Assessment of the Role of Leptin and Adiponectinas Biomarkers in Pancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment of Serum Biomarkers
2.3. Statistical Analysis
3. Results
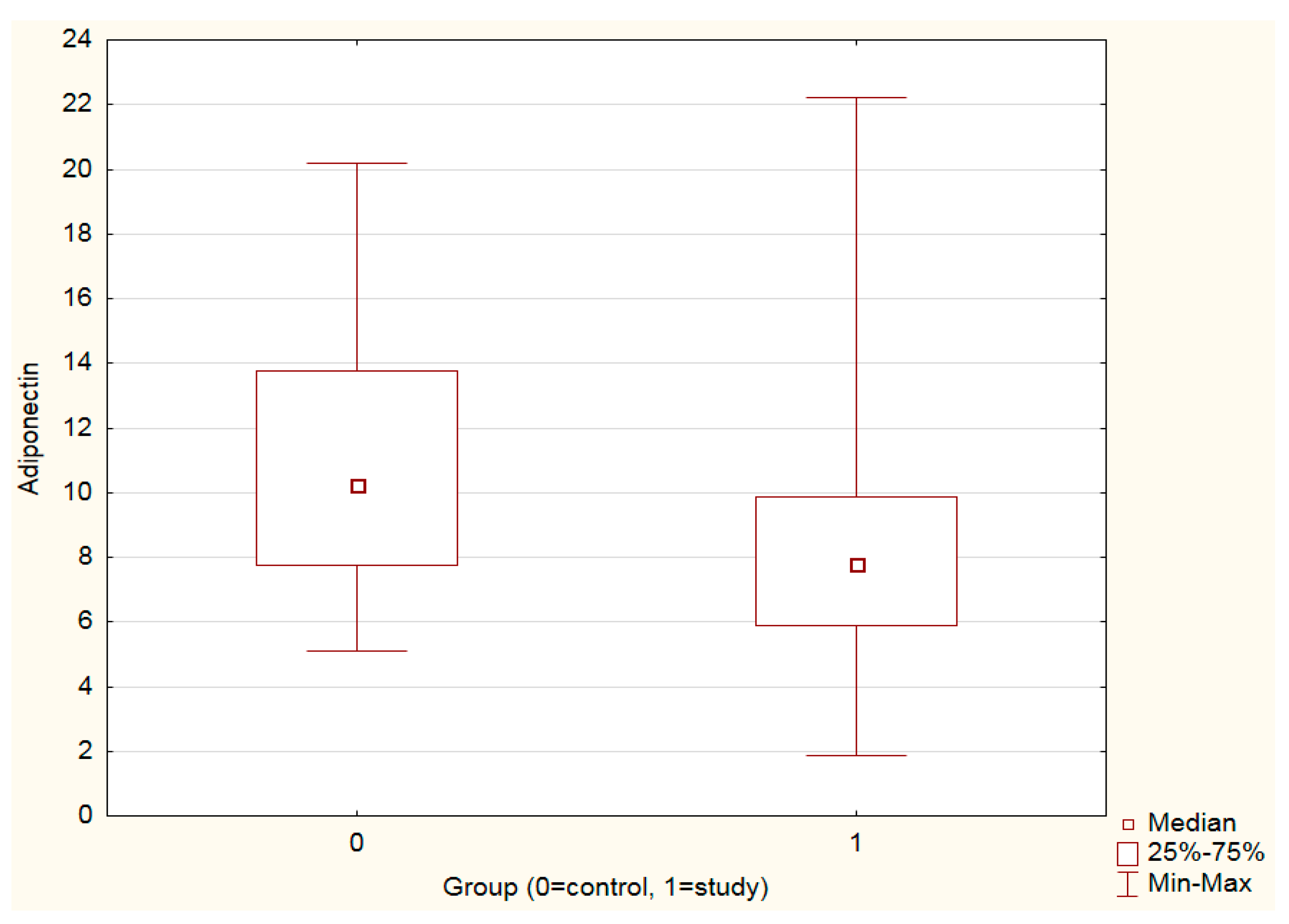
3.1. Leptin, Adiponectin Levels and Leptin–Adiponectin Ratio in the Study vs. Control Group
3.2. Leptin, Adiponectin Levels and Leptin–Adiponectin Ratio in the Study vs. Control Group—Sex Analysis
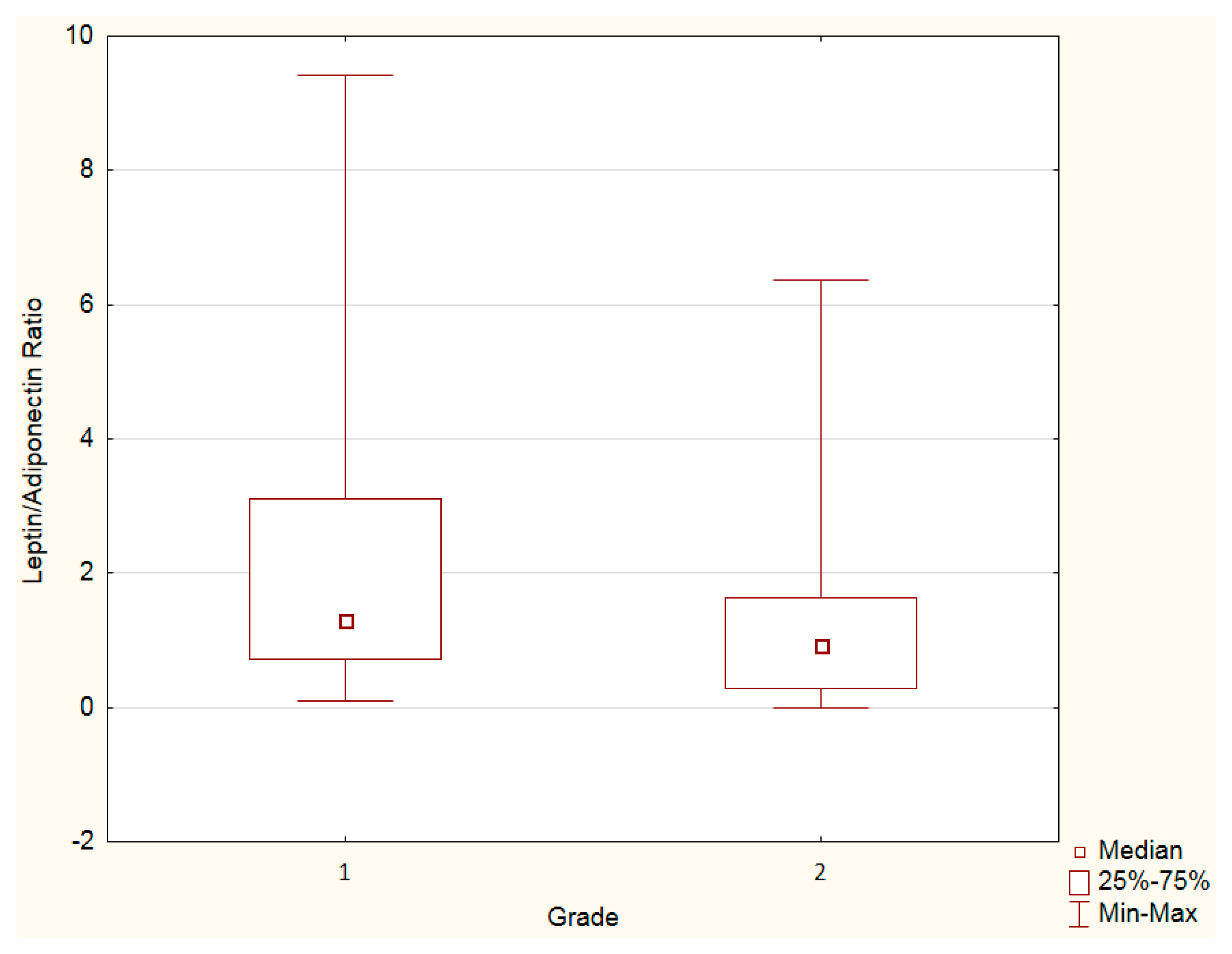
3.3. Leptin, Adiponectin Levels and Leptin–Adiponectin Ratio—Grade Analysis
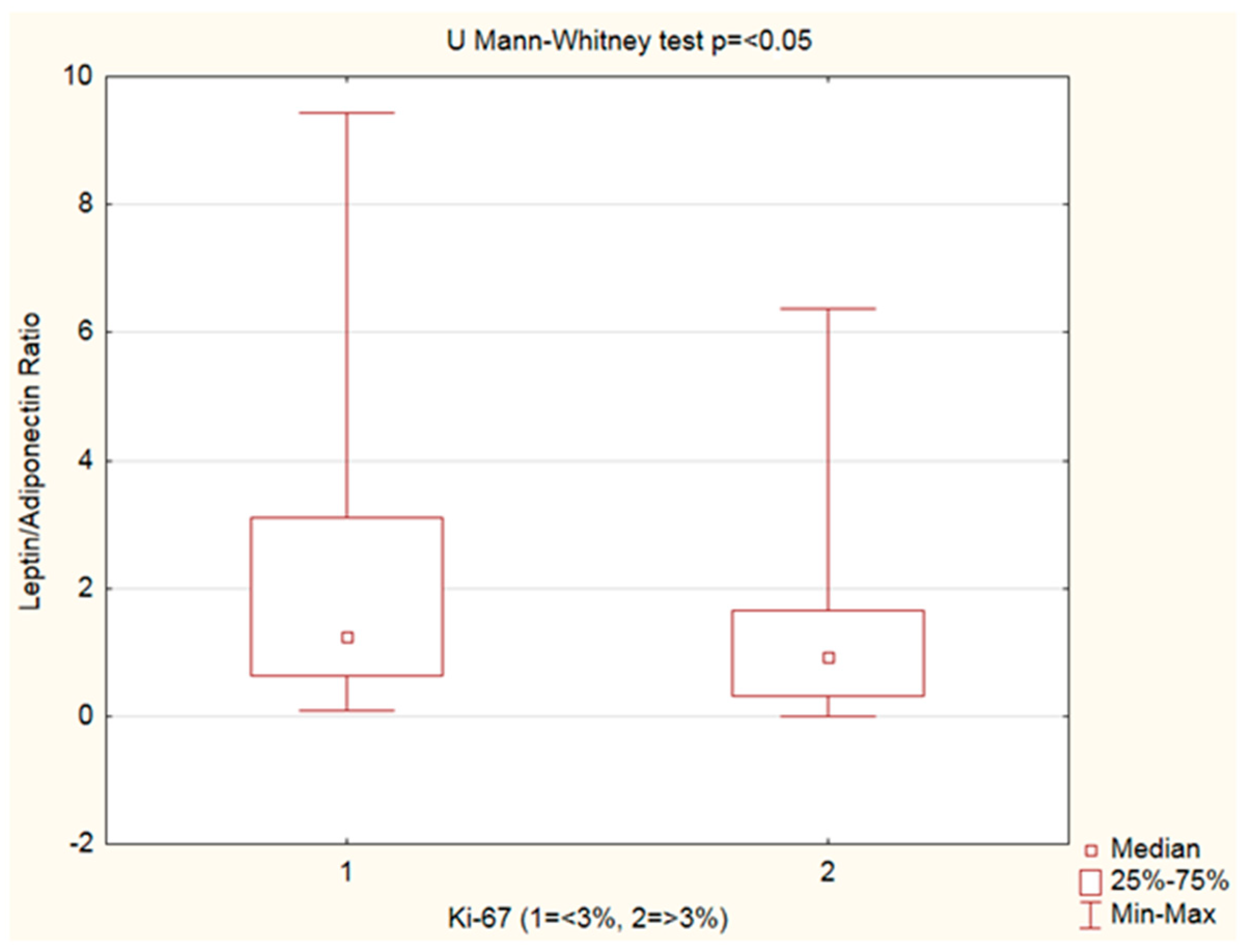
3.4. Leptin, Adiponectin Levels and Leptin–Adiponectin Ratio—Ki-67 Analysis
3.5. Leptin, Adiponectin Levels and Leptin–adiponectin Ratio—Analysis by Disease Extent
3.6. R Spearman’s Correlation
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, R.; Merchant, J.; Conrad, N.; Keutgen, C.; Hallet, X.; Drebin, J.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Kuo, J.H.; Lee, J.A.; Chabot, J.A. Nonfunctional pancreatic neuroendocrine tumors. Surg. Clin. N. Am. 2014, 94, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Bałdys-Waligórska, A.; Nowak, A. Neuroendocrine neoplasms of the digestive system—Current classification and terminology. Nowotwory 2021, 71, 26–37. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Perrier, M.; Polazzi, S.; Lemelin, A.; Fernandez, V.; Labonne, S.; Maucort-Boulch, D.; Lombard-Bohas, C.; Duclos, A.; Walter, T. Healthcare cost by primary tumour, functioning status and treatment among patients with metastatic neuroendocrine tumours: The LyREMeNET study. J. Neuroendocrinol. 2022, 34, e13092. [Google Scholar] [CrossRef]
- Foulfoin, M.; Graillot, E.; Adham, M.; Rousset, P.; Forestier, J.; Hervieu, V.; Robinson, P.; Scoazec, J.Y.; Lombard-Bohas, C.; Walter, T. Treatment of metastatic pancreatic neuroendocrine tumors: Relevance of ENETS 2016 guidelines. Endocr. Relat. Cancer 2017, 24, 71–81. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, H.L.; Conlon, K.C. Pancreatic neuroendocrine tumours. Eur. J. Surg. Oncol. 2008, 34, 324–332. [Google Scholar] [CrossRef]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St Peter, J.; Haas, T.; Lebwohl, D.; et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Ohtsuka, T.; Mori, Y.; Fujino, M.; Yasui, T.; Aishima, S.; Takahata, S.; Nakamura, M.; Ito, T.; Tanaka, M. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J. Gastroenterol. 2012, 47, 678–685. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic neuroendocrine neoplasms—Update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef]
- Van Loon, K.; Zhang, L.; Keiser, J.; Carrasco, C.; Glass, K.; Ramirez, M.T.; Bobiak, S.; Nakakura, E.K.; Venook, A.P.; Shah, M.H.; et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 2015, 4, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Hursting, S.D.; Berger, N.A. Energy balance, host-related factors, and cancer progression. J. Clin. Oncol. 2010, 28, 4058–4065. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Sena, C.M.; Seiça, R. Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab. Res. Rev. 2013, 29, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Begier, B.; Rafał, K.; Edyta, S. Obesity and the Risk of Gastrointestinal Cancers. Dig. Dis. Sci. 2019, 64, 2740–2749. [Google Scholar] [CrossRef] [Green Version]
- Vansaun, M.N. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin. Cancer Res. 2013, 19, 1926–1932. [Google Scholar] [CrossRef] [Green Version]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, J.M.; Pidgeon, G.P.; Reynolds, J.V. Leptin and gastro-intestinal malignancies. Obes. Rev. 2010, 11, 863–874. [Google Scholar] [CrossRef]
- Zhang, J.; Hochwald, S.N. Plasma adiponectin: A possible link between fat metabolism and pancreatic cancer risk. J. Natl. Cancer Inst. 2013, 105, 79–80. [Google Scholar] [CrossRef] [Green Version]
- Paz-Filho, G.; Lim, E.L.; Wong, M.L.; Licinio, J. Associations between adipokines and obesity-related cancer. Front. Biosci. 2011, 16, 1634–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpilla, G.B.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Pancreatic neuroendocrine tumors: Therapeutic challenges and research limitations. World J. Gastroenterol. 2020, 26, 4036–4054. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef]
