Pre-Referral Primary Care Blood Tests and Symptom Presentation before Cancer Diagnosis: National Cancer Diagnosis Audit Data
Abstract
Simple Summary
Abstract
1. Background
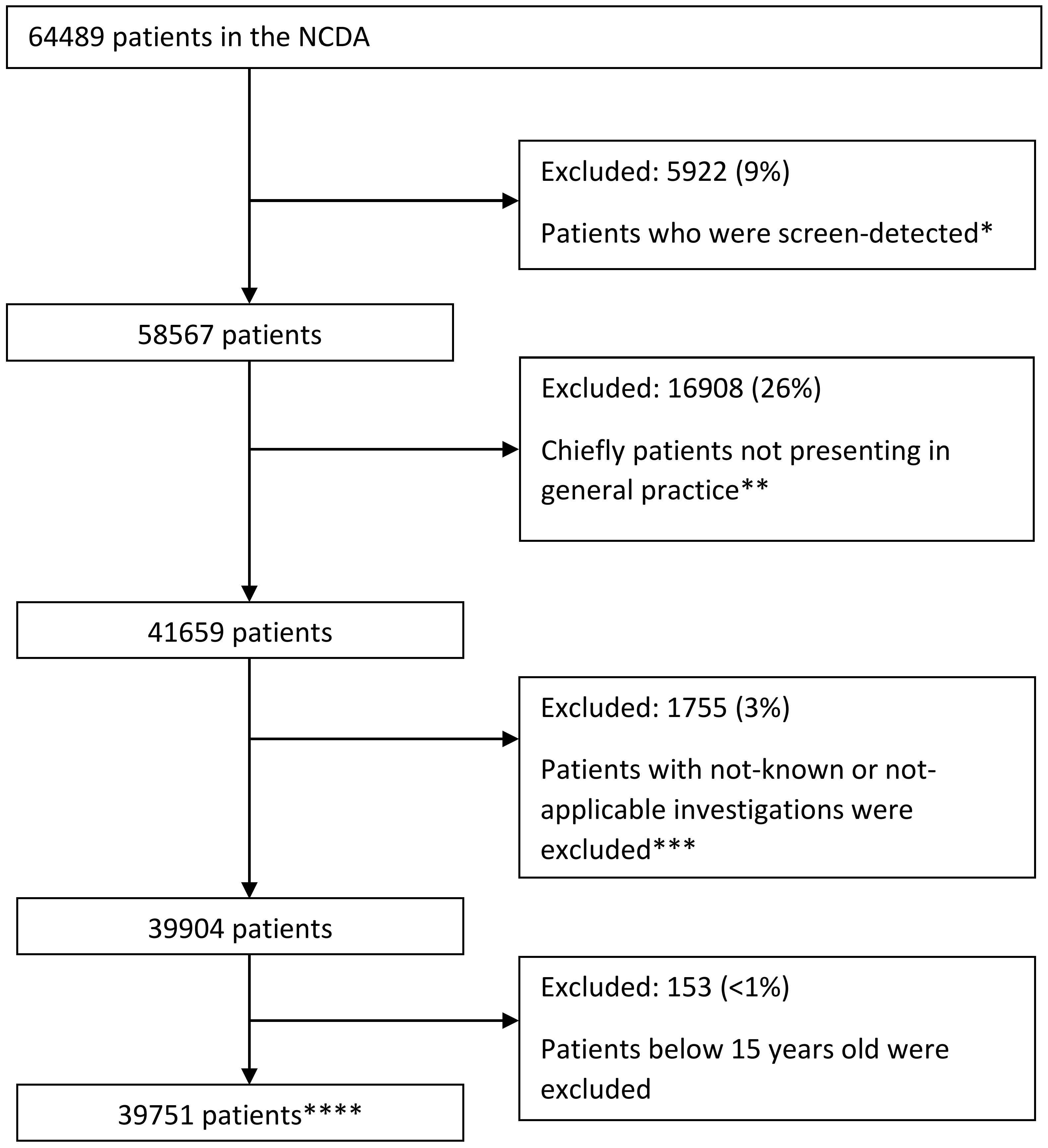
2. Methods
2.1. Study Design and Participants
2.2. Variables of Interest
2.3. Analysis
3. Results
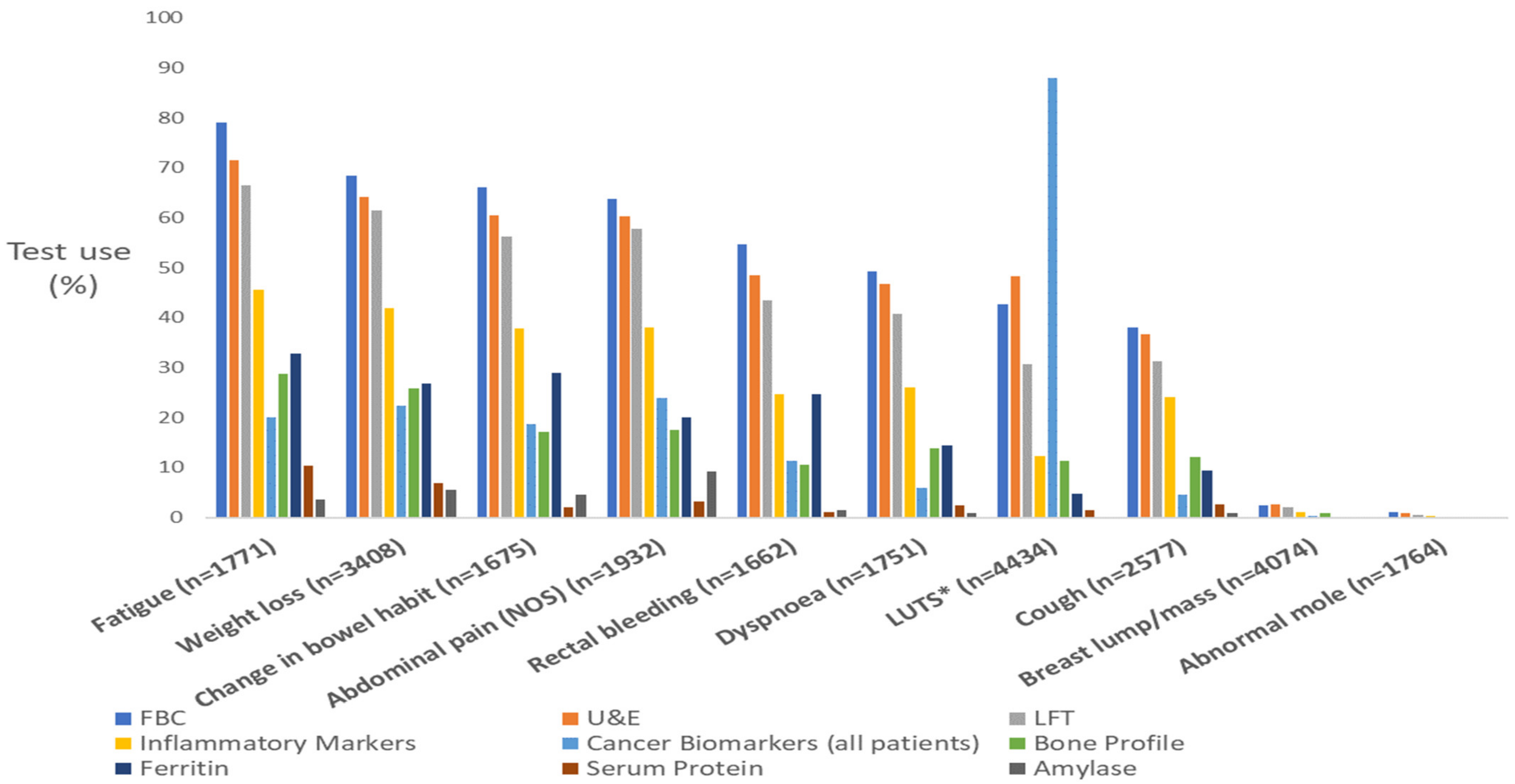
3.1. Generic Blood Tests
3.2. Specific Blood Tests
3.3. Blood Biomarker Tests
3.4. Supplementary Analysis: Generic and Less Common Blood Test Use by Symptom PPV Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emery, J.D.; Shaw, K.; Williams, B.; Mazza, D.; Fallon-Ferguson, J.; Varlow, M.; Trevena, L.J. The role of primary care in early detection and follow-up of cancer. Nat. Rev. Clin. Oncol. 2014, 11, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.; Berendsen, A.; Crawford, S.M.; Dommett, R.; Earle, C.; Emery, J.; Fahey, T.; Grassi, L.; Grunfeld, E.; Gupta, S.; et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015, 16, 1231–1272. [Google Scholar] [CrossRef] [PubMed]
- NICE Guidelines. Suspected Cancer: Recognition and Referral. Guidance and Guidelines; NICE Guidance: Nice, France, 2015; Available online: https://www.nice.org.uk/guidance/ng12/chapter/1-Recommendations-organised-by-site-of-cancer#upper-gastrointestinal-tract-cancers (accessed on 18 February 2022).
