Intramedullary Nailing with and without the Use of Bone Cement for Impending and Pathologic Fractures of the Humerus in Multiple Myeloma and Metastatic Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Demographics
2.2. Perioperative Protocols and after Care
2.3. Collected Variables
2.4. Statistical Analyses
3. Results
3.1. Survivorship
3.2. Functional Outcomes
3.3. Perioperative Outcomes and Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Laitinen, M.; Nieminen, J.; Pakarinen, T.-K. Treatment of Pathological Humerus Shaft Fractures with Intramedullary Nails with or without Cement Fixation. Arch. Orthop. Trauma Surg. 2011, 131, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight Opinion to Surgically Treated Metastatic Bone Disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry Report of 1195 Operated Skeletal Metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Wolf, H.; Funovics, P.; Pajenda, G.; Hausmann, J.T.; Vécsei, V. Surgical Treatment of Pathological Fractures of the Shaft of the Humerus. J. Trauma Inj. Infect. Crit. Care 2009, 66, 789–794. [Google Scholar] [CrossRef]
- Dijkstra, S.; Stapert, J.; Boxma, H.; Wiggers, T. Treatment of Pathological Fractures of the Humeral Shaft Due to Bone Metastases: A Comparison of Intramedullary Locking Nail and Plate Osteosynthesis with Adjunctive Bone Cement. Eur. J. Surg. Oncol. 1996, 22, 621–626. [Google Scholar] [CrossRef]
- Flemming, J.; Beals, R. Pathologic Fracture of the Humerus. Clin. Orthop. Relat. Res. 1986, 203, 258–260. [Google Scholar] [CrossRef]
- Frassica, F.J.; Frassica, D.A. Evaluation and Treatment of Metastases to the Humerus. Clin. Orthop. Relat. Res. 2003, 415, S212–S218. [Google Scholar] [CrossRef]
- Piccioli, A.; Maccauro, G.; Rossi, B.; Scaramuzzo, L.; Frenos, F.; Capanna, R. Surgical Treatment of Pathologic Fractures of Humerus. Injury 2010, 41, 1112–1116. [Google Scholar] [CrossRef]
- Bryson, D.J.; Wicks, L.; Ashford, R.U. The Investigation and Management of Suspected Malignant Pathological Fractures: A Review for the General Orthopaedic Surgeon. Injury 2015, 46, 1891–1899. [Google Scholar] [CrossRef]
- Weiss, K.R.; Bhumbra, R.; Biau, D.J.; Griffin, A.M.; Deheshi, B.; Wunder, J.S.; Ferguson, P.C. Fixation of Pathological Humeral Fractures by the Cemented Plate Technique. J. Bone Jt. Surg. Br. 2011, 93-B, 1093–1097. [Google Scholar] [CrossRef]
- Camnasio, F.; Scotti, C.; Peretti, G.M.; Fontana, F.; Fraschini, G. Prosthetic Joint Replacement for Long Bone Metastases: Analysis of 154 Cases. Arch. Orthop. Trauma Surg. 2008, 128, 787–793. [Google Scholar] [CrossRef] [PubMed]
