The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management
Abstract
Simple Summary
Abstract
1. Introduction
2. Initial Presentation of APL
3. Pathophysiology of Hemorrhage in APL
4. Hemorrhagic Events in APL
5. Risk Stratification for Hemorrhage and Hemorrhagic Death
6. Pathophysiology of Hypercoagulability in APL
7. Characteristics of Thrombotic Events in APL and Markers of Thrombotic Risk
8. Interaction between Induction Therapy and Coagulopathy in APL
9. Current Strategies to Prevent and Treat Coagulopathy
10. Conclusions and Future Directions
- Improve the early recognition of APL across all health care systems based on clinical symptoms and laboratory abnormalities, and, when possible, transfer patients to a center with experience in the care of individuals with acute leukemia
- Risk-stratify patients utilizing common laboratory parameter cutoffs
- Minimize the time to induction treatment, including ATRA at first suspicion of diagnosis of APL and supportive blood product transfusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Chen, Y.; Kantarjian, H.; Wang, H.; Cortes, J.; Ravandi, F. Acute Promyelocytic Leukemia: A Population-Based Study on Incidence and Survival in the United States, 1975–2008. Cancer 2012, 118, 5811–5818. [Google Scholar] [CrossRef] [PubMed]
- Dores, G.M.; Devesa, S.S.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Acute Leukemia Incidence and Patient Survival among Children and Adults in the United States, 2001–2007. Blood 2012, 119, 34–43. [Google Scholar] [CrossRef]
- Golomb, H.M.; Vardiman, J.; Rowley, J.D. Acute Nonlymphocytic Leukemia in Adults: Correlations with Q-Banded Chromosomes. Blood 1976, 48, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Suvajdzic, N.; Bogdanovic, A.; Kurtovic, N.K.; Sretenovic, A.; Elezovic, I.; Tomin, D. International Society of Thrombosis and Hemostasis Scoring System for Disseminated Intravascular Coagulation ≥ 6: A New Predictor of Hemorrhagic Early Death in Acute Promyelocytic Leukemia. Med. Oncol. Northwood Lond. Engl. 2013, 30, 478. [Google Scholar] [CrossRef]
- Tallman, M.S.; Andersen, J.W.; Schiffer, C.A.; Appelbaum, F.R.; Feusner, J.H.; Ogden, A.; Shepherd, L.; Willman, C.; Bloomfield, C.D.; Rowe, J.M.; et al. All-Trans-Retinoic Acid in Acute Promyelocytic Leukemia. N. Engl. J. Med. 1997, 337, 1021–1028. [Google Scholar] [CrossRef]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Iland, H.J.; Collins, M.; Bradstock, K.; Supple, S.G.; Catalano, A.; Hertzberg, M.; Browett, P.; Grigg, A.; Firkin, F.; Campbell, L.J.; et al. Use of Arsenic Trioxide in Remission Induction and Consolidation Therapy for Acute Promyelocytic Leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 Study: A Non-Randomised Phase 2 Trial. Lancet Haematol. 2015, 2, e357–e366. [Google Scholar] [CrossRef] [PubMed]
- Rahmé, R.; Thomas, X.; Recher, C.; Vey, N.; Delaunay, J.; Deconinck, E.; Hirsch, P.; Bordessoule, D.; Micol, J.-B.; Stamatoullas, A.; et al. Early Death in Acute Promyelocytic Leukemia (APL) in French Centers: A Multicenter Study in 399 Patients. Leukemia 2014, 28, 2422–2424. [Google Scholar] [CrossRef]
- Lehmann, S.; Deneberg, S.; Antunovic, P.; Rangert-Derolf, Å.; Garelius, H.; Lazarevic, V.; Myhr-Eriksson, K.; Möllgård, L.; Uggla, B.; Wahlin, A.; et al. Early Death Rates Remain High in High-Risk APL: Update from the Swedish Acute Leukemia Registry 1997–2013. Leukemia 2017, 31, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Qiao, B.; Panageas, K.S.; Schymura, M.J.; Jurcic, J.G.; Rosenblat, T.L.; Altman, J.K.; Douer, D.; Rowe, J.M.; Tallman, M.S. Early Death Rate in Acute Promyelocytic Leukemia Remains High despite All-Trans Retinoic Acid. Blood 2011, 118, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, C.; Yin, C.; Jiang, X.; Jiang, L.; Wang, Z.; Meng, F. Analysis of Early Death in Newly Diagnosed Acute Promyelocytic Leukemia Patients. Medicine 2017, 96, e9324. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.S.; Munikoty, V.; Trehan, A.; Jain, R.; Bhatia, P.; Naseem, S.; Varma, N.; Bansal, D. Early Mortality Continues to Be a Barrier to Excellent Survival in Childhood Acute Promyelocytic Leukemia: A Retrospective Study of 62 Patients Spanning 17 Years. Pediatr. Hematol. Oncol. 2023, 40, 117–130. [Google Scholar] [CrossRef]
- de la Serna, J.; Montesinos, P.; Vellenga, E.; Rayón, C.; Parody, R.; León, A.; Esteve, J.; Bergua, J.M.; Milone, G.; Debén, G.; et al. Causes and Prognostic Factors of Remission Induction Failure in Patients with Acute Promyelocytic Leukemia Treated with All-Trans Retinoic Acid and Idarubicin. Blood 2008, 111, 3395–3402. [Google Scholar] [CrossRef]
- Mitrovic, M.; Suvajdzic, N.; Elezovic, I.; Bogdanovic, A.; Djordjevic, V.; Miljic, P.; Djunic, I.; Gvozdenov, M.; Colovic, N.; Virijevic, M.; et al. Thrombotic Events in Acute Promyelocytic Leukemia. Thromb. Res. 2015, 135, 588–593. [Google Scholar] [CrossRef]
- Breccia, M.; Avvisati, G.; Latagliata, R.; Carmosino, I.; Guarini, A.; De Propris, M.S.; Gentilini, F.; Petti, M.C.; Cimino, G.; Mandelli, F.; et al. Occurrence of Thrombotic Events in Acute Promyelocytic Leukemia Correlates with Consistent Immunophenotypic and Molecular Features. Leukemia 2007, 21, 79–83. [Google Scholar] [CrossRef]
