Changes in Peripheral Immune Cells after the Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine and Disease Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Prospective Analysis of the Vax-on-Third-Profile Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Peripheral Blood Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and General Outcomes
3.2. Variations in Absolute Counts of Circulating Lymphocytes
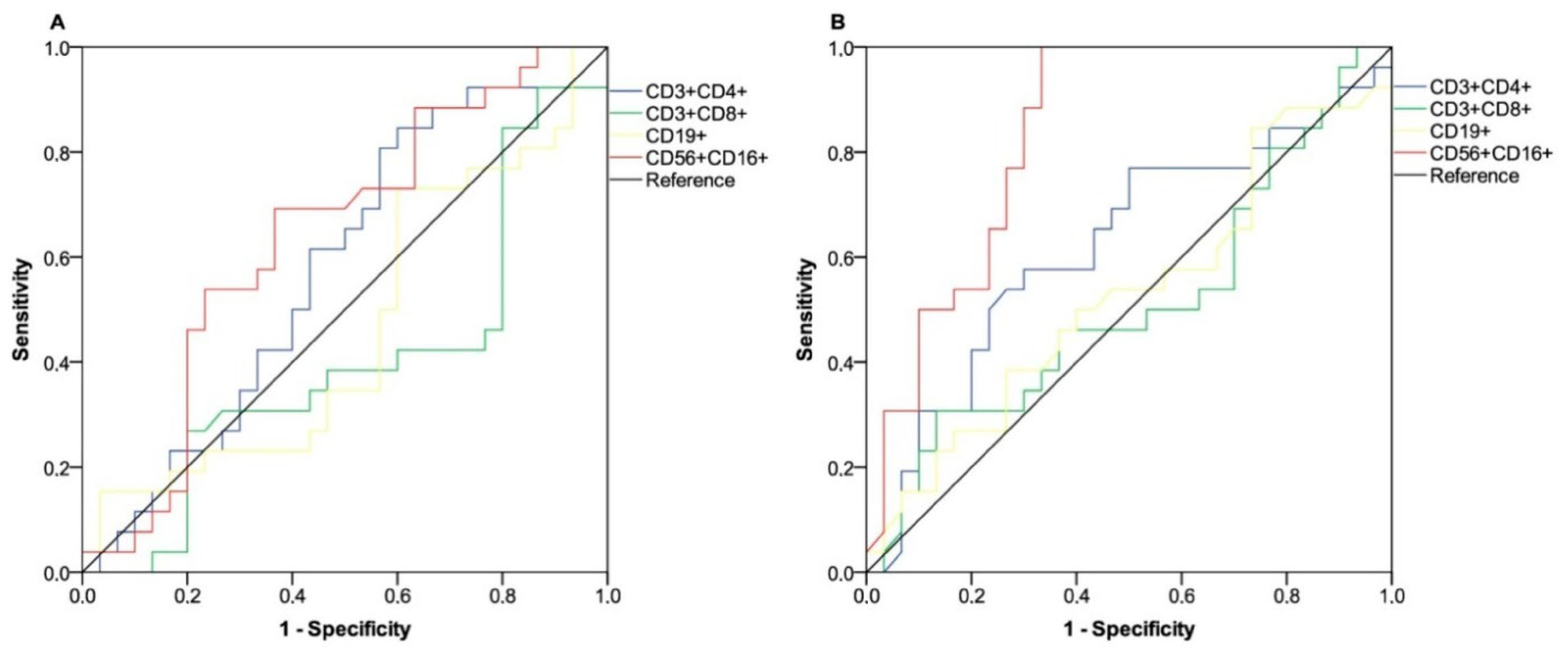
3.3. Clinical Benefit Outcome
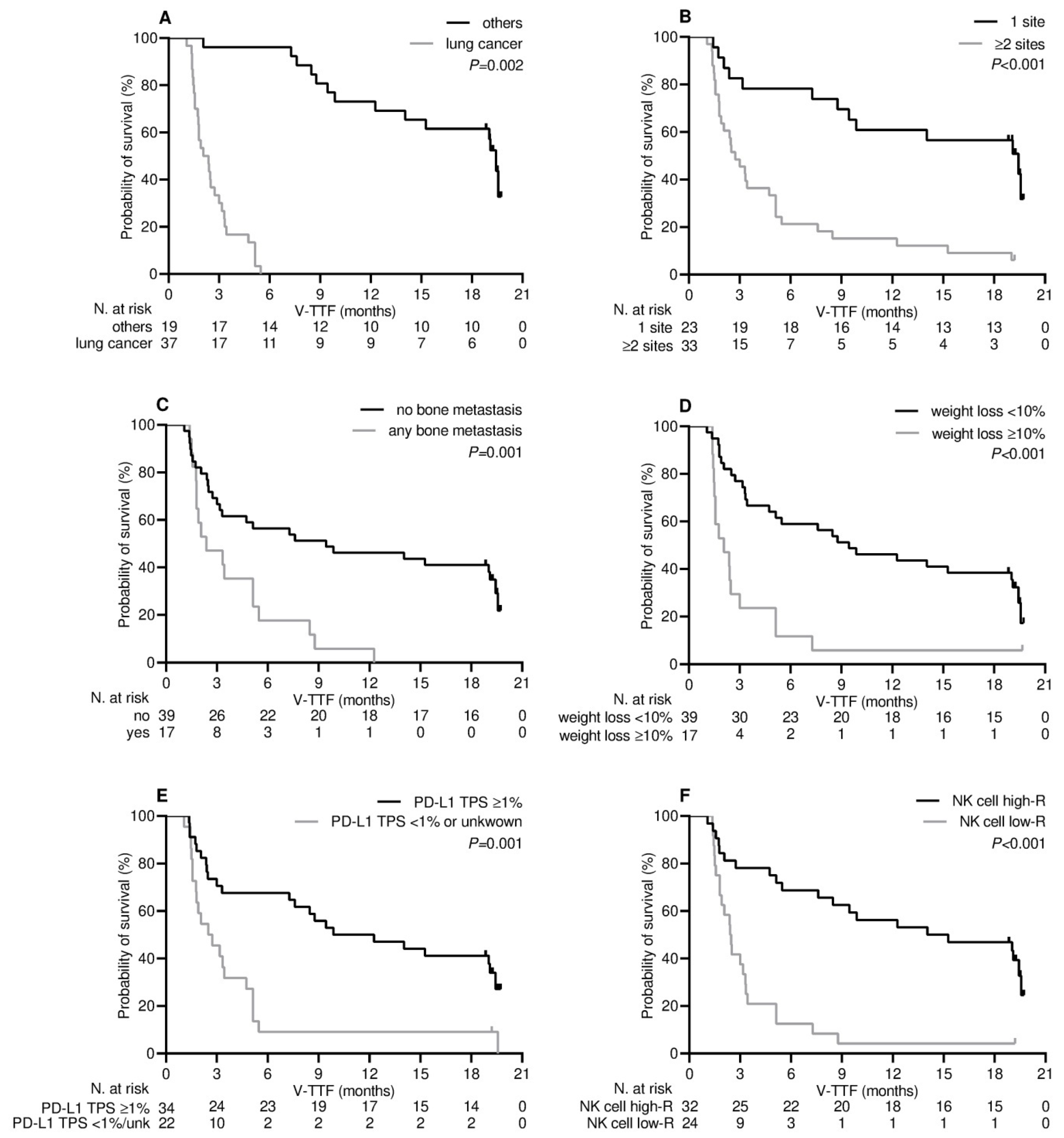
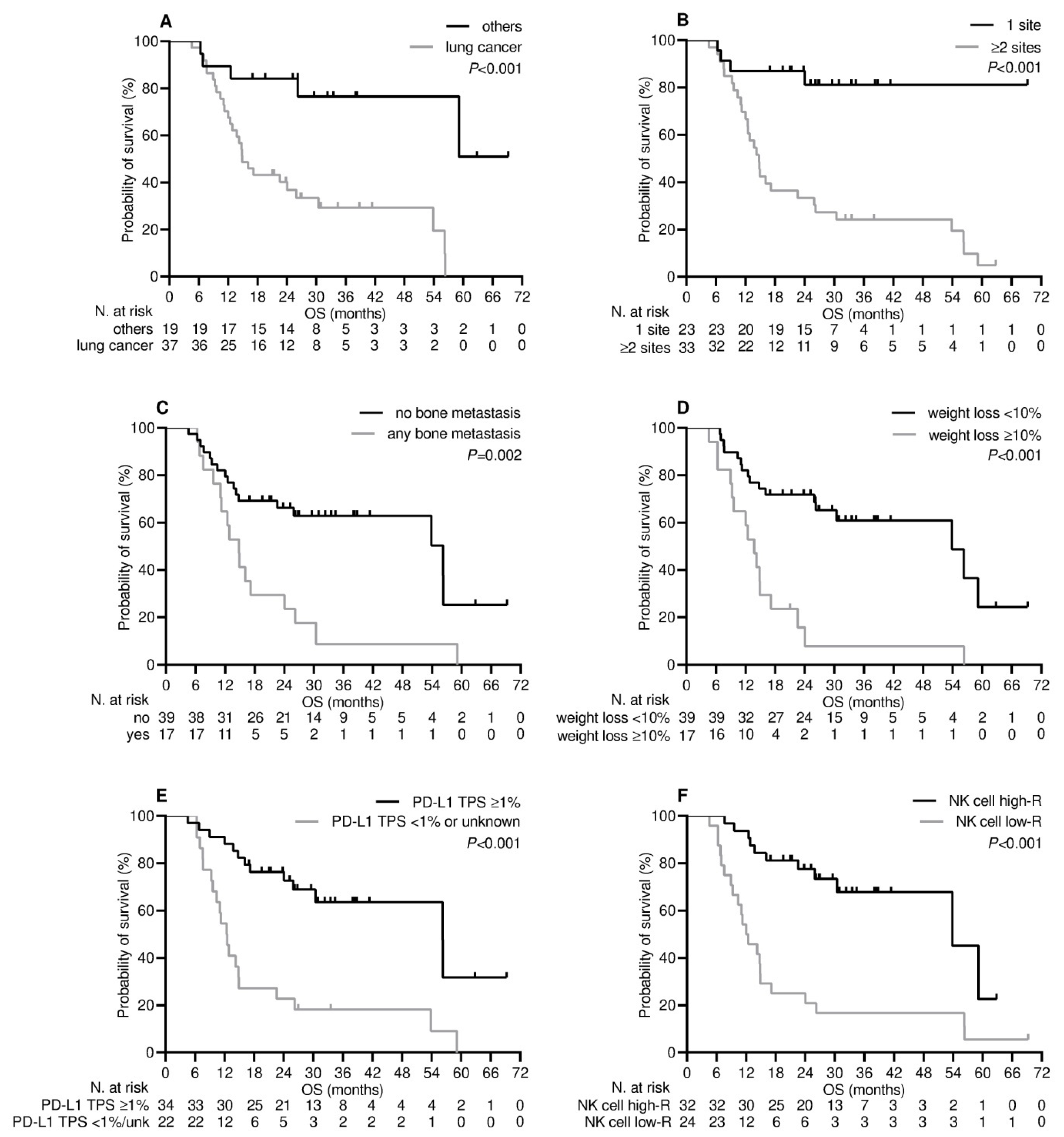
3.4. Survival Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 4 June 2023).