- Sook, Y.; Kwon, A.R.; Kyung, Y.; Woo, S. Obesity Research & Clinical Practice Circulating adipokines and risk of obesity related cancers: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2019, 13, 329–339. [Google Scholar] [CrossRef]
- Katz, L.H.; Levi, Z.; Twig, G.; Kark, J.D.; Leiba, A.; Derazne, E.; Liphshiz, I.; Keinan-Boker, L.; Eisenstein, S.; Afek, A. Risk factors associated with gastroenteropancreatic neuroendocrine tumors in a cohort of 2.3 million Israeli adolescents. Int. J. Cancer 2018, 143, 1876–1883. [Google Scholar] [CrossRef] [Green Version]
- Feola, T.; Puliani, G.; Sesti, F.; Modica, R.; Centello, R.; Minotta, R.; Cannavale, G.; Di Meglio, S.; Di Vito, V.; Lauretta, R.; et al. Risk factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A three-centric case-control study. J. Endocrinol. Investig. 2022, 45, 849–857. [Google Scholar] [CrossRef]
- Santos, A.P.; Santos, A.C.; Castro, C.; Raposo, L.; Pereira, S.S.; Torres, I.; Henrique, R.; Cardoso, H.; Monteiro, M.P. Visceral obesity and metabolic syndrome are associated with well-differentiated gastroenteropancreatic neuroendocrine tumors. Cancers 2018, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Muscogiuri, G.; Modica, R.; Altieri, B.; Pugliese, G.; Minotta, R.; Faggiano, A.; Colao, A.; Savastano, S. Cardio-Metabolic Indices and Metabolic Syndrome as Predictors of Clinical Severity of Gastroenteropancreatic Neuroendocrine Tumors. Front. Endocrinol. 2021, 12, 649496. [Google Scholar] [CrossRef]
- Ranallo, N.; Iamurri, A.P.; Foca, F.; Liverani, C.; De Vita, A.; Mercatali, L.; Calabrese, C.; Spadazzi, C.; Fabbri, C.; Cavaliere, D.; et al. Prognostic and Predictive Role of Body Composition in Metastatic Neuroendocrine Tumor Patients Treated with Everolimus: A Real-World Data Analysis. Cancers 2022, 14, 3231. [Google Scholar] [CrossRef]
- Leoncini, E.; Carioli, G.; La Vecchia, C.; Boccia, S.; Rindi, G. Risk factors for neuroendocrine neoplasms: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Halfdanarson, T.R.; Bamlet, W.R.; McWilliams, R.R.; Hobday, T.J.; Burch, P.A.; Rabe, K.G.; Petersen, G.M. Risk factors for pancreatic neuroendocrine tumors a clinic-based case-control study. Pancreas 2014, 43, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Zhan, H.; Cong, L.; Zhao, Y.; Zhang, T.; Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 324–328. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Valente, R.; Hayes, A.J.; Haugvik, S.P.; Hedenström, P.; Siuka, D.; Korsæth, E.; Kämmerer, D.; Robinson, S.M.; Maisonneuve, P.; DelleFave, G.; et al. Risk and protective factors for the occurrence of sporadic pancreatic endocrine neoplasms. Endocr. Relat. Cancer 2017, 24, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, Q.; Zhong, J.; Fei, J.; Chen, H.; Yv, L.; Tan, J.; Yuan, Y. Risk Factors for Sporadic Pancreatic Neuroendocrine Tumors: A Case-Control Study. Sci. Rep. 2016, 26. [Google Scholar] [CrossRef] [PubMed]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef]
- Gallo, M.; Ruggeri, R.M.; Muscogiuri, G.; Pizza, G.; Faggiano, A.; Colao, A. NIKE Group. Diabetes and pancreatic neuroendocrine tumours: Which interplays, if any? Cancer Treat. Rev. 2018, 67, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Prinzi, N.; Torchio, M.; Corti, F.; Coppa, J.; Buzzoni, R.; Di Bartolomeo, M.; Milione, M.; et al. Impact of Diabetes and Metformin Use on Enteropancreatic Neuroendocrine Tumors: Post Hoc Analysis of the CLARINET Study. Cancers 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, X.; Chen, C.; Liu, X.; Chen, Y.; Tan, C. Prognostic value of preoperative diabetes mellitus in patients with non-functional pancreatic neuroendocrine neoplasms. Am. J. Surg. 2022, 224, 1162–1167. [Google Scholar] [CrossRef]