- Rashidi, A.; Silverberg, M.L.; Conkling, P.R.; Fisher, S.I. Thrombosis in Acute Promyelocytic Leukemia. Thromb. Res. 2013, 131, 281–289. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and His Triad: A Question of Attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Hambley, B.C.; Tomuleasa, C.; Ghiaur, G. Coagulopathy in Acute Promyelocytic Leukemia: Can We Go Beyond Supportive Care? Front. Med. 2021, 8, 722614. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Undas, A.; Ariëns, R.A.S. Fibrin Clot Structure and Function. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arter. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Beck, E.A.; Shainoff, J.R.; Vogel, A.; Jackson, D.P. Functional Evaluation of an Inherited Abnormal Fibrinogen: Fibrinogen “Baltimore”. J. Clin. Investig. 1971, 50, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Brunclikova, M.; Simurda, T.; Zolkova, J.; Sterankova, M.; Skornova, I.; Dobrotova, M.; Kolkova, Z.; Loderer, D.; Grendar, M.; Hudecek, J.; et al. Heterogeneity of Genotype–Phenotype in Congenital Hypofibrinogenemia—A Review of Case Reports Associated with Bleeding and Thrombosis. J. Clin. Med. 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.A.; Brun, N.C.; Begtrup, K.; Broderick, J.; Davis, S.; Diringer, M.N.; Skolnick, B.E.; Steiner, T. Recombinant Activated Factor VII for Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2005, 352, 777–785. [Google Scholar] [CrossRef]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; Al-Samkari, H.; DeLoughery, T.G.; Shatzel, J.J. The Many Roles of Tranexamic Acid: An Overview of the Clinical Indications for TXA in Medical and Surgical Patients. Eur. J. Haematol. 2020, 104, 79–87. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. Prothrombin Complex Concentrates: An Update. Blood Transfus. 2010, 8, 149–154. [Google Scholar] [CrossRef]
- Garcia, D.; Libby, E.; Crowther, M.A. The New Oral Anticoagulants. Blood 2010, 115, 15–20. [Google Scholar] [CrossRef]
- Mantha, S.; Tallman, M.S.; Soff, G.A. What’s New in the Pathogenesis of the Coagulopathy in Acute Promyelocytic Leukemia? Curr. Opin. Hematol. 2016, 23, 121–126. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Keating, M.J.; Walters, R.S.; Estey, E.H.; McCredie, K.B.; Smith, T.L.; Dalton, W.T.; Cork, A.; Trujillo, J.M.; Freireich, E.J. Acute Promyelocytic Leukemia: M.D. Anderson Hospital Experience. Am. J. Med. 1986, 80, 789–797. [Google Scholar] [CrossRef]
- Cunningham, I.; Gee, T.; Reich, L.; Kempin, S.; Naval, A.; Clarkson, B. Acute Promyelocytic Leukemia: Treatment Results during a Decade at Memorial Hospital. Blood 1989, 73, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Awisati, G.; Lo Coco, F.; Mandelli, F. Acute Promyelocytic Leukemia: Clinical and Morphologic Features and Prognostic Factors. Semin. Hematol. 2001, 38, 4–12. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Avvisati, G.; Castaman, G.; Barbui, T.; Mandelli, F. Early Deaths and Anti-Hemorrhagic Treatments in Acute Promyelocytic Leukemia. A GIMEMA Retrospective Study in 268 Consecutive Patients. Blood 1990, 75, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, J.-H.; Lee, J.-H.; Kim, S.-D.; Lim, S.-N.; Choi, Y.; Lee, Y.-S.; Kang, Y.-A.; Seol, M.; Jeon, M.; et al. Significance of Fibrinogen, D-Dimer, and LDH Levels in Predicting the Risk of Bleeding in Patients with Acute Promyelocytic Leukemia. Leuk. Res. 2011, 35, 152–158. [Google Scholar] [CrossRef]
- Yan, J.; Wang, K.; Dong, L.; Liu, H.; Chen, W.; Xi, W.; Ding, Q.; Kieffer, N.; Caen, J.P.; Chen, S.; et al. PML/RARalpha Fusion Protein Transactivates the Tissue Factor Promoter through a GAGC-Containing Element without Direct DNA Association. Proc. Natl. Acad. Sci. USA 2010, 107, 3716–3721. [Google Scholar] [CrossRef]
- Falanga, A.; Consonni, R.; Marchetti, M.; Locatelli, G.; Garattini, E.; Passerini, C.G.; Gordon, S.G.; Barbui, T. Cancer Procoagulant and Tissue Factor Are Differently Modulated by All-Trans-Retinoic Acid in Acute Promyelocytic Leukemia Cells. Blood 1998, 92, 143–151. [Google Scholar] [CrossRef]
- Koyama, T.; Hirosawa, S.; Kawamata, N.; Tohda, S.; Aoki, N. All-Trans Retinoic Acid Upregulates Thrombomodulin and Downregulates Tissue-Factor Expression in Acute Promyelocytic Leukemia Cells: Distinct Expression of Thrombomodulin and Tissue Factor in Human Leukemic Cells. Blood 1994, 84, 3001–3009. [Google Scholar] [CrossRef]
- Stein, E.; McMahon, B.; Kwaan, H.; Altman, J.K.; Frankfurt, O.; Tallman, M.S. The Coagulopathy of Acute Promyelocytic Leukaemia Revisited. Best Pract. Res. Clin. Haematol. 2009, 22, 153–163. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Mannucci, P.M.; Viganò, S.; Barbui, T.; Gugliotta, L.; Cortellaro, M.; Dini, E. Liver Dysfunction Rather than Intravascular Coagulation as the Main Cause of Low Protein C and Antithrombin III in Acute Leukemia. Blood 1984, 63, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Scrobohaci, M.L.; Ghorra, P.; Zini, J.M.; Daniel, M.T.; Castaigne, S.; Degos, L. Coagulation Disorders Associated with Acute Promyelocytic Leukemia: Corrective Effect of All-Trans Retinoic Acid Treatment. Leukemia 1993, 7, 2–9. [Google Scholar] [PubMed]