- Jensen, H.; Tørring, M.L.; Olesen, F.; Overgaard, J.; Vedsted, P. Cancer suspicion in general practice, urgent referral and time to diagnosis: A population-based GP survey and registry study. BMC Cancer 2014, 14, 636. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Wardle, J.; Rubin, G. Rethinking diagnostic delay in cancer: How difficult is the diagnosis? BMJ 2014, 349, g7400. [Google Scholar] [CrossRef]
- Pearson, C.; Poirier, V.; Fitzgerald, K.; Rubin, G.; Hamilton, W. Cross-sectional study using primary care and cancer registration data to investigate patients with cancer presenting with non-specific symptoms. BMJ Open 2020, 10, e033008. [Google Scholar] [CrossRef]
- Zhou, Y.; Mendonca, S.C.; Abel, G.A.; Hamilton, W.; Walter, F.M.; Johnson, S.; Shelton, J.; Elliss-Brookes, L.; McPhail, S.; Lyratzopoulos, G. Variation in ‘fast-track’ referrals for suspected cancer by patient characteristic and cancer diagnosis: Evidence from 670 000 patients with cancers of 35 different sites. Br. J. Cancer 2018, 118, 24–31. [Google Scholar] [CrossRef]
- Bailey, S.E.; Ukoumunne, O.C.; Shephard, E.A.; Hamilton, W. Clinical relevance of thrombocytosis in primary care: A prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2017, 67, e405–e413. [Google Scholar] [CrossRef]
- Hopkins, R.; Bailey, S.E.; Hamilton, W.T.; Shephard, E.A. Microcytosis as a risk marker of cancer in primary care: A cohort study using electronic patient records. Br. J. Gen. Pract. 2020, 70, e457–e462. [Google Scholar] [CrossRef]
- Koshiaris, C.; Van den Bruel, A.; Oke, J.L.; Nicholson, B.D.; Shephard, E.; Braddick, M.; Hamilton, W. Early detection of multiple myeloma in primary care using blood tests: A case-control study in primary care. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2018, 68, e586–e593. [Google Scholar] [CrossRef] [PubMed]
- Mounce, L.T.; Hamilton, W.; Bailey, S.E. Cancer incidence following a high-normal platelet count: Cohort study using electronic healthcare records from English primary care. Br. J. Gen. Pract. 2020, 70, e622–e628. [Google Scholar] [CrossRef] [PubMed]
- Næser, E.; Møller, H.; Fredberg, U.; Frystyk, J.; Vedsted, P. Routine blood tests and probability of cancer in patients referred with non-specific serious symptoms: A cohort study. BMC Cancer 2017, 17, 817. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Schultz, M.; Gaardsting, A.; Ladelund, S.; Garred, P.; Iversen, K.; Eugen-Olsen, J.; Helms, M.; David, K.P.; Kjaer, A.; et al. Inflammatory biomarkers and cancer: CRP and suPAR as markers of incident cancer in patients with serious nonspecific symptoms and signs of cancer. Int. J. Cancer 2017, 141, 191–199. [Google Scholar] [CrossRef]