- Kusne, Y.N.; Kosiorek, H.E.; Buras, M.R.; Verona, P.M.; Coppola, K.E.; Rone, K.A.; Cook, C.B.; Karlin, N.J. Implications of neuroendocrine tumor and diabetes mellitus on patient outcomes and care: A matched case-control study. Future Sci. OA. 2021, 7, 684. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Dziemidok, P.; Kiełczykowska, M.; Hordyjewska, A.; Szcześniak, G.; Musik, I. Adipokine Profile in Patients with Type 2 Diabetes Depends on Degree of Obesity. Med. Sci. Monit. 2017, 23, 4995–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.P.; Laurencikiene, J.; Acosta, J.R.; Rydén, M.; Arner, P. Circulating and Adipose Levels of Adipokines Associated With Insulin Sensitivity in Nonobese Subjects With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3765–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidulescu, A.; Dinh, P.C., Jr.; Sarwary, S.; Forsyth, E.; Luetke, M.C.; King, D.B.; Liu, J.; Davis, S.K.; Correa, A. Associations of leptin and adiponectin with incident type 2 diabetes and interactions among African Americans: The Jackson heart study. BMC Endocr. Disord. 2020, 20, 31. [Google Scholar] [CrossRef] [Green Version]
- Alfaqih, M.A.; Al-Hawamdeh, A.; Amarin, Z.O.; Khader, Y.S.; Mhedat, K.; Allouh, M.Z. Single Nucleotide Polymorphism in the ADIPOQ Gene Modifies Adiponectin Levels and Glycemic Control in Type Two Diabetes Mellitus Patients. Biomed. Res. Int. 2022, 27, 6632442. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, L.; Evans, A.; Rao, R.G.; Jenkinson, C.; Purewal, T.; Psarelli, E.E.; Menon, U.; Timms, J.F.; Pereira, S.P.; Ghaneh, P.; et al. Blood levels of adiponectin and IL-1Ra distinguish type 3c from type 2 diabetes: Implications for earlier pancreatic cancer detection in new-onset diabetes. EBioMedicine 2022, 75, 103802. [Google Scholar] [CrossRef]
- Pusceddu, S.; Vernieri, C.; Prinzi, N.; Torchio, M.; Coppa, J.; Antista, M.; Niger, M.; Milione, M.; Giacomelli, L.; Corti, F.; et al. The potential role of metformin in the treatment of patients with pancreatic neuroendocrine tumors: A review of preclinical to clinical evidence. Therap. Adv. Gastroenterol. 2020, 13, 1756284820927271. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef]
- Szekeres, Z.; Sandor, B.; Bognar, Z.; Ramadan, F.H.J.; Palfi, A.; Bodis, B.; Toth, K.; Szabados, E. Clinical Study of Metabolic Parameters, Leptin and the SGLT2 Inhibitor Empagliflozin among Patients with Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 4405. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. Adiponectin: A manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc. Diabetol. 2014, 13, 103. [Google Scholar] [CrossRef] [Green Version]
- Natalicchio, A.; Faggiano, A.; Zatelli, M.C.; Argentiero, A.; D’Oronzo, S.; Marrano, N.; Beretta, G.D.; Acquati, S.; Adinolfi, V.; Di Bartolo, P.; et al. Metabolic disorders and gastroenteropancreatic-neuroendocrine tumors (GEP-NENs): How do they influence each other? An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit. Rev. Oncol.Hematol. 2022, 169, 103572. [Google Scholar] [PubMed]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Marconcini, R.; Spada, F.; Massironi, S.; Ibrahim, T.; Brizzi, M.P.; Campana, D.; Faggiano, A.; et al. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 2018, 155, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.L.; Yang, Z.; Yang, S.S. Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression. Int. J. Mol. Sci. 2020, 21, 8308. [Google Scholar] [CrossRef] [PubMed]
- Gukovsky, I.; Li, N.; Todoric, J.; Gukovskaya, A.; Karin, M. Inflammation, autophagy, and obesity: Common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1199–1209.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, J.; Lagou, V.; Goveia, J.; Ulrich, A.; Rohlenova, K.; Heirman, N.; Karakach, T.; Lampi, Y.; Khan, S.; Wang, J.; et al. Heterogeneous Effects of Calorie Content and Nutritional Components Underlie Dietary Influence on Pancreatic Cancer Susceptibility. Cell Rep. 2020, 32, 107880. [Google Scholar] [CrossRef]
- Mendonsa, A.M.; Chalfant, M.C.; Gorden, L.D.; VanSaun, M.N. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS ONE 2015, 10, e0126686. [Google Scholar] [CrossRef]
- Aune, D.; Greenwood, D.C.; Chan, D.S.; Vieira, R.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Cade, J.E.; Burley, V.J.; Norat, T. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 843–852. [Google Scholar] [CrossRef]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Davey Smith, G.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109, djx012. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188326. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Bobin-Dubigeon, C.; Lefrançois, A.; Vansteene, D.; Dupé, M.; Joalland, M.P.; Bard, J.M. Leptin and adiponectin as new markers of undernutrition in cancer. Clin. Biochem. 2017, 50, 525–528. [Google Scholar] [CrossRef]