- El Beaino, M.; Liu, J.; Lewis, V.O.; Lin, P.P. Do Early Results of Proximal Humeral Allograft-Prosthetic Composite Reconstructions Persist at 5-Year Followup? Clin. Orthop. Relat. Res. 2019, 477, 758–765. [Google Scholar] [CrossRef]
- Janssen, S.J.; Teunis, T.; Hornicek, F.J.; Bramer, J.A.M.; Schwab, J.H. Outcome of Operative Treatment of Metastatic Fractures of the Humerus: A Systematic Review of Twenty Three Clinical Studies. Int. Orthop. 2015, 39, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Thai, D.M.; Kitagawa, Y.; Choong, P.F. Outcome of Surgical Management of Bony Metastases to the Humerus and Shoulder Girdle: A Retrospective Analysis of 93 Patients. Int. Semin. Surg. Oncol. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wedin, R.; Hansen, B.H.; Laitinen, M.; Trovik, C.; Zaikova, O.; Bergh, P.; Kalén, A.; Schwarz-Lausten, G.; Vult von Steyern, F.; Walloe, A.; et al. Complications and Survival after Surgical Treatment of 214 Metastatic Lesions of the Humerus. J. Shoulder Elbow. Surg. 2012, 21, 1049–1055. [Google Scholar] [CrossRef]
- Hunt, K.J.; Gollogly, S.; Randall, R.L. Surgical Fixation of Pathologic Fractures: An Evaluation of Evolving Treatment Methods. Bull. Hosp. Jt. Dis. 2006, 63, 77–82. [Google Scholar]
- Janssen, S.J.; van Dijke, M.; Lozano-Calderón, S.A.; Ready, J.E.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Schwab, J.H. Complications after Surgery for Metastatic Humeral Lesions. J. Shoulder Elbow. Surg. 2016, 25, 207–215. [Google Scholar] [CrossRef]
- Pretell, J.; Rodriguez, J.; Blanco, D.; Zafra, A.; Resines, C. Treatment of Pathological Humeral Shaft Fractures with Intramedullary Nailing. A Retrospective Study. Int. Orthop. 2010, 34, 559–563. [Google Scholar] [CrossRef]
- Noger, M.; Berli, M.C.; Fasel, J.H.D.; Hoffmeyer, P.J. The Risk of Injury to Neurovascular Structures from Distal Locking Screws of the Unreamed Humeral Nail (UHN): A Cadaveric Study. Injury 2007, 38, 954–957. [Google Scholar] [CrossRef]
- Atesok, K.; Liebergall, M.; Sucher, E.; Temper, M.; Mosheiff, R.; Peyser, A. Treatment of Pathological Humeral Shaft Fractures with Unreamed Humeral Nail. Ann. Surg. Oncol. 2007, 14, 1493–1498. [Google Scholar] [CrossRef]
- Choi, E.-S.; Han, I.; Cho, H.S.; Park, I.W.; Park, J.W.; Kim, H.-S. Intramedullary Nailing for Pathological Fractures of the Proximal Humerus. Clin. Orthop. Surg. 2016, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Barker, M.E.; Hamlin, B.H. Thermal Effects of Acrylic Cementation at Bone Tumour Sites. Int. J. Hyperth. 1997, 13, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.J.; Lopez-Ben, R.; Mann, J.P.; Ponce, B.A. Pathological Fractures of the Proximal Humerus Treated with a Proximal Humeral Locking Plate and Bone Cement. J. Bone Jt. Surg. Br. 2010, 92-B, 707–712. [Google Scholar] [CrossRef]