- Asakura, H.; Saito, M.; Ito, K.; Jokaji, Y.; Uotani, C.; Kumabashiri, I.; Matsuda, T. Levels of Thrombin-Antithrombin III Complex in Plasma in Cases of Acute Promyelocytic Leukemia. Thromb. Res. 1988, 50, 895–899. [Google Scholar] [CrossRef]
- Breen, K.A.; Grimwade, D.; Hunt, B.J. The Pathogenesis and Management of the Coagulopathy of Acute Promyelocytic Leukaemia. Br. J. Haematol. 2012, 156, 24–36. [Google Scholar] [CrossRef]
- Kolev, K.; Longstaff, C. Bleeding Related to Disturbed Fibrinolysis. Br. J. Haematol. 2016, 175, 12–23. [Google Scholar] [CrossRef]
- Simurda, T.; Vilar, R.; Zolkova, J.; Ceznerova, E.; Kolkova, Z.; Loderer, D.; Neerman-Arbez, M.; Casini, A.; Brunclikova, M.; Skornova, I.; et al. A Novel Nonsense Mutation in FGB (c.1421G>A; p.Trp474Ter) in the Beta Chain of Fibrinogen Causing Hypofibrinogenemia with Bleeding Phenotype. Biomedicines 2020, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J.; Segal, H. Hyperfibrinolysis. J. Clin. Pathol. 1996, 49, 958. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Yang, H.; Hou, W.; Jin, B.; Hou, J.; Li, H.; Zhao, H.; Zhou, J. Characteristics of Fibrinolytic Disorders in Acute Promyelocytic Leukemia. Hematology 2018, 23, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Menell, J.S.; Cesarman, G.M.; Jacovina, A.T.; McLaughlin, M.A.; Lev, E.A.; Hajjar, K.A. Annexin II and Bleeding in Acute Promyelocytic Leukemia. N. Engl. J. Med. 1999, 340, 994–1004. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Jiang, M.; Dai, L.; Zhang, W.; Wu, D.; Ruan, C. The Expression of Annexin II and Its Role in the Fibrinolytic Activity in Acute Promyelocytic Leukemia. Leuk. Res. 2011, 35, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, G.M.; Guevara, C.A.; Hajjar, K.A. An Endothelial Cell Receptor for Plasminogen/Tissue Plasminogen Activator (t-PA). II. Annexin II-Mediated Enhancement of t-PA-Dependent Plasminogen Activation. J. Biol. Chem. 1994, 269, 21198–21203. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Wang, J.; Weiss, I. Expression of Receptors for Plasminogen Activators on Endothelial Cell Surface Depends on Their Origin. J. Thromb. Haemost. 2004, 2, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.; Booth, N.A.; Croll, A.; Dawson, A.A. The Bleeding Disorder in Acute Promyelocytic Leukaemia: Fibrinolysis Due to u-PA Rather than Defibrination. Br. J. Haematol. 1989, 71, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Aref, S.; El-Sherbiny, M.; Mabed, M.; Menessy, A.; El-Refaei, M. Urokinase Plasminogen Activator Receptor and Soluble Matrix Metalloproteinase-9 in Acute Myeloid Leukemia Patients: A Possible Relation to Disease Invasion. Hematology 2003, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Avvisati, G.; Ten Cate, J.W.; Sturk, A.; Lamping, R.; Petti, M.G.; Mandelli, F. Acquired Alpha-2-Antiplasmin Deficiency in Acute Promyelocytic Leukaemia. Br. J. Haematol. 1988, 70, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Zuo, N.; Fang, S.; Shi, J. CD44–Fibrinogen Binding Promotes Bleeding in Acute Promyelocytic Leukemia by in Situ Fibrin(Ogen) Deposition. Blood Adv. 2022, 6, 4617–4633. [Google Scholar] [CrossRef]
- Madoiwa, S.; Tanaka, H.; Nagahama, Y.; Dokai, M.; Kashiwakura, Y.; Ishiwata, A.; Sakata, A.; Yasumoto, A.; Ohmori, T.; Mimuro, J.; et al. Degradation of Cross-Linked Fibrin by Leukocyte Elastase as Alternative Pathway for Plasmin-Mediated Fibrinolysis in Sepsis-Induced Disseminated Intravascular Coagulation. Thromb. Res. 2011, 127, 349–355. [Google Scholar] [CrossRef]
- Oudijk, E.J.; Nieuwenhuis, H.K.; Bos, R.; Fijnheer, R. Elastase Mediated Fibrinolysis in Acute Promyelocytic Leukemia. Thromb. Haemost. 2000, 83, 906–908. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, L.; Xiong, L.; Dan, W.; Li, J.; Ye, J.; Wan, P.; Luo, X.; Chu, X.; Liu, C.; et al. Neutrophil Elastase-Mediated Proteolysis of the Tumor Suppressor P200 CUX1 Promotes Cell Proliferation and Inhibits Cell Differentiation in APL. Life Sci. 2020, 242, 117229. [Google Scholar] [CrossRef]
- Montaner, J.; Alvarez-Sabín, J.; Molina, C.A.; Anglés, A.; Abilleira, S.; Arenillas, J.; Monasterio, J. Matrix Metalloproteinase Expression Is Related to Hemorrhagic Transformation after Cardioembolic Stroke. Stroke 2001, 32, 2762–2767. [Google Scholar] [CrossRef]
- Lattanzi, S.; Di Napoli, M.; Ricci, S.; Divani, A.A. Matrix Metalloproteinases in Acute Intracerebral Hemorrhage. Neurotherapeutics 2020, 17, 484–496. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Chaudhary, S.; Ghosh, K.; Shanmukaiah, C.; Nadkarni, A.H. Secretion and Expression of Matrix Metalloproteinase-2 and 9 from Bone Marrow Mononuclear Cells in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Asian Pac. J. Cancer Prev. 2016, 17, 1519–1529. [Google Scholar] [CrossRef]