- Klempel, M.C.; Varady, K.A. Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutr. Rev. 2011, 69, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.H.; Zhang, L.L.; Xu, Y.; Chen, X.; Zhang, B.; Li, L.X.; Li, S.; Shang, D. Circulating leptin and adiponectin levels in patients with pancreatic cancer. Chin. Med. J. 2021, 134, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kobayashi, T.; Chayahara, N.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Mukohara, T.; Nishiumi, S.; Azuma, T.; Yoshida, M.; et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS ONE 2014, 20, 113259. [Google Scholar] [CrossRef] [Green Version]
- Dalamaga, M.; Migdalis, I.; Fargnoli, J.L.; Papadavid, E.; Bloom, E.; Mitsiades, N.; Karmaniolas, K.; Pelecanos, N.; Tseleni-Balafouta, S.; Dionyssiou-Asteriou, A.; et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: A case-control study. Cancer Causes Control 2009, 20, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marroquí, L.; Gonzalez, A.; Ñeco, P.; Caballero-Garrido, E.; Vieira, E.; Ripoll, C.; Nadal, A.; Quesada, I. Role of leptin in the pancreatic β-cell: Effects and signaling pathways. J. Mol. Endocrinol. 2012, 49, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Magkos, F.; Mantzoros, C.S.; Kang, E.S. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism 2011, 60, 1664–1672. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Gerst, F.; Wagner, R.; Oquendo, M.B.; Siegel-Axel, D.; Fritsche, A.; Heni, M.; Staiger, H.; Häring, H.U.; Ullrich, S. What role do fat cells play in pancreatic tissue? Mol. Metab. 2019, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuehnen, P.; Laubner, K.; Raile, K.; Schöfl, C.; Jakob, F.; Pilz, I.; Päth, G.; Seufert, J. Protein phosphatase 1 (PP-1)-dependent inhibition of insulin secretion by leptin in INS-1 pancreatic β-cells and human pancreatic islets. Endocrinology 2011, 152, 1800–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.G.; Pendharkar, S.A.; Gillies, N.A.; Miranda-Soberanis, V.; Plank, L.D.; Petrov, M.S. Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin. Exp. Med. 2017, 17, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Wos-Wroniewicz, E.; Caban, M.; Malecka-Panas, E. Role of adipokines in the assessment of severity and predicting the clinical course of acute pancreatitis. J. Physiol. Pharmacol. 2020, 71, 605–614. [Google Scholar] [CrossRef]
- White, D.L.; Hoogeveen, R.C.; Chen, L.; Richardson, P.; Ravishankar, M.; Shah, P.; Tinker, L.; Rohan, T.; Whitsel, E.A.; El-Serag, H.B.; et al. A prospective study of soluble receptor for advanced glycation end products and adipokines in association with pancreatic cancer in postmenopausal women. Cancer Med. 2018, 7, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Simpson, R.; Carr, R.A.; Wu, H.; Fan, H.; Liu, Z.; Korc, M.; Zhang, J.; Schmidt, C.M. Circulating Leptin and Branched Chain Amino Acids-Correlation with Intraductal Papillary Mucinous Neoplasm Dysplastic Grade. J. Gastrointest. Surg. 2019, 23, 966–974. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Ding, Y.; Wan, M.; Xu, M. The Role of Adipokines in Pancreatic Cancer. Front. Oncol. 2022, 8, 926230. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, J. Obesity and pancreatic cancer: Possible role of the PI3K/Akt pathway. Surgery 2010, 147, 596. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, M.; Tian, X.; Zhang, J.; Zhang, J.; Chen, J.; Xu, W.; Sheng, H. Leptin receptor mediates the proliferation and glucose metabolism of pancreatic cancer cells via AKT pathway activation. Mol. Med. Rep. 2020, 21, 945–952. [Google Scholar] [CrossRef]
- Harbuzariu, A.; Rampoldi, A.; Daley-Brown, D.S.; Candelaria, P.; Harmon, T.L.; Lipsey, C.C.; Beech, D.J.; Quarshie, A.; Ilies, G.O.; Gonzalez-Perez, R.R. Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget 2017, 31, 7740–7752. [Google Scholar] [CrossRef] [Green Version]