- Bauze, A.; Clayer, M. Treatment of Pathological Fractures of the Humerus with a Locked Intramedullary Nail. J. Orthop. Surg. 2003, 11, 34–37. [Google Scholar] [CrossRef]
- Ingman, A.M.; Waters, D.A. Locked Intramedullary Nailing of Humeral Shaft Fractures. Implant Design, Surgical Technique, and Clinical Results. J. Bone Jt. Surg. Br. 1994, 76, 23–29. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, S.; Lin, P.P.; Kim, H.S. Closed Intramedullary Nailing with Percutaneous Cement Augmentation for Long Bone Metastases. Bone Jt. J. 2016, 98-B, 703–709. [Google Scholar] [CrossRef]
- Moura, D.L.; Alves, F.; Fonseca, R.; Freitas, J.; Casanova, J. Tratamento de Fraturas Patológicas Tumorais Diafisárias do Úmero Com Haste Intramedular Rígida Bloqueada Estática—Experiência de 22 Anos. Rev. Bras. Ortop. 2019, 54, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ofluoglu, O.; Erol, B.; Ozgen, Z.; Yildiz, M. Minimally Invasive Treatment of Pathological Fractures of the Humeral Shaft. Int. Orthop. 2009, 33, 707–712. [Google Scholar] [CrossRef]
- Pizzo, R.A.; Hoskins, T.; Patel, J.N.; Miller, J.M.; Goyette, D.; Mazzei, C.; Wittig, J.C. Distally Unlocked Intramedullary Nailing With Cement Fixation for Impending and Actual Pathologic Humerus Fractures: A Retrospective Case Series. JAAOS Glob. Res. Rev. 2020, 4, e20.00090. [Google Scholar] [CrossRef]
- Redmond, B.J.; Biermann, J.S.; Blasier, R.B. Interlocking Intramedullary Nailing of Pathological Fractures of the Shaft of the Humerus. J. Bone Jt. Surg. 1996, 78, 891–896. [Google Scholar] [CrossRef]
- Rommens, P.M.; Blum, J. Retrograde Locked Nailing of Humeral Shaft Fractures Using the Unreamed Humeral Nail (UHN). Orthop. Traumatol. 1999, 7, 251–259. [Google Scholar] [CrossRef]
- Spencer, S.J.; Holt, G.; Clarke, J.V.; Mohammed, A.; Leach, W.J.; Roberts, J.L.B. Locked Intramedullary Nailing of Symptomatic Metastases in the Humerus. J. Bone Jt. Surg. Br. 2010, 92-B, 142–145. [Google Scholar] [CrossRef]
- Tomé, J.; Carsi, B.; García-Fernández, C.; Marco, F.; López-Durán Stern, L. Treatment of Pathologic Fractures of the Humerus with Seidel Nailing. Clin. Orthop. Relat. Res. 1998, 350, 51–55. [Google Scholar]
- Vandeweyer, E.; Gebhart, M. Treatment of Humeral Pathological Fractures by Internal Fixation and Methylmetacrylate Injection. Eur. J. Surg. Oncol. (EJSO) 1997, 23, 238–242. [Google Scholar] [CrossRef]
- Gebhart, M.; Dequanter, D.; Vandeweyer, E. Metastatic Involvement of the Humerus: A Retrospective Study of 51 Cases. Acta Orthop. Belg. 2001, 67, 456–463. [Google Scholar]