- Watson, J.; Mounce, L.; Bailey, S.E.R.; Cooper, S.L.; Hamilton, W. Blood markers for cancer. BMJ 2019, 367, l5774. [Google Scholar] [CrossRef]
- Watson, J.; Salisbury, C.; Banks, J.; Whiting, P.; Hamilton, W. Predictive value of inflammatory markers for cancer diagnosis in primary care: A prospective cohort study using electronic health records. Br. J. Cancer 2019, 120, 1045–1051. [Google Scholar] [CrossRef]
- Cranfield, B.M.; Koo, M.M.; Abel, G.A.; Swann, R.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Primary care blood tests before cancer diagnosis: National Cancer Diagnosis Audit data. Br. J. Gen. Pract. 2022, 73, e95–e103. [Google Scholar] [CrossRef]
- Swann, R.; McPhail, S.; Shand, B.; Rashbass, J.; Witt, J.; Abel, G.A.; Hiom, S.; Lyratzopoulos, G.; Rubin, G. Diagnosing cancer in primary care: Results from the National Cancer Diagnosis Audit. Br. J. Gen. Pract. 2018, 68, e63–e72. [Google Scholar] [CrossRef]
- Moore, S.F.; Price, S.J.; Chowienczyk, S.; Bostock, J.; Hamilton, W. The impact of changing risk thresholds on the number of people in England eligible for urgent investigation for possible cancer: An observational cross-sectional study. Br. J. Cancer 2021, 125, 1593–1597. [Google Scholar] [CrossRef]
- Swann, R.; McPhail, S.; Abel, G.A.; Witt, J.; Wills, L.; Hiom, S.; Lyratzopoulos, G.; Rubin, G. National Cancer Diagnosis Audits for England 2018 versus 2014: A comparative analysis. Br. J. Gen. Pract. 2023, 1–9. [Google Scholar] [CrossRef]
- Shephard, E.A.; Neal, R.D.; Rose, P.; Walter, F.M.; Litt, E.J.; Hamilton, W.T. Quantifying the risk of multiple myeloma from symptoms reported in primary care patients: A large case-control study using electronic records. Br. J. Gen. Pract. 2015, 65, e106–e113. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Hyde, C.; Hamilton, W. Risk of breast cancer in symptomatic women in primary care: A case–control study using electronic records. Br. J. Gen. Pract. 2014, 64, e788. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.; Hajioff, S.; Graham, J.; Schmidt-Hansen, M. Suspected cancer (part 2—Adults): Reference tables from updated NICE guidance. BMJ 2015, 350, h3044. [Google Scholar] [CrossRef] [PubMed]
- Wiering, B.; Lyratzopoulos, G.; Hamilton, W.; Campbell, J.; Abel, G. Concordance with urgent referral guidelines in patients presenting with any of six “alarm” features of possible cancer: A retrospective cohort study using linked primary care records. BMJ Qual. Saf. 2022, 31, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Thulesius, H.; Neves, A.L.; Harker, S.; Koskela, T.; Petek, D.; Hoffman, R.; Brekke, M.; Buczkowski, K.; Buono, N.; et al. How European primary care practitioners think the timeliness of cancer diagnosis can be improved: A thematic analysis. BMJ Open 2019, 9, e030169. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.; Vedsted, P.; Emery, J. Improving cancer outcomes: Better access to diagnostics in primary care could be critical. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2011, 61, 317–318. [Google Scholar] [CrossRef]