- White, P.B.; True, E.M.; Ziegler, K.; Wang, S.S.; Swartz-Basile, D.A.; Pitt, H.A.; Zyromski, N.J. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J. Gastrointest. Surg. 2010, 14, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Lanza-Jacoby, S.; Yan, G.; Radice, G.; LePhong, C.; Baliff, J.; Hess, R. Calorie restriction delays the progression of lesions to pancreatic cancer in the LSL-KrasG12D.; Pdx-1/Cre mouse model of pancreatic cancer. Exp. Biol. Med. 2013, 238, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Man, T.; Seicean, R.; Lucaciu, L.; Leucuta, D.; Ilies, M.; Iuga, C.; Petrusel, L.; Seicean, A. Leptin involvement in the survival of pancreatic adenocarcinoma patients with obesity and diabetes. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Kimura, H.; Sekijima, M.; Matsumoto, K.; Arao, T.; Chikugo, T.; Yamada, Y.; Kitano, M.; Ito, A.; Takeyama, Y.; et al. Plasma concentrations of angiogenesis-related molecules in patients with pancreatic cancer. Jpn. J. Clin. Oncol. 2012, 42, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Dimou, N.L.; Papadimitriou, N.; Mariosa, D.; Johansson, M.; Brennan, P.; Peters, U.; Chanock, S.J.; Purdue, M.; Bishop, D.T.; Gago-Dominquez, M.; et al. Circulating adipokine concentrations and risk of five obesity-related cancers: A Mendelian randomization study. Int. J. Cancer 2021, 148, 1625–1636. [Google Scholar] [CrossRef]
- Karabulut, S.; Afsar, C.U.; Karabulut, M.; Alis, H.; Erturk, K.; Karaman, S.; Kones, O.; Bilgin, E.; Tas, F. Serum leptin levels may have diagnostic and predictive roles in patients with pancreatic adenocarcinoma treated with gemcitabine-based chemotherapy. J. BUON 2016, 21, 895–902. [Google Scholar]
- Stolzenberg-Solomon, R.Z.; Newton, C.C.; Silverman, D.T.; Pollak, M.; Nogueira, L.M.; Weinstein, S.J.; Albanes, D.; Männistö, S.; Jacobs, E.J. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am. J. Epidemiol. 2015, 182, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Babic, A.; Bao, Y.; Qian, Z.R.; Yuan, C.; Giovannucci, E.L.; Aschard, H.; Kraft, P.; Amundadottir, L.T.; Stolzenberg-Solomon, R.; Morales-Oyarvide, V.; et al. Pancreatic Cancer Risk Associated with Prediagnostic Plasma Levels of Leptin and Leptin Receptor Genetic Polymorphisms. Cancer Res. 2016, 76, 7160–7167. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Gan, Y.; Shen, Y.; Cai, X.; Song, Y.; Zhao, F.; Yao, M.; Gu, J.; Tu, H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget 2015, 6, 16120–16134. [Google Scholar] [CrossRef] [Green Version]
- Kadri Colakoglu, M.; Bostanci, E.B.; Ozdemir, Y.; Dalgic, T.; Aksoy, E.; Ozer, I.; Ozogul, Y.; Oter, V. Roles of adiponectin and leptin as diagnostic markers in pancreatic cancer. Bratisl. Lek. Listy 2017, 118, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorowska, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E. Role of adipocytokines and its correlation with endocrine pancreatic function in patients with pancreatic cancer. Pancreatology 2013, 13, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Barassi, A.; Corsi, M.M.; Morselli-Labate, A.M.; Campana, D.; Casadei, R.; Santini, D.; Corinaldesi, R.; D’Eril, G.M. Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand. J. Gastroenterol. 2010, 45, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Giovannucci, E.L.; Kraft, P.; Stampfer, M.J.; Ogino, S.; Ma, J.; Buring, J.E.; Sesso, H.D.; Lee, I.M.; Gaziano, J.M.; et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J. Natl. Cancer Inst. 2013, 105, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fan, Y.; Zhang, W.; Shen, Y.; Liu, T.; Yao, M.; Gu, J.; Tu, H.; Gan, Y. Adiponectin Suppresses Human Pancreatic Cancer Growth through Attenuating the β-Catenin Signaling Pathway. Int. J. Biol. Sci. 2019, 15, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Manley, S.J.; Olou, A.A.; Jack, J.L.; Ruckert, M.T.; Walsh, R.M.; Eades, A.E.; Bye, B.A.; Ambrose, J.; Messaggio, F.; Anant, S.; et al. Synthetic adiponectin-receptor agonist, AdipoRon, induces glycolytic dependence in pancreatic cancer cells. Cell Death Dis. 2022, 13, 114. [Google Scholar] [CrossRef]
- Kato, M.; Watabe, K.; Tsujii, M.; Funahashi, T.; Shimomura, I.; Takehara, T. Adiponectin inhibits murine pancreatic cancer growth. Dig. Dis. Sci. 2014, 59, 1192–1196. [Google Scholar] [CrossRef]
