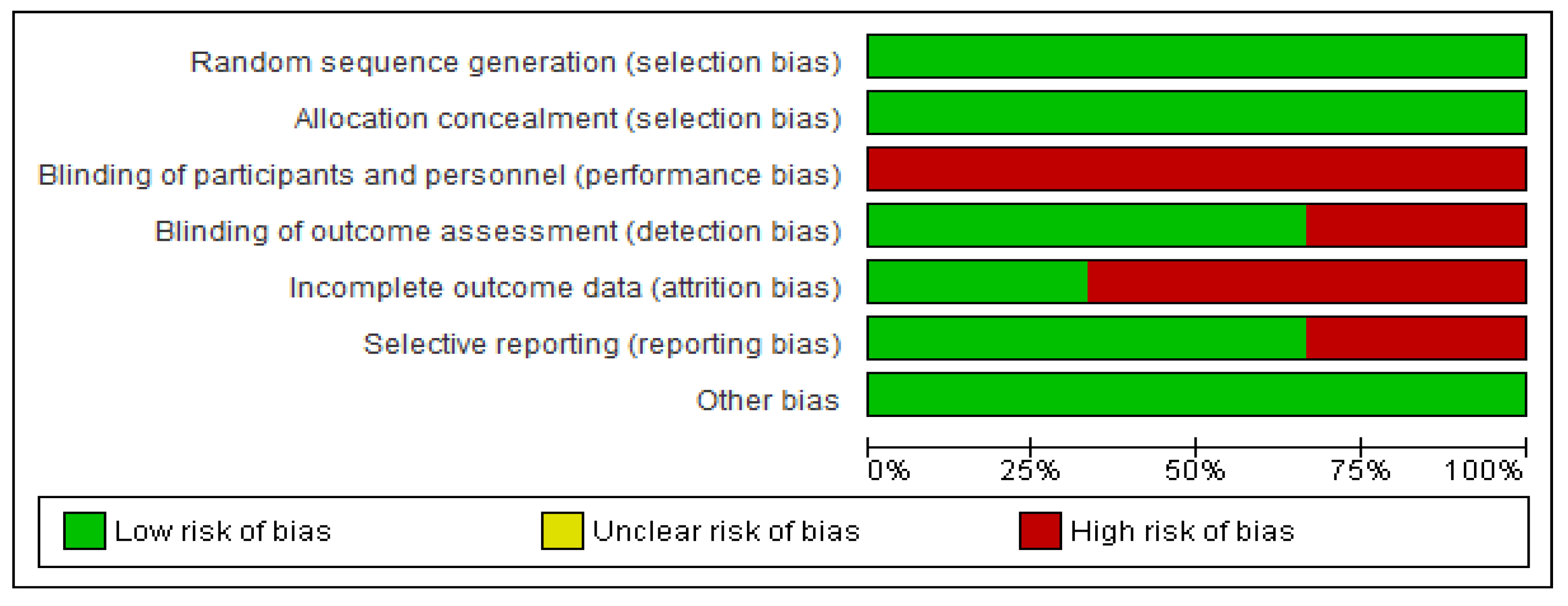
3. Results
3.1. Selected Studies
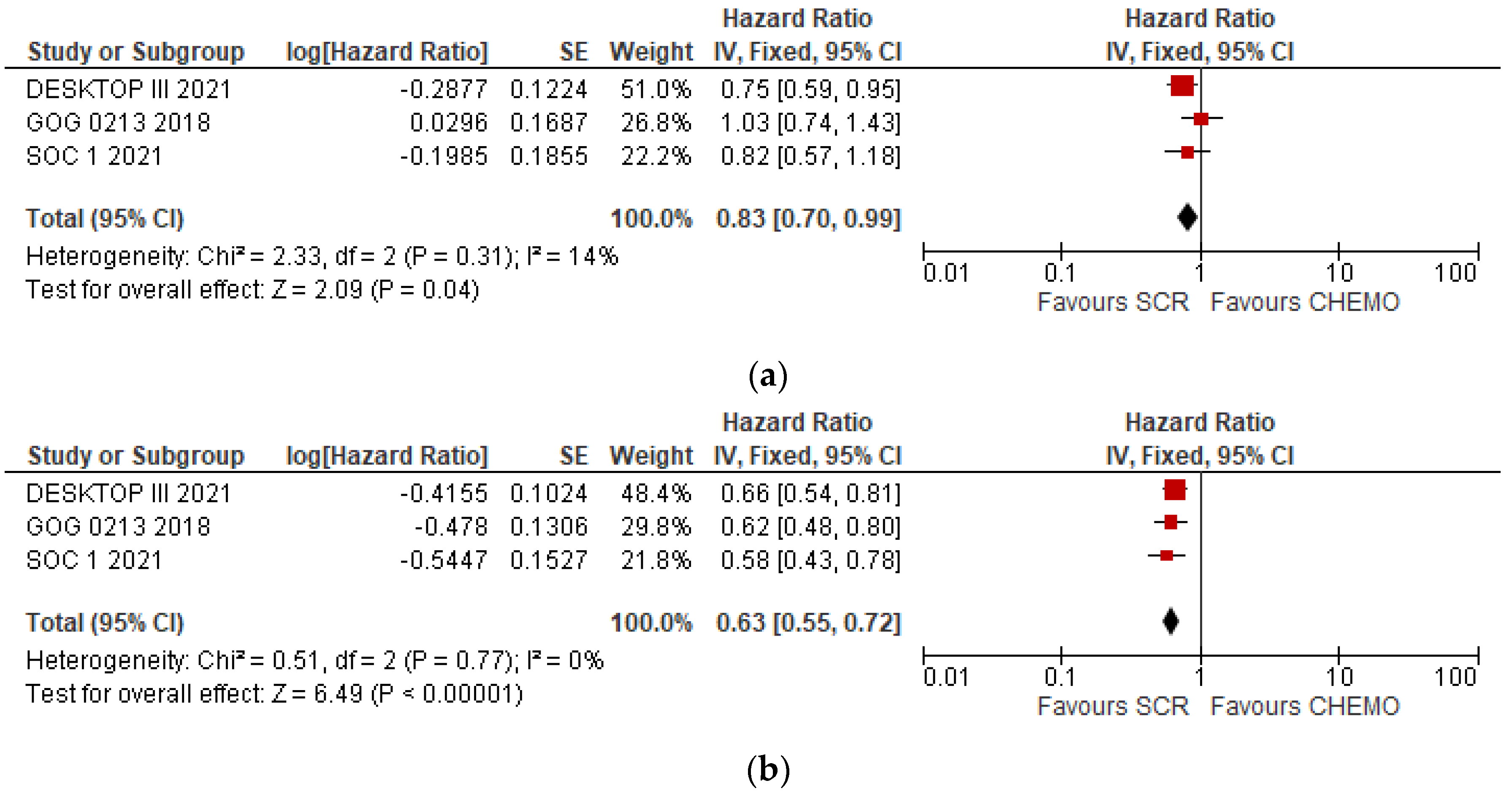
3.2. Overall Meta-Analyses of OS and DFS
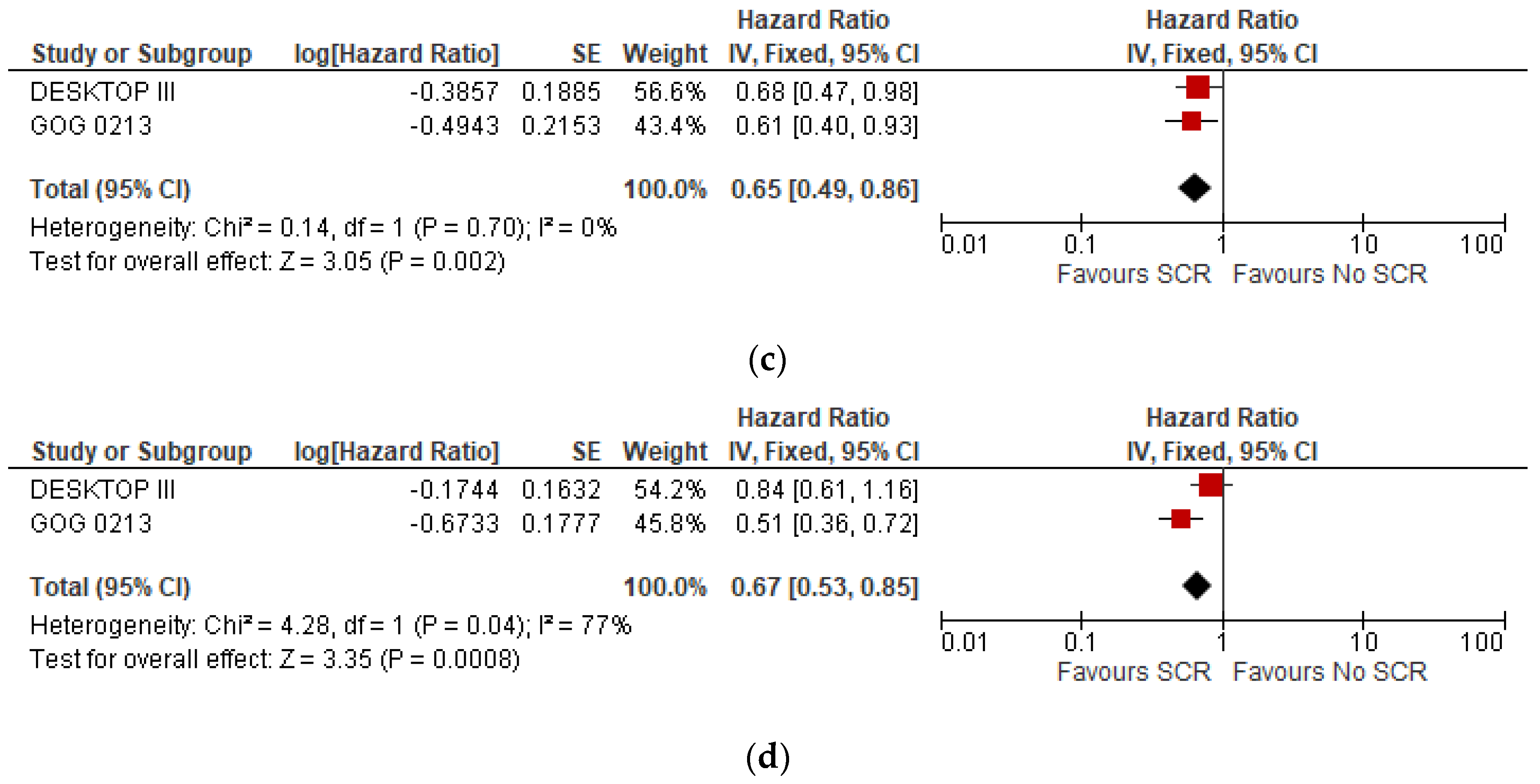
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, W.J.; Chi, D.S.; Sehouli, J.; Tropé, C.G.; Jiang, R.; Ayhan, A.; Cormio, G.; Xing, Y.; Breitbach, G.P.; Braicu, E.I.; et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: An evidence-based proposal for patient selection. Ann. Surg. Oncol. 2012, 14, 597–604. [Google Scholar] [CrossRef]
- Ding, T.; Tang, D.; Xi, M. The survival outcome and complication of secondary cytoreductive surgery plus chemotherapy in recurrent ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P.; et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Wood-Bouwens, C.M.; Haslem, D.; Moulton, B.; Almeda, A.F.; Lee, H.; Heestand, G.M.; Nadauld, L.D.; Ji, H.P. Therapeutic Monitoring of Circulating DNA Mutations in Metastatic Cancer with Personalized Digital PCR. J. Mol. Diagn. 2019, 22, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Mughal, T.I.; Razavi, P.; Dawson, S.-J.; Moss, E.L.; Govindan, R.; Tan, I.B.; Yap, Y.-S.; Robinson, W.A.; Morris, C.D.; et al. Liquid biopsies for residual disease and recurrence. Med 2021, 2, 1292–1313. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Du Bois, A.; Hahmann, M.; Hasenburg, A.; Burges, A.; Loibl, S.; Gropp, M.; Huober, J.; Fink, D.; Schröder, W.; et al. Surgery in Recurrent Ovarian Cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR Trial. Ann. Surg. Oncol. 2006, 13, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Yin, S.; Zhu, J.; Zhang, P.; Liu, J.; Xiang, L.; Zhu, Y.; Wu, S.; Chen, X.; Wang, X.; et al. A phase II trial of cytoreductive surgery combined with niraparib maintenance in platinum-sensitive, secondary recurrent ovarian cancer: SGOG SOC-3 study. J. Gynecol. Oncol. 2020, 31, e61. [Google Scholar] [CrossRef]