- Song, Y.; Peng, P.; Qiao, C.; Zhang, R.; Li, J.; Lu, H. Low Platelet Count Is Potentially the Most Important Contributor to Severe Bleeding in Patients Newly Diagnosed with Acute Promyelocytic Leukemia. OncoTargets Ther. 2017, 10, 4917–4924. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Bussel, J.B.; Frelinger, A.L.; Babula, B.; Linden, M.D.; Li, Y.; Barnard, M.R.; Tate, C.; Feldman, E.J.; Michelson, A.D. Differences in Platelet Function in Patients with Acute Myeloid Leukemia and Myelodysplasia Compared to Equally Thrombocytopenic Patients with Immune Thrombocytopenia. J. Thromb. Haemost. JTH 2011, 9, 2302–2310. [Google Scholar] [CrossRef]
- Bumbea, H.; Vladareanu, A.M.; Dumitru, I.; Popov, V.M.; Ciufu, C.; Nicolescu, A.; Onisai, M.; Marinescu, C.; Cisleanu, D.; Voican, I.; et al. Platelet Defects in Acute Myeloid Leukemia—Potential for Hemorrhagic Events. J. Clin. Med. 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Ravn, A.; Carlsson, L.; Antunovic, P.; Deneberg, S.; Mollgard, L.; Derolf, A.R.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; et al. Continuing High Early Death Rate in Acute Promyelocytic Leukemia: A Population-Based Report from the Swedish Adult Acute Leukemia Registry. Leukemia 2011, 25, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Kwaan, H.C. Reassessing the Hemostatic Disorder Associated With Acute Promyelocytic Leukemia. Blood 1992, 79, 543–553. [Google Scholar] [CrossRef]
- Cordonnier, C.; Vernant, J.P.; Brun, B.; Heilmann, M.G.; Kuentz, M.; Bierling, P.; Farcet, J.P.; Rodet, M.; Duedari, N.; Imbert, M.; et al. Acute Promyelocytic Leukemia in 57 Previously Untreated Patients. Cancer 1985, 55, 18–25. [Google Scholar] [CrossRef]
- Sobas, M.; Czyż, A.; Montesinos, P.; Armatys, A.; Helbig, G.; Hołowiecka, A.; Pluta, A.; Zarzycka, E.; Piątkowska-Jakubas, B.; Majcherek, M.; et al. Outcome of a Real-Life Population of Patients With Acute Promyelocytic Leukemia Treated According to the PETHEMA Guidelines: The Polish Adult Leukemia Group (PALG) Experience. Clin. Lymphoma Myeloma Leuk. 2020, 20, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Goldman, D.A.; Devlin, S.M.; Lee, J.-W.; Zannino, D.; Collins, M.; Douer, D.; Iland, H.J.; Litzow, M.R.; Stein, E.M.; et al. Determinants of Fatal Bleeding during Induction Therapy for Acute Promyelocytic Leukemia in the ATRA Era. Blood 2017, 129, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Li, Q.; Brunson, A.; Jonas, B.A.; Wun, T.; Keegan, T.H.M. Complications and Early Mortality in Patients with Acute Promyelocytic Leukemia Treated in California. Am. J. Hematol. 2018, 93, E370–E372. [Google Scholar] [CrossRef]
- Da Silva, W.F.; da Rosa, L.I.; Marquez, G.L.; Bassolli, L.; Tucunduva, L.; Silveira, D.R.A.; Buccheri, V.; Bendit, I.; Rego, E.M.; Rocha, V.; et al. Real-Life Outcomes on Acute Promyelocytic Leukemia in Brazil—Early Deaths Are Still a Problem. Clin. Lymphoma Myeloma Leuk. 2019, 19, e116–e122. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhou, P.; Liu, Y.; Wei, S.; Li, D.; Li, W.; Niu, X.; Niu, J.; Zhang, Y.; Cao, W.; et al. Predictive Factors for Differentiating Thrombohemorrhagic Disorders in High-Risk Acute Promyelocytic Leukemia. Thromb. Res. 2022, 210, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tai, C.-H.; Tsay, W.; Chen, P.-Y.; Tien, H.-F. Prediction of Fatal Intracranial Hemorrhage in Patients with Acute Myeloid Leukemia. Ann. Oncol. 2009, 20, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.S.; Kohrt, H.E.; Coutre, S.; Gotlib, J.R.; Majeti, R.; Alizadeh, A.A.; Medeiros, B.C. Treatment Advances Have Not Improved the Early Death Rate in Acute Promyelocytic Leukemia. Haematologica 2012, 97, 133–136. [Google Scholar] [CrossRef]
- Naymagon, L.; Mascarenhas, J. Hemorrhage in Acute Promyelocytic Leukemia: Can It Be Predicted and Prevented? Leuk. Res. 2020, 94, 106356. [Google Scholar] [CrossRef]
- Yanada, M.; Matsushita, T.; Asou, N.; Kishimoto, Y.; Tsuzuki, M.; Maeda, Y.; Horikawa, K.; Okada, M.; Ohtake, S.; Yagasaki, F.; et al. Severe Hemorrhagic Complications during Remission Induction Therapy for Acute Promyelocytic Leukemia: Incidence, Risk Factors, and Influence on Outcome. Eur. J. Haematol. 2007, 78, 213–219. [Google Scholar] [CrossRef]
- Chang, H.; Kuo, M.-C.; Shih, L.-Y.; Dunn, P.; Wang, P.-N.; Wu, J.-H.; Lin, T.-L.; Hung, Y.-S.; Tang, T.-C. Clinical Bleeding Events and Laboratory Coagulation Profiles in Acute Promyelocytic Leukemia. Eur. J. Haematol. 2012, 88, 321–328. [Google Scholar] [CrossRef]
- Breccia, M.; Latagliata, R.; Cannella, L.; Minotti, C.; Meloni, G.; Lo-Coco, F. Early Hemorrhagic Death before Starting Therapy in Acute Promyelocytic Leukemia: Association with High WBC Count, Late Diagnosis and Delayed Treatment Initiation. Haematologica 2010, 95, 853–854. [Google Scholar] [CrossRef]