- Messaggio, F.; Mendonsa, A.M.; Castellanos, J.; Nagathihalli, N.S.; Gorden, L.; Merchant, N.B.; VanSaun, M.N. Adiponectin receptor agonists inhibit leptin induced pSTAT3 and in vivo pancreatic tumor growth. Oncotarget 2017, 49, 85378–85391. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Xu, S.; Piao, C.; Qiu, S.; Li, H.; Du, J. Adiponectin induces CXCL1 secretion from cancer cells and promotes tumor angiogenesis by inducing stromal fibroblast senescence. Mol. Carcinog. 2016, 55, 1796–1806. [Google Scholar] [CrossRef]
- Grote, V.A.; Rohrmann, S.; Dossus, L.; Nieters, A.; Halkjaer, J.; Tjønneland, A.; Overvad, K.; Stegger, J.; Chabbert-Buffet, N.; Boutron-Ruault, M.C.; et al. The association of circulating adiponectin levels with pancreatic cancer risk: A study within the prospective EPIC cohort. Int. J. Cancer 2012, 130, 2428–2437. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Weinstein, S.; Pollak, M.; Tao, Y.; Taylor, P.R.; Virtamo, J.; Albanes, D. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am. J. Epidemiol. 2008, 168, 1047–1055. [Google Scholar] [CrossRef]
- Nogueira, L.M.; Newton, C.C.; Pollak, M.; Silverman, D.T.; Albanes, D.; Männistö, S.; Weinstein, S.J.; Jacobs, E.J.; Stolzenberg-Solomon, R.Z. Serum C-peptide, Total and High Molecular Weight Adiponectin, and Pancreatic Cancer: Do Associations Differ by Smoking? Cancer Epidemiol. Biomark. Prev. 2017, 26, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Kuruma, S.; Egawa, N.; Kurata, M.; Honda, G.; Kamisawa, T.; Ueda, J.; Ishii, H.; Ueno, M.; Nakao, H.; Mori, M.; et al. Case-control study of diabetes-related genetic variants and pancreatic cancer risk in Japan. World J. Gastroenterol. 2014, 46, 17456–17462. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Omar, H.; Ghaffar, M.F.A.; Marie, M.S.; Ramadan, M.E.A.; Talima, S.M.; Daly, M.; Mahmoud, S. Single Nucleotide Polymorphism in Adiponectin Gene and Risk of Pancreatic Adenocarcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Cheng, X.; Wang, D.; Peng, M.; Xue, Z.; Da, Y.; Zhang, N.; Yao, Z.; Li., M.; Xu, A.; et al. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1α signaling. Oncotarget 2014, 5, 4732–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasiorowska, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E. Subclinical Inflammation and Endothelial Dysfunction in Patients with Chronic Pancreatitis and Newly Diagnosed Pancreatic Cancer. Dig. Dis. Sci. 2016, 61, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Dranka-Bojarowska, D.; Lekstan, A.; Olakowski, M.; Jablonska, B.; Lewinski, A.; Musialski, P.; Sobczyk, W.; Kapalka, A.; Lampe, P. The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J. Physiol. Pharmacol. 2015, 66, 653–663. [Google Scholar]
- Yusof, K.M.; Groen, K.; Rosli, R.; Abdullah, M.; Mahmud, R.; Avery-Kiejda, K.A. Evaluation of Circulating MicroRNAs and Adipokines in Breast Cancer Survivors with Arm Lymphedema. Int. J. Mol. Sci. 2022, 23, 11359. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [Green Version]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential effects of leptin and adiponectin in endothelial angiogenesis. J. Diabetes Res. 2015, 2015, 648239. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Mishra, S.; Mishra, S.; Kumar, S.; Gupta, V. Association of Leptin: Adiponectin ratio and metabolic risk markers in postmenopausal women. Immunol. Lett. 2018, 196, 63–67. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, M.E.; Cleary, M.P. The balance between leptin and adiponectin in the control of carcinogenesis—Focus on mammary tumorigenesis. Biochimie 2012, 94, 2164–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashizawa, N.; Yahata, T.; Quan, J.; Adachi, S.; Yoshihara, K.; Tanaka, K. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol. Oncol. 2010, 119, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.V.; Park, P.H. Tumor Metabolic Reprogramming by Adipokines as a Critical Driver of Obesity-Associated Cancer Progression. Int. J. Mol. Sci. 2021, 22, 1444. [Google Scholar] [CrossRef] [PubMed]
- Cairat, M.; Rinaldi, S.; Navionis, A.S.; Romieu, I.; Biessy, C.; Viallon, V.; Olsen, A.; Tjønneland, A.; Fournier, A.; Severi, G.; et al. Circulating inflammatory biomarkers, adipokines and breast cancer risk-a case-control study nested within the EPIC cohort. BMC Med. 2022, 20, 118. [Google Scholar] [CrossRef]