- Bickels, J.; Kollender, Y.; Wittig, J.C.; Meller, I.; Malawer, M.M. Function after Resection of Humeral Metastases. Clin. Orthop. Relat. Res. 2005, 437, 201–208. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, H.G.; Kim, J.R.; Lin, P.P.; Kim, H.S. Minimally Invasive Surgery of Humeral Metastasis Using Flexible Nails and Cement in High-Risk Patients with Advanced Cancer. Surg. Oncol. 2011, 20, e32–e37. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yeh, T.-T.; Pan, R.-Y.; Wu, C.-C. Ante-Grade Intramedullary Nailing for the Treatment of Humeral Shaft Metastatic Bone Tumor. J. Med. Sci. 2014, 34, 247. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, H.S. Joint-Preserving Palliative Surgery Using Self-Locking Screws of Intramedullary Nail and Percutaneous Cementoplasty for Proximal Humeral Metastasis in the Advanced Cancer Patients. World J. Surg. Oncol. 2018, 16, 93. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, H.S. Preliminary Results: Use of Multi-Hole Injection Nails for Intramedullary Nailing with Simultaneous Bone Cement Injection in Long-Bone Metastasis. Skeletal. Radiol. 2019, 48, 219–225. [Google Scholar] [CrossRef]
- Ricard, M.-A.M.; Stavropoulos, N.A.; Nooh, A.; Ste-Marie, N.; Goulding, K.; Turcotte, R. Intramedullary Nailing Versus Plate Osteosynthesis for Humeral Shaft Metastatic Lesions. Cureus 2021, 13, e13788. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.V.; Kobryn, A.; Alam, J.S.; Tretiakov, M. Single-Stage versus Multi-Stage Intramedullary Nailing for Multiple Synchronous Long Bone Impending and Pathologic Fractures in Metastatic Bone Disease and Multiple Myeloma. Cancers 2023, 15, 1227. [Google Scholar] [CrossRef] [PubMed]
- Mirels, H. Metastatic Disease in Long Bones. A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 1989, 249, 256–264. [Google Scholar] [CrossRef]
- Beickert, R.; Buckle, R.; Buhren, V.; Mason, M.D. T2 Humeral Nailing System. In Shoulder and Elbow Trauma and Its Complications; Woodhead Publishing: Sawston, UK, 2010; pp. 1–52. [Google Scholar]
- Cai, D.F.; Fan, Q.H.; Zhong, H.H.; Peng, S.; Song, H. The Effects of Tourniquet Use on Blood Loss in Primary Total Knee Arthroplasty for Patients with Osteoarthritis: A Meta-Analysis. J. Orthop. Surg. Res. 2019, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A System for the Functional Evaluation of Reconstructive Procedures after Surgical Treatment of Tumors of the Musculoskeletal System. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- National Center for Health Statistics. How to Use the National Death Index: Steps in the Process. Available online: https://www.cdc.gov/nchs/ndi/portal.htm (accessed on 1 June 2023).