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Generic Blood Tests | ||||||||
| Symptom Name | FBC | % | U&E | % | LFT | % | IM | % |
| Fatigue (n = 1771) b | 1398 | 79 | 1267 | 72 | 1177 | 66 | 808 | 46 |
| Loss of appetite (n = 1264) a | 939 | 74 | 887 | 70 | 842 | 67 | 583 | 46 |
| Weight loss (n = 3408) c | 2331 | 68 | 2183 | 64 | 2090 | 61 | 1428 | 42 |
| Upper abdominal pain (n = 1192) b | 805 | 68 | 741 | 62 | 736 | 62 | 492 | 41 |
| Diarrhoea (n = 1013) b | 673 | 66 | 621 | 61 | 574 | 57 | 404 | 40 |
| Change in bowel habit (n = 1675) d | 1107 | 66 | 1013 | 60 | 941 | 56 | 633 | 38 |
| Nausea and/or vomiting (n = 1067) b | 693 | 65 | 666 | 62 | 648 | 61 | 433 | 41 |
| Abdominal pain (NOS) (n = 1932) c | 1233 | 64 | 1163 | 60 | 1114 | 58 | 736 | 38 |
| Lower abdominal pain (n = 1060) | 683 | 64 | 631 | 60 | 584 | 55 | 407 | 38 |
| Constipation (n = 831) b | 527 | 63 | 484 | 58 | 448 | 54 | 297 | 36 |
| Distension (n = 980) c | 609 | 62 | 593 | 61 | 539 | 55 | 352 | 36 |
| Back pain (n = 1405) a | 837 | 60 | 797 | 57 | 719 | 51 | 578 | 41 |
| Dyspepsia (n = 805) b | 468 | 58 | 436 | 54 | 414 | 51 | 247 | 31 |
| Rectal bleeding (n = 1662) e | 907 | 55 | 805 | 48 | 723 | 44 | 411 | 25 |
| Bone pain (n = 490) a | 262 | 53 | 249 | 51 | 222 | 45 | 185 | 38 |
| Other symptom (n = 2131) | 1093 | 51 | 1021 | 48 | 926 | 43 | 589 | 28 |
| Dyspnoea (n = 1751) b | 863 | 49 | 818 | 47 | 714 | 41 | 457 | 26 |
| Dysuria (n = 551) b | 255 | 46 | 263 | 48 | 199 | 36 | 110 | 20 |
| Urinary tract infection (n = 477) a | 209 | 44 | 217 | 45 | 148 | 31 | 93 | 19 |
| LUTS (nocturia, frequency, hesitancy, urgency, retention) (n = 4434) e | 1893 | 43 | 2139 | 48 | 1360 | 31 | 545 | 12 |
| Haematuria (n = 1465) d | 599 | 41 | 588 | 40 | 367 | 25 | 186 | 13 |
| Neck lump/mass (n = 1201) | 498 | 41 | 422 | 35 | 367 | 31 | 325 | 27 |
| Not applicable (n = 2795) | 1129 | 40 | 912 | 33 | 734 | 26 | 326 | 12 |
| Chest pain (n = 960) b | 387 | 40 | 365 | 38 | 330 | 34 | 236 | 25 |
| Not known (n = 687) | 270 | 39 | 219 | 32 | 200 | 29 | 76 | 11 |
| Dysphagia (n = 997) e | 383 | 39 | 366 | 37 | 341 | 35 | 190 | 19 |
| Cough (n = 2577) b | 982 | 38 | 944 | 37 | 807 | 31 | 620 | 24 |
| All other symptoms (n = 6828) | 2310 | 37 | 2115 | 34 | 1900 | 30 | 1316 | 21 |
| Chest infection (n = 686) a | 251 | 37 | 239 | 35 | 212 | 31 | 159 | 23 |