- Gutiérrez-Salmerón, M.; Lucena, S.R.; Chocarro-Calvo, A.; García-Martínez, J.M.; Martín Orozco, R.M.; García-Jiménez, C. Metabolic and hormonal remodeling of colorectal cancer cell signalling by diabetes. Endocr. Relat. Cancer. 2021, 28, R191–R206. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Wu, Q.J.; Wang, Y.L.; Ma, X.X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int. J. Cancer 2015, 137, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Siemińska, L.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Warakomski, J.; Kos-Kudła, B. Serum concentrations of adipokines in men with prostate cancer and benign prostate hyperplasia. Endokrynol. Pol. 2018, 69, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, T.; Trompet, S.; Noordam, R.; van Klinken, J.B.; van Dijk, K.W.; Lamb, H.J.; Cobbaert, C.M.; den Heijer, M.; Jazet, I.M.; Jukema, J.W.; et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018, 107, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Selthofer-Relatić, K.; Radić, R.; Stupin, A.; Šišljagić, V.; Bošnjak, I.; Bulj, N.; Selthofer, R.; DelićBrkljačić, D. Leptin/adiponectin ratio in overweight patients—Gender differences. Diab. Vasc. Dis. Res. 2018, 15, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ramos, C.M.; Quackenbush, J.; DeMeo, D.L. Genome-Wide Sex and Gender Differences in Cancer. Front. Oncol. 2020, 23, 597788. [Google Scholar] [CrossRef] [PubMed]
- Zyromski, N.J.; Mathur, A.; Pitt, H.A.; Wade, T.E.; Wang, S.; Nakshatri, P.; Swartz-Basile, D.A.; Nakshatri, H. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery 2009, 146, 258–263. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.; Patterson, L.; Dubin, J.A.; Mourtzakis, M. Elevated C-Peptides, Abdominal Obesity, and Abnormal Adipokine Profile are Associated With Higher Gleason Scores in Prostate Cancer. Prostate 2017, 77, 211–221. [Google Scholar] [CrossRef]
- Słomian, G.J.; Nowak, D.; Buczkowska, M.; Głogowska-Gruszka, A.; Słomian, S.P.; Roczniak, W.; Janyga, S.; Nowak, P. The role of adiponectin and leptin in the treatment of ovarian cancer patients. Endokrynol. Pol. 2019, 70, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Brocco, D.; Florio, R.; De Lellis, L.; Veschi, S.; Grassadonia, A.; Tinari, N.; Cama, A. The Role of Dysfunctional Adipose Tissue in Pancreatic Cancer: A Molecular Perspective. Cancers 2020, 12, 1849. [Google Scholar] [CrossRef]
- Warakomski, J.; Romuk, E.; Jarząb, B.; Krajewska, J.; Siemińska, L. Concentrations of Selected Adipokines, Interleukin-6, and Vitamin D in Patients with Papillary Thyroid Carcinoma in Respect to Thyroid Cancer Stages. Int. J. Endocrinol. 2018, 2018, 4921803. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Jia, L.; Zhao, T.; Zhang, H.; Chen, J.; Yang, S.; Liu, J.; Yu, M.; Hao, J. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014, 354, 172–180. [Google Scholar] [CrossRef]
- Lin, T.C.; Hsiao, M. Leptin and Cancer: Updated Functional Roles in Carcinogenesis, Therapeutic Niches, and Developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef]
- Ma, L.; Fan, Z.; Du, G.; Wang, H. Leptin-elicited miRNA-342-3p potentiates gemcitabine resistance in pancreatic ductal adenocarcinoma. Biochem. Biophys. Res. Commun. 2019, 509, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin-Notch axis impairs 5-fluorouracil effects on pancreatic cancer. Oncotarget 2018, 26, 18239–18253. [Google Scholar] [CrossRef] [Green Version]
- Cifarelli, V.; Lashinger, L.M.; Devlin, K.L.; Dunlap, S.M.; Huang, J.; Kaaks, R.; Pollak, M.N.; Hursting, S.D. Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct MicroRNA-Regulated Mechanisms. Diabetes 2015, 64, 1632–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevellin, E.; Scarpa, M.; Carraro, A.; Lunardi, F.; Kotsafti, A.; Porzionato, A.; Saadeh, L.; Cagol, M.; Alfieri, R.; Tedeschi, U.; et al. Esophageal adenocarcinoma and obesity: Peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 2015, 6, 11203–11215. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Matheny, M.; Strehler, K.Y.; Toklu, H.Z.; Kirichenko, N.; Carter, C.S.; Morgan, D.; Tümer, N. Rapamycin Normalizes Serum Leptin by Alleviating Obesity and Reducing Leptin Synthesis in Aged Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Motallebnezhad, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Nickho, H.; Samadi-Kafil, H.; Shamsasenjan, K.; Yousefi, M. The insulin-like growth factor-I receptor (IGF-IR) in breast cancer: Biology and treatment strategies. Tumour Biol. 2016, 37, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.; Liu, Z.; Zhu, S.; Shen, P.; Zhang, H.; Zhang, M.; Chen, N.; Zhao, J.; Chen, J.; et al. The Adiponectin-AdipoR1 Axis Mediates Tumor Progression and Tyrosine Kinase Inhibitor Resistance in Metastatic Renal Cell Carcinoma. Neoplasia 2019, 21, 921–931. [Google Scholar] [CrossRef] [PubMed]

| Variables | Study Group n = 83 | Control Group n = 39 |
|---|---|---|
| Females/Males | 52/31 | 31/8 |
| Age [years] | 50.39 ± 13.79 | 38 ± 10.52 |
| BMI [kg/m2] | 24.35 ± 4.14 | 24.19 ± 3.52 |
| Functionality status | ||
| Non-functional | 81 | N/A |
| Functional | 2 | N/A |
| Grade | ||
| G1 | 44 | N/A |
| G2 | 39 | N/A |
| Ki-67 | ||
| <3% | 45 | N/A |
| ≥3% | 38 | N/A |
| TNM stage | ||
| I | 27 | N/A |
| IIA | 14 | N/A |
| IIB | 5 | N/A |
| IIIA | 2 | N/A |
| IIIB | 8 | N/A |
| IV | 27 | N/A |
| Disease extent | ||
| Local | 46 | N/A |
| Regional | 8 | N/A |
| Distal | 29 | N/A |
| Variables | Study Group n = 83 | Control Group n = 39 | p Value 1 |
|---|---|---|---|
| Leptin (ng/mL) | 8.05 | 7.96 | 0.822 |
| Adiponectin (µg/mL) | 7.79 | 10.26 | <0.001 |
| Leptin–adiponectin ratio | 1.03 | 0.82 | 0.356 |
| Study Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Variables | Females n = 52 | Males n = 31 | p Value 1 | Females n = 31 | Males n = 8 | p Value 1 |
| Leptin (ng/mL) | 9.63 | 7.15 | <0.01 | 8.82 | 7.21 | 0.414 |
| Adiponectin (µg/mL) | 8.37 | 7.14 | 0.137 | 10.97 | 7.60 | <0.01 |
| Leptin–adiponectin ratio | 1.20 | 0.92 | 0.102 | 0.72 | 1.14 | 0.654 |
| Variables | PanNENs G1 n = 44 | PanNENs G2 n = 39 | p Value 1 |
|---|---|---|---|
| Leptin (ng/mL) | 8.88 | 7.67 | 0.062 |
| Adiponectin (µg/mL) | 7.60 | 7.89 | 0.156 |
| Leptin–adiponectin ratio | 1.16 | 0.96 | <0.05 |
| Variables | Ki-67 < 3% n = 45 | Ki-67 ≥ 3% n = 38 | p Value 1 |
|---|---|---|---|
| Leptin (ng/mL) | 8.36 | 7.61 | 0.108 |
| Adiponectin (µg/mL) | 7.21 | 7.66 | 0.245 |
| Leptin–adiponectin ratio | 1.25 | 0.93 | <0.05 |
| Variables | Localized Disease n = 54 | Distal Disease n = 29 | p Value 1 |
|---|---|---|---|
| Leptin (ng/mL) | 10.20 | 6.81 | <0.001 |
| Adiponectin (µg/mL) | 7.18 | 7.89 | 0.455 |
| Leptin–adiponectin ratio | 1.25 | 0.92 | <0.01 |
| Parameter | Leptin (ng/mL) | Adiponectin (µg/mL) | Leptin–Adiponectin Ratio |
|---|---|---|---|
| Leptin (ng/mL) | R = −0.13, p = NS | R = 0.87, p ≤ 0.001 | |
| Adiponectin (µg/mL) | R = −0.13, p = NS | R = −0.55, p ≤ 0.001 | |
| Leptin–adiponectin ratio | R = 0.87, p ≤ 0.001 | R = −0.55, p ≤ 0.001 | |
| BMI (kg/m2) | R = 0.46, p ≤ 0.001 | R = −0.36, p ≤ 0.001 | R = 0.54, p ≤ 0.001 |
| AFP (µg/L) | R = −0.04, p = NS | R = −0.06, p = NS | R = −0.05, p = NS |
| CA19-9 (U/mL) | R = −0.01, p = NS | R = −0.04, p = NS | R = 0.01, p = NS |
| CEA (µg/L) | R = −0.15, p = NS | R = 0.13, p = NS | R = −0.20, p ≤ 0.05 |
| CgA (µg/L) | R = −0.23, p ≤ 0.05 | R = 0.36, p ≤ 0.001 | R = −0.34, p ≤ 0.001 |
| Serotonin (ng/mL) | R = −0.20, p ≤ 0.05 | R = 0.05, p = NS | R = −0.14, p = NS |
| 5-HIAA (mg/24 h) | R = −0.20, p ≤ 0.05 | R = 0.06, p = NS | R = −0.19, p = NS |
| Ki-67 (%) | R = −0.27, p ≤ 0.01 | R = −0.10, p = NS | R = −0.28, p ≤ 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocian-Jastrzębska, A.; Malczewska-Herman, A.; Rosiek, V.; Kos-Kudła, B. Assessment of the Role of Leptin and Adiponectinas Biomarkers in Pancreatic Neuroendocrine Neoplasms. Cancers 2023, 15, 3517. https://doi.org/10.3390/cancers15133517
Bocian-Jastrzębska A, Malczewska-Herman A, Rosiek V, Kos-Kudła B. Assessment of the Role of Leptin and Adiponectinas Biomarkers in Pancreatic Neuroendocrine Neoplasms. Cancers. 2023; 15(13):3517. https://doi.org/10.3390/cancers15133517
Chicago/Turabian StyleBocian-Jastrzębska, Agnes, Anna Malczewska-Herman, Violetta Rosiek, and Beata Kos-Kudła. 2023. "Assessment of the Role of Leptin and Adiponectinas Biomarkers in Pancreatic Neuroendocrine Neoplasms" Cancers 15, no. 13: 3517. https://doi.org/10.3390/cancers15133517