- Riddoch, G.; Rowley Bristow, W.; Cairns, H.W.B.; Carmichael, E.A.; Critchley, M.; Greenfield, J.G.; Learmonth, J.R.; Platt, H.; Seddon, H.J.; Symonds, C.P.; et al. Aids to the examination of the peripheral nervous system. Med. Res. Counc.–Nerves Inj. Comm. 1943, 45, 1–62. [Google Scholar]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.-M.; Smith, M.; Coleman, R. Pathologic Fractures Correlate with Reduced Survival in Patients with Malignant Bone Disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef]
- Galán-Olleros, M.; Marco, J.; Oteo, D.; Cristóbal-Bilbao, R.; Manrique, E.; García-Maroto, R.; Marco, F.; Cebrián-Parra, J.L. Orthopedic Surgical Treatment and Perioperative Complications in Multiple Myeloma Bone Disease: Analysis of a Series (2009–2018). Ann. Surg. Oncol. 2021, 28, 1158–1166. [Google Scholar] [CrossRef]
- Harrington, K.D.; Johnston, J.O.; Turner, R.H.; Green, D.L. The Use of Methylmethacrylate as an Adjunct in the Internal Fixation of Malignant Neoplastic Fractures. J. Bone Jt. Surg. Am. 1972, 54, 1665–1676. [Google Scholar] [CrossRef]
- Harrington, K.D. The Use of Methylmethacrylate as an Adjunct in the Internal Fixation of Unstable Comminuted Intertrochanteric Fractures in Osteoporotic Patients. J. Bone Jt. Surg. Am. 1975, 57, 744–750. [Google Scholar] [CrossRef]
- Sim, F.H.; Kruse, R.L.; Johnson, E.W. Problems in Pathologic Fractures. Minn. Med. 1974, 57, 266–269. [Google Scholar]
- Johnson, J.A.; Berkshire, A.; Leighton, R.K.; Gross, M.; Chess, D.G.; Petrie, D. Some Basic Biomechanical Characteristics of Medullary Pressure Generation during Reaming of the Femur. Injury 1995, 26, 451–454. [Google Scholar] [CrossRef]



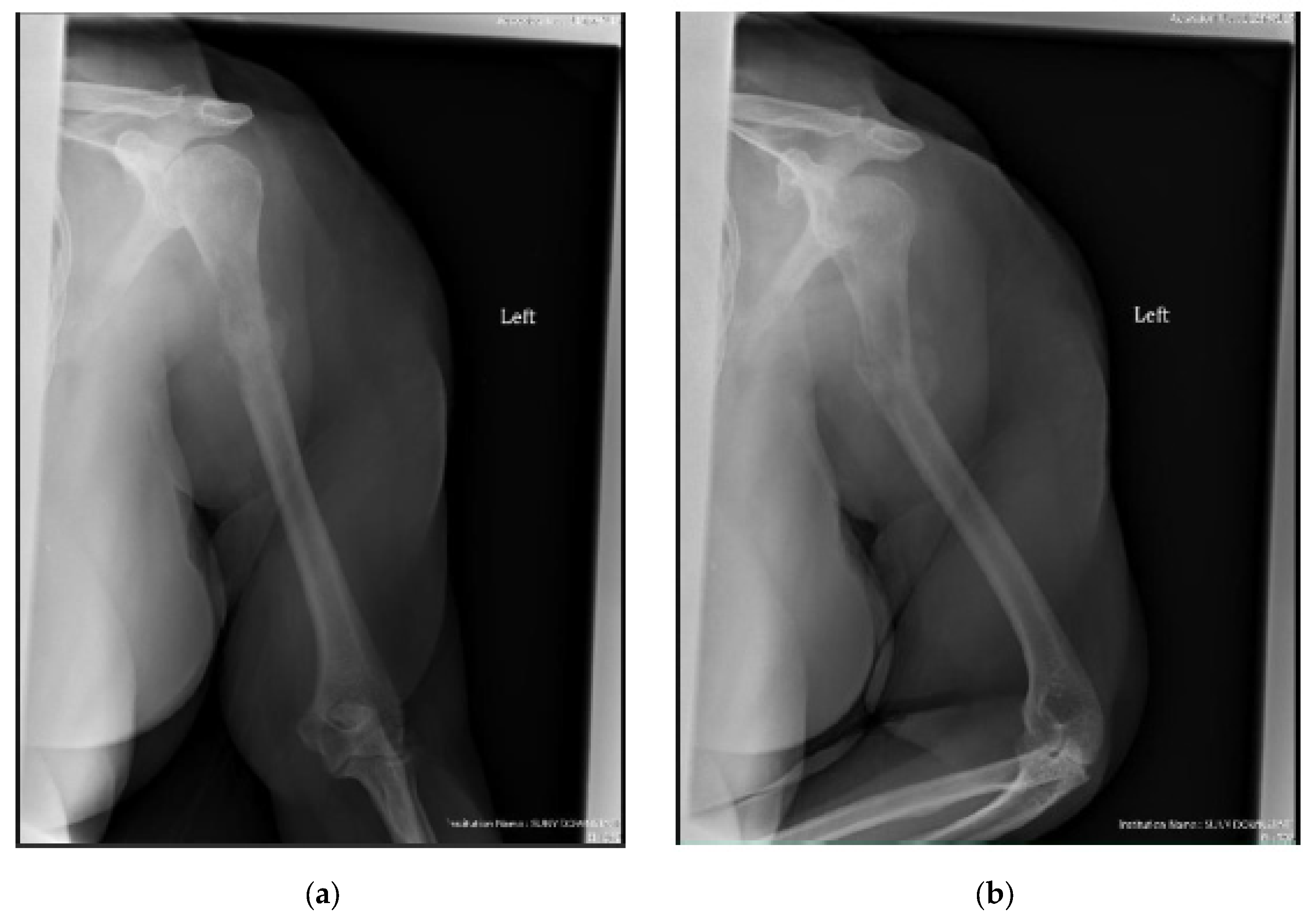


| Entire Patient Population | Cemented Cohort | Uncemented Cohort | p-Value ǁ | |
|---|---|---|---|---|
| N = 82 (100%) | N = 46 (100%) | N = 36 (100%) | ||
| Age (Year) * | 68.0 ± 11.7 (38–96) | 67.9 ± 10.9 (38–90) | 68.3 ± 13.3 (44–96) | 0.46 |
| Body Mass Index (Kg/m2) * | 27.6 ± 5.6 (17.1–45.2) | 27.2 ± 5.1 (17.2–37.6) | 28.2 ± 6.2 (21.4–45.2) | 0.07 |
| Sex § | 1.00 | |||
| Male | 41 (50.0%) | 23 (50.0%) | 18 (50.0%) | |
| Female | 41 (50.0%) | 23 (50.0%) | 18 (50.0%) | |
| Concomitant Procedure | 49 (59.8%) | 20 (43.5%) | 29 (80.6%) | 1.00 |
| Bilateral Humerii | 18 (22.0%) | 8 (17.4%) | 10 (27.8%) | |
| Another Long Bone | 31 (37.8%) | 12 (26.1%) | 19 (52.8%) | |
| Staging of Procedures | <0.001 | |||
| Single Nail | 33 (40.2%) | 26 (56.5%) | 7 (19.4%) | |
| Multiple Nails-One Setting | 38 (46.3%) | 15 (32.6%) | 23 (63.9%) | |
| Multiple Nails-Two+ Settings | 11 (13.4%) | 5 (10.9%) | 6 (16.7%) | |
| All Nails | Cemented Nails | Uncemented Nails | p-Value ǁ | |
| N = 100 (100%) | N = 53 (100%) | N = 47 (100%) | ||
| Laterality § | 0.49 | |||
| Left | 48 (48.0%) | 26 (49.1%) | 22 (46.8%) | |
| Right | 52 (52.0%) | 27 (50.9%) | 25 (53.2%) | |