- Conte, C.; Fagotti, A.; Avesani, G.; Trombadori, C.; Federico, A.; D’indinosante, M.; Giudice, M.T.; Pelligra, S.; Lodoli, C.; Marchetti, C.; et al. Update on the secondary cytoreduction in platinum-sensitive recurrent ovarian cancer: A narrative review. Ann. Transl. Med. 2021, 9, 510. [Google Scholar] [CrossRef]
- Al Rawahi, T.; Lopes, A.D.; E. Bristow, R.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, 2, CD008765. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Reuss, A.; Hasenburg, A.; Scambia, G.; Cibula, D.; Mahner, S.; Vergote, I.; Reinthaller, A.; Burges, A.; et al. Prospective Validation Study of a Predictive Score for Operability of Recurrent Ovarian Cancer: The Multicenter Intergroup Study DESKTOP II. A Project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int. J. Gynecol. Cancer 2011, 21, 289–295. [Google Scholar] [CrossRef]
- Lee, C.K.; Lord, S.; Grunewald, T.; Gebski, V.; Hardy-Bessard, A.-C.; Sehouli, J.; Woie, K.; Heywood, M.; Schauer, C.; Vergote, I.; et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: Analysis of the CALYPSO trial. Gynecol. Oncol. 2014, 136, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.Y.; Harter, P.; Chi, D.S.; Sehouli, J.; Jiang, R.; Tropé, C.G.; Ayhan, A.; Cormio, G.; Xing, Y.; Wollschlaeger, K.M.; et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br. J. Cancer 2011, 105, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Conte, C.; Giudice, M.T.; Nero, C.; Vizzielli, G.; Alletti, S.G.; Cianci, S.; Lodoli, C.; Di Giorgio, A.; De Rose, A.M.; et al. Secondary Laparoscopic Cytoreduction in Recurrent Ovarian Cancer: A Large, Single-Institution Experience. J. Minim. Invasive Gynecol. 