- Abla, O.; Ribeiro, R.C.; Testi, A.M.; Montesinos, P.; Creutzig, U.; Sung, L.; Di Giuseppe, G.; Stephens, D.; Feusner, J.H.; Powell, B.L.; et al. Predictors of Thrombohemorrhagic Early Death in Children and Adolescents with t(15;17)-Positive Acute Promyelocytic Leukemia Treated with ATRA and Chemotherapy. Ann. Hematol. 2017, 96, 1449–1456. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, Y.; Jin, B.; Cao, W.; Lu, M.; Yan, L.; Yang, H.; Tian, X.; Hou, J.; Fu, J.; et al. Factors Affecting Thrombohemorrhagic Early Death in Patients with Acute Promyelocytic Leukemia Treated with Arsenic Trioxide Alone. Blood Cells. Mol. Dis. 2019, 79, 102351. [Google Scholar] [CrossRef]
- Chu, Y.; Guo, H.; Zhang, Y.; Qiao, R. Procoagulant Platelets: Generation, Characteristics, and Therapeutic Target. J. Clin. Lab. Anal. 2021, 35, e23750. [Google Scholar] [CrossRef]
- Wang, T.-F.; Makar, R.S.; Antic, D.; Levy, J.H.; Douketis, J.D.; Connors, J.M.; Carrier, M.; Zwicker, J.I. Management of Hemostatic Complications in Acute Leukemia: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 3174–3183. [Google Scholar] [CrossRef]
- Uhl, L.; Assmann, S.F.; Hamza, T.H.; Harrison, R.W.; Gernsheimer, T.; Slichter, S.J. Laboratory Predictors of Bleeding and the Effect of Platelet and RBC Transfusions on Bleeding Outcomes in the PLADO Trial. Blood 2017, 130, 1247–1258. [Google Scholar] [CrossRef]
- Naymagon, L.; Moshier, E.; Tremblay, D.; Mascarenhas, J. Predictors of Early Hemorrhage in Acute Promyelocytic Leukemia. Leuk. Lymphoma 2019, 60, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Shi, M.; Song, J.; Niu, X.; Wei, S.; Dou, L.; Xiao, M.; Li, D.; Xu, F.; Bai, Y.; et al. Absolute Circulating Leukemic Cells as a Risk Factor for Early Bleeding Events in Patients with Non-High-Risk Acute Promyelocytic Leukemia. Cancer Manag. Res. 2021, 13, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, Y.; Li, F.; Zheng, Y.; Su, Y. Value of the FDP/FIB Ratio in Predicting Early Severe Bleeding Events in Patients with Newly Diagnosed Acute Promyelocytic Leukemia. Ann. Hematol. 2023, 102, 787–794. [Google Scholar] [CrossRef]
- Minamiguchi, H.; Fujita, H.; Atsuta, Y.; Asou, N.; Sakura, T.; Ueda, Y.; Sawa, M.; Dobashi, N.; Taniguchi, Y.; Suzuki, R.; et al. Predictors of Early Death, Serious Hemorrhage, and Differentiation Syndrome in Japanese Patients with Acute Promyelocytic Leukemia. Ann. Hematol. 2020, 99, 2787–2800. [Google Scholar] [CrossRef]
- Bassi, S.C.; Rego, E.M. Tissue Factor Pathway Inhibitor (TFPI) May Be Another Important Factor in the Coagulopathy in Acute Promyelocytic Leukemia (APL). Blood 2015, 126, 2278. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Trenor, C.C.; Furie, B.C.; Furie, B. Tissue Factor–Bearing Microparticles and Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Rego, E.M. Role of Microparticles in the Hemostatic Dysfunction in Acute Promyelocytic Leukemia. Semin. Thromb. Hemost. 2010, 36, 917–924. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Rego, E.M.; McMachon, B.; Weiss, I. Thrombin Generationand Fibrinolytic Activity in Microparticles In Acute Promyelocytic Leukemia. Blood 2013, 122, 3620. [Google Scholar] [CrossRef]
- Ma, G.; Liu, F.; Lv, L.; Gao, Y.; Su, Y. Increased Promyelocytic-Derived Microparticles: A Novel Potential Factor for Coagulopathy in Acute Promyelocytic Leukemia. Ann. Hematol. 2013, 92, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Spath, B.; Haubold, K.; Holstein, K.; Marx, G.; Wierecky, J.; Brümmendorf, T.H.; Dierlamm, J.; Bokemeyer, C.; Eifrig, B. Tissue Factor Procoagulant Activity of Plasma Microparticles in Patients with Cancer-Associated Disseminated Intravascular Coagulation. Ann. Hematol. 2008, 87, 451–457. [Google Scholar] [CrossRef]
- Griffin, J.D.; Rambaldi, A.; Vellenga, E.; Young, D.C.; Ostapovicz, D.; Cannistra, S.A. Secretion of Interleukin-1 by Acute Myeloblastic Leukemia Cells in Vitro Induces Endothelial Cells to Secrete Colony Stimulating Factors. Blood 1987, 70, 1218–1221. [Google Scholar] [CrossRef]
- Cozzolino, F.; Torcia, M.; Miliani, A.; Carossino, A.M.; Giordani, R.; Cinotti, S.; Filimberti, E.; Saccardi, R.; Bernabei, P.; Guidi, G. Potential Role of Interleukin-1 as the Trigger for Diffuse Intravascular Coagulation in Acute Nonlymphoblastic Leukemia. Am. J. Med. 1988, 84, 240–250. [Google Scholar] [CrossRef]
- Schorer, A.E.; Kaplan, M.E.; Rao, G.H.; Moldow, C.F. Interleukin 1 Stimulates Endothelial Cell Tissue Factor Production and Expression by a Prostaglandin-Independent Mechanism. Thromb. Haemost. 1986, 56, 256–259. [Google Scholar] [CrossRef]
- Schleef, R.R.; Bevilacqua, M.P.; Sawdey, M.; Gimbrone, M.A.; Loskutoff, D.J. Cytokine Activation of Vascular Endothelium. Effects on Tissue-Type Plasminogen Activator and Type 1 Plasminogen Activator Inhibitor. J. Biol. Chem. 1988, 263, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.R.; Tsiang, M.; Sadler, J.E. Regulation of Thrombomodulin by Tumor Necrosis Factor-Alpha: Comparison of Transcriptional and Posttranscriptional Mechanisms. Blood 1991, 77, 542–550. [Google Scholar] [CrossRef]
- Ikezoe, T.; Takeuchi, A.; Isaka, M.; Arakawa, Y.; Iwabu, N.; Kin, T.; Anabuki, K.; Sakai, M.; Taniguchi, A.; Togitani, K.; et al. Recombinant Human Soluble Thrombomodulin Safely and Effectively Rescues Acute Promyelocytic Leukemia Patients from Disseminated Intravascular Coagulation. Leuk. Res. 2012, 36, 1398–1402. [Google Scholar] [CrossRef]