| Fracture Type §,+ | <0.01 | |||
| Impending | 40 (40.0%) | 10 (18.9%) | 30 (63.8%) | |
| Complete | 60 (60.0%) | 43 (81.1%) | 17 (36.2%) | |
| Major lesion/Fracture Location §,† | 0.02 | |||
| Diaphysis | 55 (55.0%) | 20 (37.7%) | 35 (74.5%) | |
| Proximal | 32 (32.0%) | 26 (49.5%) | 6 (12.8%) | |
| Distal | 3 (3.0%) | 2 (3.8%) | 1 (2.1%) | |
| Diaphysis + Proximal | 7 (7.0%) | 3 (5.1%) | 4 (8.5%) | |
| Proximal + Distal | 1 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Diaphysis + Proximal + Distal | 2 (2.0%) | 1 (1.9%) | 1 (2.1%) | |
| Primary Diagnosis § | 0.24 | |||
| Multiple Myeloma | 54 (54.0%) | 31 (58.5%) | 23 (48.9%) | |
| Breast Cancer | 18 (18.0%) | 6 (11.3%) | 12 (25.5%) | |
| Prostate Cancer | 8 (8.0%) | 3 (5.7%) | 5 (10.6%) | |
| Renal Cancer | 7 (7.0%) | 5 (9.4%) | 2 (4.3%) | |
| Lung Cancer | 4 (4.0%) | 3 (5.7%) | 1 (2.1%) | |
| Hepatocellular Carcinoma | 1 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Lymphoma | 4 (4.0%) | 1 (1.9%) | 3 (6.4%) | |
| Melanoma | 1 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Cholangiocarcinoma | 1 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Giant Cell Carcinoma | 1 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Gastrointestinal Stromal Tumor | 1 (1.0%) | 0 (0.0%) | 1 (2.1%) | |
| Anesthesia Type § | 0.20 | |||
| General Anesthesia | 94 (94.0%) | 47 (88.7%) | 47 (100%) | |
| Scalene Block | 1 (1.0%) | 4 (7.5%) | 0 (0.0%) | |
| General Anesthesia + Scalene Block | 5 (5.0%) | 2 (3.8%) | 0 (0.0%) |
| All Nails | Cemented Nails | Uncemented Nails | p-Value ǁ | |
|---|---|---|---|---|
| N = 100 (100%) | N = 53 (100%) | N = 47 (100%) | ||
| Medical Complications * | 14 (14.0%) | 7 (13.2%) | 7 (14.9%) | 0.76 |
| Surgical Complications * | 8 (8.0%) | 6 (11.3%) | 2 (4.3%) | 0.47 |
| Overall Complications * | 21 + (21.0%) | 12 + (22.6%) | 9 (19.1%) | 1.00 |
| Estimated Blood Loss (mL) § | 166 ± 138 (0–1000) | 203 ± 179 (0–1000) | 126 ± 49 (50–300) | 0.003 |
| Single Nail | 184 ± 215 (0–1000) | 209 ± 238 (0–1000) | 100 ± 50 (50–200) | 0.046 |
| Multiple Nails-One Setting | 149 ± 84 (50–500) | 193 ± 110 (100–500) | 124 ± 46 (50–300) | 0.007 |
| Multiple Nails-Two+ Settings | 173 ± 44 (100–200) | 183 ± 41(100–200) | 164 ± 48 (100–200) | 0.45 |
| Perioperative Transfusion (mL) § | 236 ± 394 (0–1950) | 204 ± 318 (0–975) | 271 ± 462 (0–1950) | 0.41 |
| Single Nail | 115 ± 245 (0–650) | 135 ± 270 (0–650) | 46 ± 123 (0–325) | 0.23 |
| Multiple Nails-One Setting | 325 ± 461 (0–1950) | 293 ± 348 (0–975) | 344 ± 520 (0–1950) | 0.67 |
| Multiple Nails-Two+ Settings | 175 ± 314 (0–975) | 217 ± 394 (0–975) | 139 ± 256 (0–650) | 0.69 |