2018, 25, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Marchetti, C.; Conte, C.; Loverro, M.; Giudice, M.T.; Quagliozzi, L.; Distefano, M.; Chiantera, V.; Scambia, G.; Fagotti, A. The impact of secondary cytoreductive surgery in platinum sensitive recurrent ovarian cancer treated with upfront neoadjuvant chemotherapy and interval debulking surgery. Gynecol. Oncol. 2022, 165, 453–458. [Google Scholar] [CrossRef]
- Kim, S.I.; Cho, J.; Lee, E.J.; Park, S.; Park, S.J.; Seol, A.; Lee, N.; Yim, G.W.; Lee, M.; Lim, W.; et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: A systematic review and meta-analysis. Medicine (United States). 2019, 98, 1–9. [Google Scholar] [CrossRef]
- Matsumoto, A.; Higuchi, T.; Yura, S.; Mandai, M.; Kariya, M.; Takakura, K.; Fujii, S. Role of salvage cytoreductive surgery in the treatment of patients with recurrent ovarian cancer after platinum-based chemotherapy. J. Obstet. Gynaecol. Res. 2006, 32, 580–587. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.; Kitchener, H.; Bacon, M.; Dubois, A.; Friedlander, M.; Ledermann, J.; Marth, C.; Thigpen, T.; Trimble, E. 2010 Gynecologic Cancer InterGroup (GCIG) Consensus Statement on Clinical Trials in Ovarian Cancer: Report From the Fourth Ovarian Cancer Consensus Conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef]
- Llueca, A.; Serra, A.; Delgado, K.; Maiocchi, K.; Jativa, R.; Gomez, L.; Escrig, J. A radiologic-laparoscopic model to predict suboptimal (or complete and optimal) debulking surgery in advanced ovarian cancer: A pilot study. Int. J. Women’s Heal. 2019, 11, 333–342. [Google Scholar] [CrossRef]
- Llueca, A.; MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery); Serra, A.; Rivadulla, I.; Gomez, L.; Escrig, J. Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J. Surg. Oncol. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. New Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Fanfani, F.; Vizzielli, G.; Gallotta, V.; Ercoli, A.; Paglia, A.; Costantini, B.; Vigliotta, M.; Scambia, G.; Ferrandina, G. Should laparoscopy be included in the work-up of advanced ovarian cancer patients attempting interval debulking surgery? Gynecol. Oncol. 2010, 116, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Amadio, G.; Salutari, V.; Paris, I.; Di Stefano, M.; Ferandina, G.; Scambia, G.; Fagotti, A. Impact of bevacizumab containing first line chemotherapy on recurrent disease in epithelial ovarian cancer: A case-control study. Gynecol. Oncol. 2016, 142, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Climent, M.T.; Segarra, B.; Maazouzi, Y.; Soriano, M.; Escrig, J.; on behalf MUAPOS Working Group. Outcome quality standards in advanced ovarian cancer surgery. World J. Surg. Oncol. 2020, 18, 309. [Google Scholar] [CrossRef]
- Llueca, A.; Climent, M.T.; Escrig, J.; Carrasco, P.; Serra, A.; Gomez-Quiles, L.; Játiva, R.; Cebrian, G.; Bosso, V.; Villarin, A.; et al. Validation of three predictive models for suboptimal cytoreductive surgery in advanced ovarian cancer. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Concin, N.; Planchamp, F.; Morice, P.; Vergote, I.; Du Bois, A.; Querleu, D. Quality indicators for advanced ovarian cancer surgery from the European Society of Gynaecological Oncology (ESGO): 2020 update. Int. J. Gynecol. Cancer 2020, 30, 436–440. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.; Sonke, G.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int. J. Environ. Res. Public Heal. 2022, 19, 8577. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Rassy, E.; Moschetta, M.; Ghose, A.; Adeleke, S.; Sanchez, E.; Sheriff, M.; Chargari, C.; Pavlidis, N. BRCA Mutations in Ovarian and Prostate Cancer: Bench to Bedside. Cancers 2022, 14, 3888. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Karihtala, P.; Samartzis, E.P.; Sheriff, M.; Pappas-Gogos, G.; Ozturk, M.A.; Uccello, M.; Karathanasi, A.; Tringos, M.; et al. Development of new poly(ADP-ribose) polymerase (PARP) inhibitors in ovarian cancer: Quo Vadis? Ann Transl. Med. 2020, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Bruno, M.; Conte, C.; Giudice, M.T.; Davià, F.; Moro, F.; Zannoni, G.F.; Fagotti, A.; De Bonis, M.; Capoluongo, E.; et al. Salvage lymphadenectomy in recurrent ovarian cancer patients: Analysis of clinical outcome and BRCA1/2 gene mutational status. Eur. J. Surg. Oncol. 2020, 46, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Conte, C.; D’indinosante, M.; Capoluongo, E.; Minucci, A.; De Rose, A.M.; Ardito, F.; Giuliante, F.; Di Giorgio, A.; Zannoni, G.F.; et al. Prognostic factors value of germline and somatic brca in patients undergoing surgery for recurrent ovarian cancer with liver metastases. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 2096–2102. [Google Scholar] [CrossRef]
- Hollis, R.L.; Churchman, M.; Gourley, C. Distinct implications of different BRCA mutations: Efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. OncoTargets Ther. 2017, ume 10, 2539–2551. [Google Scholar] [CrossRef]


| Groups | N of Patients | Selection Criteria | CTC % | Survival In Scs | Survival in No Scs | |
|---|---|---|---|---|---|---|
| DESKTOP III | SCS No SCS | 206 201 | AGO score | 76% | 53.7 m (OS) | 46 m (OS) |
| GOG 0213 | SCS No SCS | 240 245 | None | 67% | 50.6 m (OS) | 64.7 m (OS) |
| SOC-1 | SCS No SCS | 182 175 | IModel + PET TC | 77% | 58.1 m (OS) | 53.9 m (OS) |
| Age | FIGO | Platinum-Free Interval | Previous Bevacizumab | Histology | |
|---|---|---|---|---|---|
| DESKTOP III | SCS 60.8 (54.2–67.3) | III 145 (70.4%) IV 16 (7.8%) | 6–12 m 48 (23.6%) >12 m 155 (76.4%) | 33 (16%) | Serous 177 (85.9%) Other 23 (14.1%) |