- Ikezoe, T. Pathogenesis of Disseminated Intravascular Coagulation in Patients with Acute Promyelocytic Leukemia, and Its Treatment Using Recombinant Human Soluble Thrombomodulin. Int. J. Hematol. 2014, 100, 27–37. [Google Scholar] [CrossRef]
- Matsuda, K.; Jo, T.; Toyama, K.; Nakazaki, K.; Matsui, H.; Fushimi, K.; Yasunaga, H. Efficacy of Recombinant Human Soluble Thrombomodulin in Induction Therapy for Acute Promyelocytic Leukemia. Thromb. Res. 2021, 202, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Watanabe, J.; Honda, G.; Mimuro, J.; Takahashi, H.; Tsuji, H.; Eguchi, Y.; Kitajima, I.; Sakata, Y. Thrombomodulin Alfa Treatment in Patients with Acute Promyelocytic Leukemia and Disseminated Intravascular Coagulation: A Retrospective Analysis of an Open-Label, Multicenter, Post-Marketing Surveillance Study Cohort. Thromb. Res. 2014, 133, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Lowenberg, B.; Naoe, T.; Lengfelder, E.; Dohner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of Acute Promyelocytic Leukemia: Updated Recommendations from an Expert Panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Montesinos, P.; de la Serna, J.; Vellenga, E.; Rayon, C.; Bergua, J.; Parody, R.; Esteve, J.; Gonzalez, M.; Brunet, S.; Sanz, M. Incidence and Risk Factors for Thrombosis in Patients with Acute Promyelocytic Leukemia. Experience of the PETHEMA LPA96 and LPA99 Protocols. Blood 2006, 108, 1503. [Google Scholar] [CrossRef]
- Chang, H.; Kuo, M.-C.; Shih, L.-Y.; Wu, J.-H.; Lin, T.-L.; Dunn, P.; Tang, T.-C.; Hung, Y.-S.; Wang, P.-N. Acute Promyelocytic Leukemia-Associated Thrombosis. Acta Haematol. 2013, 130, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Sorà, F.; Rossi, E.; Chiusolo, P.; Laurenti, L.; Fianchi, L.; Zini, G.; Pagano, L.; Sica, S.; Leone, G. The Risk of Thrombosis in Patients with Acute Leukemia: Occurrence of Thrombosis at Diagnosis and during Treatment. J. Thromb. Haemost. 2005, 3, 1985–1992. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, M.; Yang, X.; Zhang, W.; Yang, R.; Wei, X.; Wei, X.; Duan, L.; Wang, C.; Mi, R.; et al. The Value of FDP/FIB and D-Dimer/FIB Ratios in Predicting High-Risk APL-Related Thrombosis. Leuk. Res. 2019, 79, 34–37. [Google Scholar] [CrossRef]
- Watts, J.M.; Tallman, M.S. Acute Promyelocytic Leukemia: What Is the New Standard of Care? Blood Rev. 2014, 28, 205–212. [Google Scholar] [CrossRef]
- Huang, M.E.; Ye, Y.C.; Chen, S.R.; Chai, J.R.; Lu, J.X.; Zhoa, L.; Gu, L.J.; Wang, Z.Y. Use of All-Trans Retinoic Acid in the Treatment of Acute Promyelocytic Leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef]
- Fenaux, P.; Chastang, C.; Chevret, S.; Sanz, M.; Dombret, H.; Archimbaud, E.; Fey, M.; Rayon, C.; Huguet, F.; Sotto, J.-J.; et al. A Randomized Comparison of All Transretinoic Acid (ATRA) Followed by Chemotherapy and ATRA Plus Chemotherapy and the Role of Maintenance Therapy in Newly Diagnosed Acute Promyelocytic Leukemia. Blood 1999, 94, 1192–1200. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Bowen, D.; Kell, J.; Knapper, S.; Morgan, Y.G.; Lok, J.; Grech, A.; Jones, G.; et al. Arsenic Trioxide and All-Trans Retinoic Acid Treatment for Acute Promyelocytic Leukaemia in All Risk Groups (AML17): Results of a Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2015, 16, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Olwill, S.A.; McGlynn, H.; Gilmore, W.S.; Alexander, H.D. All-Trans Retinoic Acid-Induced Downregulation of Annexin II Expression in Myeloid Leukaemia Cell Lines Is Not Confined to Acute Promyelocytic Leukaemia. Br. J. Haematol. 2005, 131, 258–264. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Teofili, L.; Sica, S.; Mastrangelo, S.; Di Mario, A.; Rutella, S.; Salutari, P.; Rumi, C.; d’Onofrio, G.; Leone, G. Effect of All-Trans Retinoic Acid on Procoagulant and Fibrinolytic Activities of Cultured Blast Cells from Patients with Acute Promyelocytic Leukemia. Blood 1995, 86, 3535–3541. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Lefèbvre, P.; Baine, R.M.; Shoji, M.; Cohen, I.; Green, D.; Kwaan, H.C.; Paietta, E.; Rickles, F.R. Effects of All-Trans Retinoic Acid or Chemotherapy on the Molecular Regulation of Systemic Blood Coagulation and Fibrinolysis in Patients with Acute Promyelocytic Leukemia. J. Thromb. Haemost. 2004, 2, 1341–1350. [Google Scholar] [CrossRef]
- Falanga, A.; Diani, E.; Russo, L.; Balducci, D.; Marchetti, M. Arsenic Trioxide (ATO) and All-Trans Retinoic Acid (ATRA) Differently Affect the Thrombin Generation Potential of Acute Promyelocytic Leukemia (APL) Cells. Blood 2009, 114, 3986. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Luo, D.; Zhou, J.; Li, D. Addition of Arsenic Trioxide into Induction Regimens Could Not Accelerate Recovery of Abnormality of Coagulation and Fibrinolysis in Patients with Acute Promyelocytic Leukemia. PLoS ONE 2016, 11, e0147545. [Google Scholar] [CrossRef]