| Intraoperative Mortality | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| Mortality in the Same Admission | 2 (2.0%) | 2 (3.8%) | 0 (0.0%) | 0.22 |
| Preoperative MSTS Score (%) † | 42.4 ± 8.4 (28–60) | 40.2 ± 9.6 (16–64) | 66.7 ± 28.5 (20–100) | 0.01 |
| Postoperative MSTS Score at 3 months (%) † | 89.2 ± 5.5 (76–96) | 89.8 ± 7.0 (80–100) | 90.9 ± 1.9 (90–100) | 0.72 |
| Survival Time (months) † | 10.0 ± 14.3 (0–86) | 8.3 ± 9.3 (0–35) | 11.6 ± 17.7 (0–86) | 0.34 |
| Complication Type | Sex * | Age | Primary Diagnosis | Lesion Location | Fracture Type | Setting | Complication | Surgical Intervention | Same Admission Perioperative Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Cemented | |||||||||
| Medical | M | 77 | Multiple myeloma | Proximal | Pathologic + | Postoperative | Gastrointestinal bleeding | No | No |
| Medical | M | 65 | Multiple myeloma | Proximal | Pathologic + | Postoperative | Disseminated intravascular coagulation | No | Yes, unresponsive ventricular fibrillation at 4 weeks |
| Medical | F | 38 | Breast cancer | Proximal | Pathologic + | Postoperative | Pulmonary embolism | No | No |
| Medical | M | 68 | Hepatocellular carcinoma | Proximal | Pathologic + | Postoperative | Sepsis | No | Yes, sepsis and variceal bleeding with disseminated intravascular coagulation at 4 weeks |
| Medical/ Miscellaneous † | F | 60 | Multiple myeloma | Diaphysis | Impending | Postoperative | Quadriparesis from cervical metastasis/hypotension/respiratory distress postop day #2 | Urgent neurosurgical decompression and fixation. Regained 4 to 4+/5 power # in 6 months | No |
| Medical | M | 71 | Multiple myeloma | Proximal + Distal | Impending | Postoperative | Pneumonia | No | No |
| Medical + Surgical | F | 57 | Breast cancer | Proximal + Diaphysis + Distal | Pathologic + | Intraoperative + Postoperative | Cement extrusion into elbow joint § + Hypotension | Intraoperative elbow arthrotomy and removal using another incision during the same setting | No |
| Surgical | M | 62 | Renal cancer | Proximal + Diaphysis | Pathologic + | Intraoperative | Cement extrusion into the shoulder joint as well as broken cement piece in the deltoid soft tissue § | Intraoperative arthrotomy and removal of all extruded cement using another incision during the same setting | No |
| Surgical | M | 87 | Prostate cancer | Diaphysis | Pathologic + | Intraoperative | Broken and retained piece of drill bit | No (asymptomatic) | No |
| Surgical | F | 59 | Multiple myeloma | Proximal + Diaphysis | Pathologic + | Intraoperative | Proximal nail cutout of the lateral cortex | Intraoperative conversion to plate after aborting nail ** | No |
| Surgical | M | 64 | Renal cancer | Diaphysis | Pathologic + | Postoperative | High radial nerve palsy | No. Did not have full recovery until death at 4 months with brain metastasis | No |