| No SCS 62.2 (54.2–69.9) | III 143 (71.1%) IV 13 (6.5%) | 6–12 m 47 (23.7%) >12 m 151 (76.3%) | 31 (15.4%) | Serous 161 (80.1%) Other 40 (19.9%) | |
| GOG 0213 | SCS <60 years 135 (56.2%) >60 years 105 (43.8%) | NE | NE | 25 (10.4%) | Serous 211 (87%) Other 29 (12.3%) |
| No SCS <60 years 145 (59.1%) >60 years 100 (40.8%) | 30 (12.2%) | Serous 207 (84.5%) Other 38 (15.5%) | |||
| SOC-1 | SCS <54 years 80 >54 years 102 | III 128 IV 20 | NE | NE | Serous 158 Another 24 |
| No SCS <54 years 90 >54 years 85 | III 121 IV 25 | Serous 145 Another 30 |
| Groups | DFS (Median Months) | HR | IC 95% | P | OS (Median Months) | HR | IC 95% | P | |
|---|---|---|---|---|---|---|---|---|---|
| DESKTOP III | SCS | 18.4 | 0.66 | 12.7–20.8 | NE | 53.7 | 0.75 | 0.59–0.96 | 0.02 |
| No SCS | 14 | 46 | |||||||
| GOG 0213 | SCS | 18.9 | 0.62 | 0.48–0.80 | NE | 50.6 | 1.03 | 0.74–1.43 | 0.08 |
| No SCS | 16.2 | 64.7 | |||||||
| SOC-1 | SCS | 17.4 | 0.58 | 0.45–0.74 | <0.0001 | 58.1 | 0.82 | 0.57–1.19 | NE |
| No SCS | 11.9 | 53.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent, M.T.; Serra, A.; Llueca, M.; Llueca, A. Surgery in Recurrent Ovarian Cancer: A Meta-Analysis. Cancers 2023, 15, 3470. https://doi.org/10.3390/cancers15133470
Climent MT, Serra A, Llueca M, Llueca A. Surgery in Recurrent Ovarian Cancer: A Meta-Analysis. Cancers. 2023; 15(13):3470. https://doi.org/10.3390/cancers15133470
Chicago/Turabian StyleCliment, Maria Teresa, Anna Serra, Maria Llueca, and Antoni Llueca. 2023. "Surgery in Recurrent Ovarian Cancer: A Meta-Analysis" Cancers 15, no. 13: 3470. https://doi.org/10.3390/cancers15133470
APA StyleCliment, M. T., Serra, A., Llueca, M., & Llueca, A. (2023). Surgery in Recurrent Ovarian Cancer: A Meta-Analysis. Cancers, 15(13), 3470. https://doi.org/10.3390/cancers15133470