- Zhu, H.-H.; Wu, D.-P.; Du, X.; Zhang, X.; Liu, L.; Ma, J.; Shao, Z.-H.; Ren, H.-Y.; Hu, J.-D.; Xu, K.-L.; et al. Oral Arsenic plus Retinoic Acid versus Intravenous Arsenic plus Retinoic Acid for Non-High-Risk Acute Promyelocytic Leukaemia: A Non-Inferiority, Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 871–879. [Google Scholar] [CrossRef]
- Wartha, F.; Henriques-Normark, B. ETosis: A Novel Cell Death Pathway. Sci. Signal. 2008, 1, pe25. [Google Scholar] [CrossRef]
- Ma, R.; Li, T.; Cao, M.; Si, Y.; Wu, X.; Zhao, L.; Yao, Z.; Zhang, Y.; Fang, S.; Deng, R.; et al. Extracellular DNA Traps Released by Acute Promyelocytic Leukemia Cells through Autophagy. Cell Death Dis. 2016, 7, e2283. [Google Scholar] [CrossRef]
- Li, T.; Ma, R.; Zhang, Y.; Mo, H.; Yang, X.; Hu, S.; Wang, L.; Novakovic, V.A.; Chen, H.; Kou, J.; et al. Arsenic Trioxide Promoting ETosis in Acute Promyelocytic Leukemia through MTOR-Regulated Autophagy. Cell Death Dis. 2018, 9, 75. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zuo, N.; Yang, H.; Fang, S.; Shi, J. Extracellular Traps Increase Burden of Bleeding by Damaging Endothelial Cell in Acute Promyelocytic Leukaemia. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Liaw, P.C. The Role of Leukocytes in Thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kantarjian, H.; Ravandi, F. Acute Promyelocytic Leukemia Current Treatment Algorithms. Blood Cancer J. 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Estey, E.; Jones, D.; Faderl, S.; O’Brien, S.; Fiorentino, J.; Pierce, S.; Blamble, D.; Estrov, Z.; Wierda, W.; et al. Effective Treatment of Acute Promyelocytic Leukemia with All-Trans-Retinoic Acid, Arsenic Trioxide, and Gemtuzumab Ozogamicin. J. Clin. Oncol. 2009, 27, 504–510. [Google Scholar] [CrossRef]
- Abaza, Y.; Kantarjian, H.; Garcia-Manero, G.; Estey, E.; Borthakur, G.; Jabbour, E.; Faderl, S.; O’Brien, S.; Wierda, W.; Pierce, S.; et al. Long-Term Outcome of Acute Promyelocytic Leukemia Treated with All-Trans-Retinoic Acid, Arsenic Trioxide, and Gemtuzumab. Blood 2017, 129, 1275–1283. [Google Scholar] [CrossRef]
- Lancet, J.E.; Moseley, A.B.; Coutre, S.E.; DeAngelo, D.J.; Othus, M.; Tallman, M.S.; Litzow, M.R.; Komrokji, R.S.; Erba, H.P.; Appelbaum, F.R. A Phase 2 Study of ATRA, Arsenic Trioxide, and Gemtuzumab Ozogamicin in Patients with High-Risk APL (SWOG 0535). Blood Adv. 2020, 4, 1683–1689. [Google Scholar] [CrossRef]
- Sanz, M.A.; Grimwade, D.; Tallman, M.S.; Lowenberg, B.; Fenaux, P.; Estey, E.H.; Naoe, T.; Lengfelder, E.; Büchner, T.; Döhner, H.; et al. Management of Acute Promyelocytic Leukemia: Recommendations from an Expert Panel on Behalf of the European LeukemiaNet. Blood 2009, 113, 1875–1891. [Google Scholar] [CrossRef]
- Oh, H.; Park, H.E.; Song, M.S.; Kim, H.; Baek, J.-H. The Therapeutic Potential of Anticoagulation in Organ Fibrosis. Front. Med. 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Hambley, B.C.; Norsworthy, K.J.; Jasem, J.; Zimmerman, J.W.; Shenderov, E.; Webster, J.A.; Showel, M.M.; Gondek, L.P.; Dalton, W.B.; Prince, G.; et al. Fibrinogen Consumption and Use of Heparin Are Risk Factors for Delayed Bleeding during Acute Promyelocytic Leukemia Induction. Leuk. Res. 2019, 83, 106174. [Google Scholar] [CrossRef]
- Ito, T.; Kakihana, Y.; Maruyama, I. Thrombomodulin as an Intravascular Safeguard against Inflammatory and Thrombotic Diseases. Expert Opin. Ther. Targets 2016, 20, 151–158. [Google Scholar] [CrossRef]
- Ito, T.; Thachil, J.; Asakura, H.; Levy, J.H.; Iba, T. Thrombomodulin in Disseminated Intravascular Coagulation and Other Critical Conditions—A Multi-Faceted Anticoagulant Protein with Therapeutic Potential. Crit. Care 2019, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Maruyama, I.; Shimazaki, S.; Yamamoto, Y.; Aikawa, N.; Ohno, R.; Hirayama, A.; Matsuda, T.; Asakura, H.; Nakashima, M.; et al. Efficacy and Safety of Recombinant Human Soluble Thrombomodulin (ART-123) in Disseminated Intravascular Coagulation: Results of a Phase III, Randomized, Double-Blind Clinical Trial. J. Thromb. Haemost. 2007, 5, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Montesinos, P. Open Issues on Bleeding and Thrombosis in Acute Promyelocytic Leukemia. Thromb. Res. 2010, 125, S51–S54. [Google Scholar] [CrossRef] [PubMed]
- Zver, S.; Andoljšek, D.; Černelč, P. Effective Treatment of Life-Threatening Bleeding with Recombinant Activated Factor VII in a Patient with Acute Promyelocytic Leukaemia. Eur. J. Haematol. 2004, 72, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xue, F.; Liu, X.F.; Jiang, E.L.; Yang, D.L.; Liu, K.Q.; Xiao, Z.J.; Zhang, F.K.; Feng, S.Z.; Han, M.Z.; et al. Analysis of clinical efficacy of recombinant activated factor VII on bleeding in patients with hematologic disorders. Zhonghua Xue Ye Xue Za Zhi 2017, 38, 410–414. [Google Scholar] [CrossRef]
- Nosari, A.; Caimi, T.M.; Zilioli, V.; Molteni, A.; Mancini, V.; Morra, E. Cerebral Hemorrhage Treated with NovoSeven in Acute Promyelocytic Leukemia. Leuk. Lymphoma 2012, 53, 160–161. [Google Scholar] [CrossRef]
- Levi, M.; Levy, J.H.; Andersen, H.F.; Truloff, D. Safety of Recombinant Activated Factor VII in Randomized Clinical Trials. N. Engl. J. Med. 2010, 363, 1791–1800. [Google Scholar] [CrossRef]