| Surgical | M | 57 | Multiple myeloma | Proximal | Pathologic + | Postoperative | Painful backed out proximal locking screws back out at 2 years | Proximal screws removal two years post index procedure | No |
| Uncemented | |||||||||
| Medical | M | 83 | Multiple myeloma | Diaphysis | Pathologic + | Postoperative | Hypotension | No | No |
| Medical | F | 55 | Breast cancer | Proximal | Pathologic + | Postoperative | Septic shock with thrombocytopenia | No | No |
| Medical | F | 58 | Multiple myeloma | Diaphysis | Impending | Postoperative | Transaminitis with hyperbilirubinemia | No | No |
| Medical | F | 68 | Multiple myeloma | Diaphysis | Impending | Postoperative | Clostridium difficile infection | No | No |
| Medical | F | 71 | Multiple myeloma | Proximal Diaphysis | Impending | Postoperative | Pneumonia | No | No |
| Medical | M | 81 | Prostate | Diaphysis | Impending | Postoperative | Disseminated intravascular coagulation | No | No |
| Medical | F | 57 | Breast cancer | Proximal + Distal | Impending | Postoperative | Hypotension + Respiratory Distress | No | No |
| Surgical | F | 82 | Multiple myeloma | Diaphysis | Pathologic + | Postoperative | Proximal nail cutout of the lateral cortex | No (asymptomatic) | No |
| Surgical | F | 44 | Breast cancer | Diaphysis | Impending | Postoperative | Partial low radial nerve palsy | No. Full recovery in 3 months | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobryn, A.; Nian, P.; Baidya, J.; Li, T.L.; Maheshwari, A.V. Intramedullary Nailing with and without the Use of Bone Cement for Impending and Pathologic Fractures of the Humerus in Multiple Myeloma and Metastatic Disease. Cancers 2023, 15, 3601. https://doi.org/10.3390/cancers15143601
Kobryn A, Nian P, Baidya J, Li TL, Maheshwari AV. Intramedullary Nailing with and without the Use of Bone Cement for Impending and Pathologic Fractures of the Humerus in Multiple Myeloma and Metastatic Disease. Cancers. 2023; 15(14):3601. https://doi.org/10.3390/cancers15143601
Chicago/Turabian StyleKobryn, Andriy, Patrick Nian, Joydeep Baidya, Tai L. Li, and Aditya V. Maheshwari. 2023. "Intramedullary Nailing with and without the Use of Bone Cement for Impending and Pathologic Fractures of the Humerus in Multiple Myeloma and Metastatic Disease" Cancers 15, no. 14: 3601. https://doi.org/10.3390/cancers15143601
APA StyleKobryn, A., Nian, P., Baidya, J., Li, T. L., & Maheshwari, A. V. (2023). Intramedullary Nailing with and without the Use of Bone Cement for Impending and Pathologic Fractures of the Humerus in Multiple Myeloma and Metastatic Disease. Cancers, 15(14), 3601. https://doi.org/10.3390/cancers15143601