| Other vaginal bleeding (n = 421) | 131 | 31 | 91 | 22 | 74 | 18 | 37 | 9 |
| Haemoptysis (n = 469) e | 143 | 30 | 131 | 28 | 111 | 24 | 80 | 17 |
| Sore throat (n = 478) | 132 | 28 | 113 | 24 | 96 | 20 | 88 | 18 |
| Hoarseness (n = 468) c | 109 | 23 | 102 | 22 | 97 | 21 | 62 | 13 |
| Post-menopausal bleeding (n = 896) e | 166 | 19 | 145 | 16 | 124 | 14 | 61 | 7 |
| Breast pain (n = 768) b | 21 | 3 | 24 | 3 | 20 | 3 | 9 | 1 |
| Breast lump/mass (n = 4074) e | 102 | 3 | 109 | 3 | 80 | 2 | 44 | 1 |
| Abnormal mole (n = 1764) | 18 | 1 | 17 | 1 | 8 | 0 | 5 | 0 |
| All patients (n = 39,751) | 15,540 | 39 | 14,555 | 37 | 12,414 | 31 | 7598 | 19 |
| (b) | ||||||||
| Less Common Blood Tests | ||||||||
| Symptom Name | Bone Profile | % | Ferritin | % | Serum Protein Electrophoresis * | % | Amylase | % |
| Fatigue (n = 1771) b | 509 | 29 | 581 | 33 | 185 | 10 | 62 | 4 |
| Loss of appetite (n = 1264) a | 367 | 29 | 379 | 30 | 92 | 7 | 88 | 7 |
| Weight loss (n = 3408) c | 878 | 26 | 913 | 27 | 236 | 7 | 187 | 5 |
| Upper abdominal pain (n = 1192) b | 210 | 18 | 252 | 21 | 44 | 4 | 172 | 14 |
| Diarrhoea (n = 1013) b | 162 | 16 | 279 | 28 | 21 | 2 | 64 | 6 |
| Change in bowel habit (n = 1675) d | 287 | 17 | 483 | 29 | 35 | 2 | 75 | 4 |
| Nausea and/or vomiting (n = 1067) b | 229 | 21 | 238 | 22 | 37 | 3 | 97 | 9 |
| Abdominal pain (NOS) (n = 1932) c | 339 | 18 | 387 | 20 | 61 | 3 | 179 | 9 |
| Lower abdominal pain (n = 1060) | 195 | 18 | 233 | 22 | 23 | 2 | 39 | 4 |
| Constipation (n = 831) b | 185 | 22 | 204 | 25 | 34 | 4 | 39 | 5 |
| Distension (n = 980) c | 169 | 17 | 179 | 18 | 17 | 2 | 55 | 6 |
| Back pain (n = 1405) a | 425 | 30 | 207 | 15 | 251 | 18 | 37 | 3 |
| Dyspepsia (n = 805) b | 120 | 15 | 175 | 22 | 22 | 3 | 59 | 7 |
| Rectal bleeding (n = 1662) e | 175 | 11 | 411 | 25 | 18 | 1 | 24 | 1 |
| Bone pain (n = 490) a | 155 | 32 | 60 | 12 | 79 | 16 | 10 | 2 |
| Other symptom (n = 2131) | 368 | 17 | 368 | 17 | 136 | 6 | 56 | 3 |
| Dyspnoea (n = 1751) b | 243 | 14 | 254 | 15 | 44 | 3 | 14 | 1 |
| Dysuria (n = 551) b | 60 | 11 | 33 | 6 | 11 | 2 | 11 | 2 |
| Urinary tract infection (n = 477) a | 42 | 9 | 33 | 7 | 11 | 2 | 5 | 1 |
| LUTS ** (nocturia, frequency, hesitancy, urgency, retention) (n = 4434) e | 498 | 11 | 213 | 5 | 69 | 2 | 18 | <1% |
| Haematuria (n = 1465) d | 118 | 8 | 79 | 5 | 11 | 1 | 9 | 1 |
| Neck lump/mass (n = 1201) | 125 | 10 | 78 | 6 | 30 | 2 | 5 | <1% |
| Not applicable (n = 2795) | 259 | 9 | 334 | 12 | 151 | 5 | 20 | 1 |
| Chest pain (n = 960) b | 138 | 14 | 96 | 10 | 49 | 5 | 19 | 2 |
| Not known (n = 687) | 49 | 7 | 69 | 10 | 29 | 4 | 2 | <1% |
| Dysphagia (n = 997) e | 98 | 10 | 136 | 14 | 5 | 1 | 22 | 2 |
| Cough (n = 2577) b | 312 | 12 | 240 | 9 | 68 | 3 | 25 | 1 |