- Downey, L.; Brown, M.L.; Faraoni, D.; Zurakowski, D.; DiNardo, J.A. Recombinant Factor VIIa Is Associated With Increased Thrombotic Complications in Pediatric Cardiac Surgery Patients. Anesth. Analg. 2017, 124, 1431–1436. [Google Scholar] [CrossRef]
- Warren, B.L.; Eid, A.; Singer, P.; Pillay, S.S.; Carl, P.; Novak, I.; Chalupa, P.; Atherstone, A.; Pénzes, I.; Kübler, A.; et al. High-Dose Antithrombin III in Severe SepsisA Randomized Controlled Trial. JAMA 2001, 286, 1869–1878. [Google Scholar] [CrossRef]
- Eisele, B.; Heinrichs, H.; Delvos, U.; Lamy, M.; Thijs, L.G.; Keinecke, H.O.; Schuster, H.P.; Matthias, F.R.; Fourrier, F. Antithrombin III in Patients with Severe Sepsis. Intensive Care Med. 1998, 24, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, B.; Villoutreix, B.O. The Anticoagulant Protein C Pathway. FEBS Lett. 2005, 579, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T. Thrombomodulin/Activated Protein C System in Septic Disseminated Intravascular Coagulation. J. Intensiv. Care 2015, 3, 1. [Google Scholar] [CrossRef]
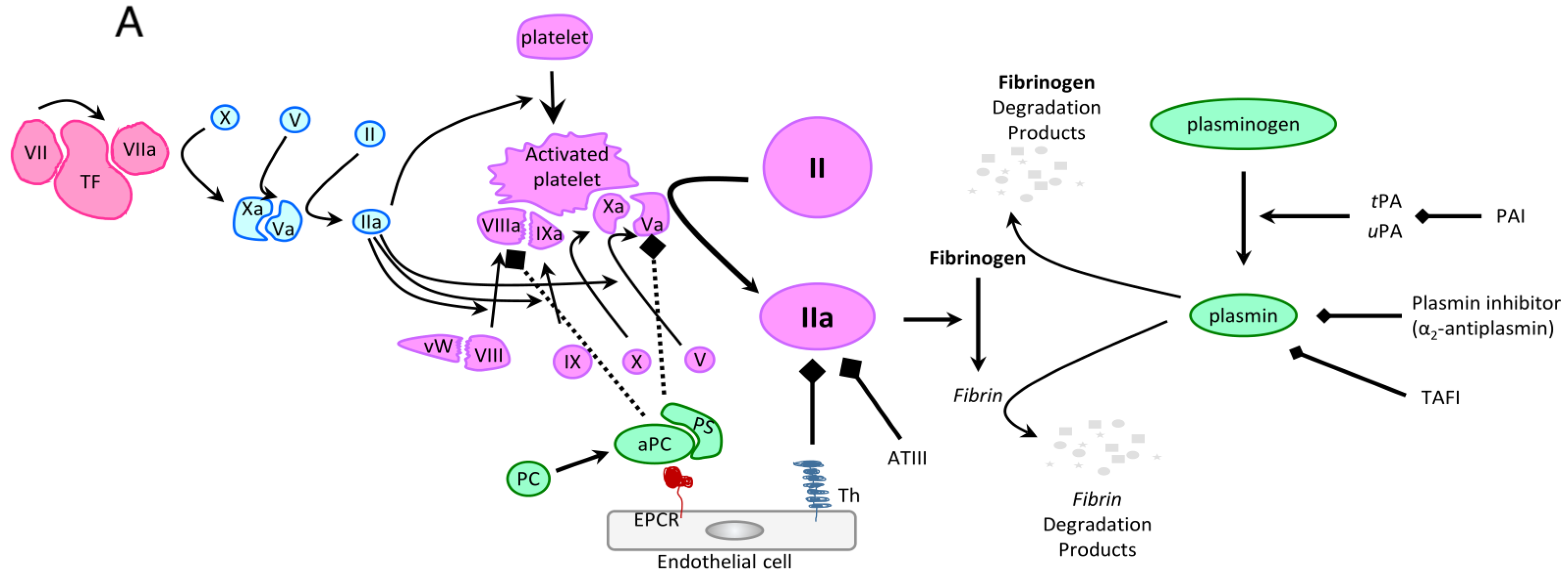
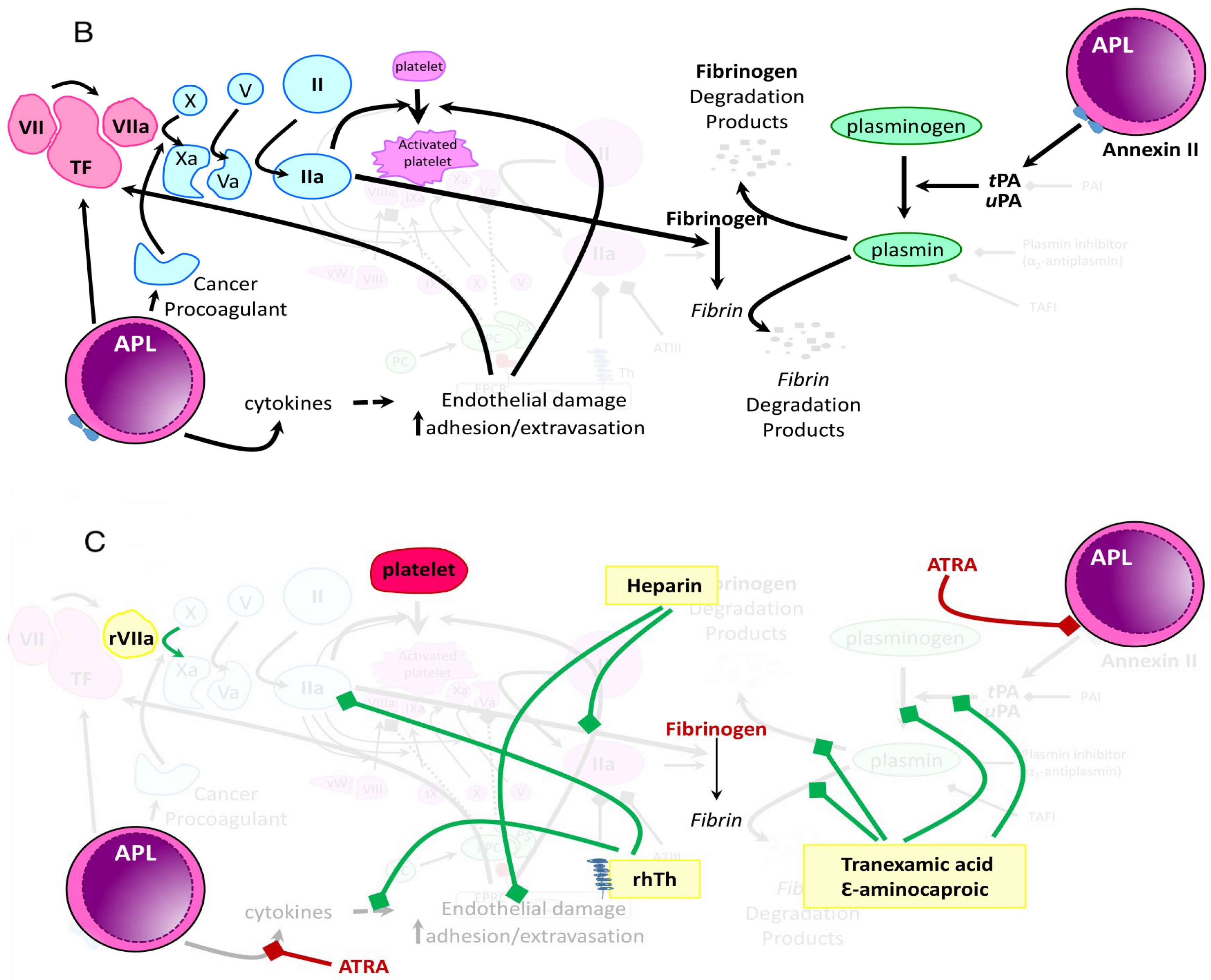
| Study/Year | Incidence of Early Death | Incidence of Early Hemorrhagic Death | Types of Hemorrhage |
|---|---|---|---|
| Lehmann et al., 2011 [65] | 28.5% (30/105) | 11.4% (12/105) | Intracranial 11/12 Pulmonary 1/12 |
| Park et al., 2011 [12] | 17.3% (242/1400) | N/A | Not reported |
| McClellan et al., 2012 [74] | 18.6% (13/70) | 11.4% (8/70) | Intracranial 7/8 Pulmonary 1/8 |
| Lehmann et al., 2017 [11] | 25.1% (49/195) | 11.3% (22/195) | Intracranial 21/22 Pulmonary 1/22 |
| Xu et al., 2017 [13] | 23.1% (49/212) | 17.5% (37/212) | Not reported |
| Mantha et al., 2017 [69] a | N/A | 3.7% (37/995) | Intracranial 24/37 Pulmonary 12/37 |
| Ho et al., 2018 [70] | 16.7% (161/963) | 9.0% (87/963) | Only reported on intracranial events |
| Silva et al., 2019 [71] | 29.5% (18/61) | 9.8% (6/61) | Intracranial 5/6 Pulmonary 1/6 |
| Xiao et al., 2022 [72] b | Not reported | Overall rate of bleeding events (15/83) Hemorrhagic death rate 8.4% (7/83) | Intracranial 8/15 Pulmonary 2/15 Gastrointestinal 2/15 Other 2/15 |
| Parameter | Supporting Studies | Suggested Cutoff Value |
|---|---|---|
| Leukocytosis | [11,13,15,35,62,69,72,76,77,78,79,80] | >10 × 109/L >20 × 109/L [69] |
| Fibrinogen | [6,35,62,76,80,81] | <1600 mg/L [81] <1000 mg/L [6,82] |
| Thrombocytopenia | [11,13,14,35,62] | <5 × 109/L [83] <30 × 109/L [11] |
| Lactate Dehydrogenase | [13,35,62,78,84] | >700 U/L [84] |
| Creatinine | [11,13,15,78,80] | >90 umol/L (1.02 mg/dL) [11] |
| Performance Status | [6,13,69,76] | ECOG 3–4 [13,69] |
| PT | [6,13,72,77] | N/A |
| D-Dimer | [62,80] | >4 mg/L [80] |
| Age | [13,69] | >55 years old [11] |
| Circulating Promyeloblast Level | [69,85] | >1.00–2.59 × 109/L [69,85] |
| Study | Type of Thrombosis | Incidence of Thrombosis |
|---|---|---|
| De Stefano et al., 2005 [106] | DVT/PE 1 Intracranial 2 | 9.6% (3/31) |
| Montesinos et al., 2006 [104] | DVT 17/39 PE 5/39 Cardiac 4/39 Intracranial 10/39 Other 3/39 | 5.1% (39/759) |
| Breccia et al., 2007 [17] | DVT 5/11 Cardiac 4/11 Intracranial 2/11 | 8.9% (11/124) |
| Chang et al., 2013 [105] | DVT 5/10 Cardiac 1/10 Intracranial 5/10 | 7.9% (10/127) a |
| Mitrovic et al., 2015 [16] | DVT 7/13 Cardiac 2/13 Intracranial 2/13 Other 2/13 | 5.1% (13/63) |
| Bai et al., 2019 [107] b | DVT 2/6 Intracranial 4/6 | 18.2% (6/33) |
| Xiao et al., 2022 [72] b | DVT 4/10 Cardiac 0/10 Intracerebral 5/10 Other 3/10 | 24.5% (12/83) |
| Rashidi et al., 2013 [18] c | DVT 27/94 Cardiac 25/94 Intracranial 27/94 | N/A |
| Agent/Procedure | Indication |
|---|---|
| ATRA | Immediate initiation if APL is suspected as a diagnosis |
| Cryoprecipitate/Fibrinogen Concentrate 1 | Initiation if APL is suspected as a diagnosis and fibrinogen < 100–150 mg/dL |
| Platelets | Initiation if APL is suspected as a diagnosis and platelet count < 30–50 × 109/L |
| Fresh Frozen Plasma (FFP)/Cryoprecipitate | Initiation if APL is suspected as a diagnosis and INR > 1.5 |
| Heparin | Not recommended unless severe thrombotic events |
| Recombinant Thrombomodulin | DIC—used in Japan, not currently recommended for broad clinical practice pending additional trials |
| Antifibrinolytic/Anticoagulant Therapy | Not recommended outside clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermsen, J.; Hambley, B. The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management. Cancers 2023, 15, 3477. https://doi.org/10.3390/cancers15133477
Hermsen J, Hambley B. The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management. Cancers. 2023; 15(13):3477. https://doi.org/10.3390/cancers15133477
Chicago/Turabian StyleHermsen, Jack, and Bryan Hambley. 2023. "The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management" Cancers 15, no. 13: 3477. https://doi.org/10.3390/cancers15133477
APA StyleHermsen, J., & Hambley, B. (2023). The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management. Cancers, 15(13), 3477. https://doi.org/10.3390/cancers15133477