| All other symptoms *** (n = 6828) | 722 | 11 | 551 | 9 | 233 | 4 | 153 | 2 |
| Chest infection (n = 686) a | 74 | 11 | 48 | 7 | 15 | 2 | 8 | 1 |
| Other vaginal bleeding (n = 421) | 20 | 5 | 46 | 11 | 5 | 1 | 1 | <1% |
| Haemoptysis (n = 469) e | 38 | 8 | 29 | 6 | 3 | 1 | 3 | 1 |
| Sore throat (n = 478) | 27 | 6 | 28 | 6 | 6 | 1 | 3 | 1 |
| Hoarseness (n = 468) c | 26 | 6 | 30 | 6 | 5 | 1 | 2 | <1% |
| Post-menopausal bleeding (n = 896) e | 34 | 4 | 44 | 5 | 2 | <1% | 1 | <1% |
| Breast pain (n = 768) b | 4 | 1 | 7 | 1 | 3 | <1% | 0 | 0 |
| Breast lump/mass (n = 4074) e | 35 | 1 | 17 | <1% | 6 | <1% | 2 | <1% |
| Abnormal mole (n = 1764) | 4 | <1% | 4 | <1% | 0 | 0 | 0 | 0 |
| All patients (n = 39,752) | 4367 | 11 | 4299 | 11 | 1240 | 3 | 761 | 2 |
| Symptom Name | Cancer Biomarkers | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Number of Men with Symptom | Biomarker Use | % | Number of Women with Symptom | Biomarker Use | % | |
| Fatigue (n = 1771) b | 939 | 249 | 27 | 832 | 105 | 13 |
| Loss of appetite (n = 1264) a | 673 | 155 | 23 | 591 | 136 | 23 |
| Weight loss (n = 3408) c | 2042 | 500 | 24 | 1366 | 259 | 19 |
| Upper abdominal pain (n = 1192) b | 619 | 80 | 13 | 573 | 105 | 18 |
| Diarrhoea (n = 1013) b | 563 | 92 | 16 | 450 | 74 | 16 |
| Nausea and/or vomiting (n = 1067) b | 461 | 44 | 10 | 606 | 110 | 18 |
| Change in bowel habit (n = 1675) d | 939 | 149 | 16 | 736 | 163 | 22 |
| Abdominal pain (NOS) (n = 1932) c | 936 | 159 | 17 | 996 | 302 | 30 |
| Lower abdominal pain (n = 1060) | 438 | 132 | 30 | 622 | 199 | 32 |
| Constipation (n = 831) b | 443 | 101 | 23 | 388 | 102 | 26 |
| Distension (n = 980) c | 351 | 53 | 15 | 629 | 332 | 53 |
| Back pain (n = 1405) a | 851 | 404 | 47 | 554 | 64 | 12 |
| Dyspepsia (n = 805) b | 475 | 43 | 9 | 330 | 45 | 14 |
| Bone pain (n = 490) a | 305 | 108 | 35 | 185 | 13 | 7 |
| Rectal bleeding (n = 1662) e | 954 | 118 | 12 | 708 | 69 | 10 |
| Other symptom (n = 2131) | 1232 | 353 | 29 | 899 | 81 | 9 |
| LUTS * (nocturia, frequency, hesitancy, urgency, retention) (n = 4434) e | 4242 | 3716 | 88 | 192 | 50 | 26 |
| Dyspnoea (n = 1751) b | 973 | 73 | 8 | 778 | 30 | 4 |
| Dysuria (n = 551) b | 408 | 261 | 64 | 143 | 19 | 13 |
| Urinary tract infection (n = 477) a | 293 | 186 | 63 | 184 | 26 | 14 |
| N/A (n = 2795) | 2197 | 1231 | 56 | 598 | 33 | 6 |
| Haematuria (n = 1465) d | 1156 | 410 | 35 | 309 | 8 | 3 |
| N/K (n = 687) | 532 | 336 | 63 | 155 | 7 | 5 |
| Neck lump/mass (n = 1201) | 680 | 25 | 4 | 521 | 8 | 2 |
| Chest pain (n = 960) b | 536 | 47 | 9 | 424 | 19 | 4 |
| Cough (n = 2577) b | 1441 | 86 | 6 | 1136 | 29 | 3 |
| Dysphagia (n = 997) e | 670 | 29 | 4 | 307 | 16 | 5 |
| All other symptoms (n = 6828) ** | 3332 | 715 | 21 | 2949 | 239 | 8 |
| Chest infection (n = 686) a | 374 | 23 | 6 | 312 | 9 | 3 |
| Other vaginal bleeding (n = 421) | 0 | ** ≤3 | ≤0 | 421 | 56 | 13 |
| Haemoptysis (n = 469) e | 302 | 7 | 2 | 167 | ≤3 | 1 |
| Sore throat (n = 478) | 328 | ≤3 | ≤1 | 150 | 4 | 3 |
| Hoarseness (n = 468) c | 351 | 9 | 3 | 117 | ≤3 | 2 |
| Post-menopausal bleeding (n = 896) e | 0 | ≤3 | ≤0 | 896 | 76 | 8 |
| Breast pain (n = 768) b | 11 | ≤3 | ≤27 | 757 | ≤3 | <1 |
| Breast lump/mass (n = 4074) e | 52 | ≤3 | ≤6 | 4022 | 12 | <1 |
| Abnormal mole (n = 1764) | 811 | ≤3 | ≤1 | 953 | 0 | 0 |
| All patients (n = 39,752) | 21,854 | 7828 | 36 | 17,898 | 1461 | 8 |
| Population Total, n (Column %) | Received a Blood Test *, n (Row %) | Crude OR (95% CI) and p-Value | Adjusted OR *** (Excluding Cancer Site) and p-Value | Adjusted OR (Including Cancer Site) and p-Value | |
|---|---|---|---|---|---|
| Total: | 29,043 (100%) ** | 12,998 (45%) | |||
| Symptom PPV * | **** <0.001 | **** <0.001 | **** <0.001 | ||
| 0.01–0.99% | 3162 (11%) | 1656 (52%) | 2.41 (2.22–2.61) | 2.40 (2.21–2.60) | 1.57 (1.43–1.73) |
| 1.00–1.99% | 7454 (26%) | 3779 (51%) | 2.25 (2.12–2.39) | 2.33 (2.19–2.48) | 1.96 (1.81–2.12) |
| 2.00–2.99% | 4846 (17%) | 3113 (64%) | 3.94 (3.67–4.23) | 3.89 (3.61–4.18) | 2.79 (2.55–3.05) |
| 3.00–4.99% | 2101 (7%) | 887 (42%) | 1.60 (1.45–1.76) | 1.47 (1.34–1.62) | 1.25 (1.11–1.40) |
| ≥5.00% | 11,480 (40%) | 3593 (31%) | ref | ref | ref |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cranfield, B.M.; Abel, G.A.; Swann, R.; Moore, S.F.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Pre-Referral Primary Care Blood Tests and Symptom Presentation before Cancer Diagnosis: National Cancer Diagnosis Audit Data. Cancers 2023, 15, 3587. https://doi.org/10.3390/cancers15143587
Cranfield BM, Abel GA, Swann R, Moore SF, McPhail S, Rubin GP, Lyratzopoulos G. Pre-Referral Primary Care Blood Tests and Symptom Presentation before Cancer Diagnosis: National Cancer Diagnosis Audit Data. Cancers. 2023; 15(14):3587. https://doi.org/10.3390/cancers15143587
Chicago/Turabian StyleCranfield, Ben M., Gary A. Abel, Ruth Swann, Sarah F. Moore, Sean McPhail, Greg P. Rubin, and Georgios Lyratzopoulos. 2023. "Pre-Referral Primary Care Blood Tests and Symptom Presentation before Cancer Diagnosis: National Cancer Diagnosis Audit Data" Cancers 15, no. 14: 3587. https://doi.org/10.3390/cancers15143587
APA StyleCranfield, B. M., Abel, G. A., Swann, R., Moore, S. F., McPhail, S., Rubin, G. P., & Lyratzopoulos, G. (2023). Pre-Referral Primary Care Blood Tests and Symptom Presentation before Cancer Diagnosis: National Cancer Diagnosis Audit Data. Cancers, 15(14), 3587. https://doi.org/10.3390/cancers15143587