- Al Hajji, Y.; Taylor, H.; Starkey, T.; Lee, L.Y.W.; Tilby, M. Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: A systematic review. Br. J. Cancer 2022, 127, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Labaki, C.; Bakouny, Z.; Hsu, C.Y.; Schmidt, A.L.; de Lima Lopes, G., Jr.; Hwang, C.; Singh, S.R.K.; Jani, C.; Weissmann, L.B.; et al. Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: A retrospective observational study from the COVID-19 and Cancer Consortium. Lancet Reg. Health Am. 2023, 19, 100445. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Ionescu, M.C.; Starkey, T.; Little, M.; Tilby, M.; Tripathy, A.R.; Mckenzie, H.S.; Al-Hajji, Y.; Appanna, N.; Barnard, M.; et al. COVID-19: Third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: A population-based study. Eur. J. Cancer 2022, 175, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Debie, Y.; Van Audenaerde, J.R.M.; Vandamme, T.; Croes, L.; Teuwen, L.A.; Verbruggen, L.; Vanhoutte, G.; Marcq, E.; Verheggen, L.; Le Blon, D.; et al. Humoral and Cellular Immune Responses against SARS-CoV-2 after Third Dose BNT162b2 following Double-Dose Vaccination with BNT162b2 versus ChAdOx1 in Patients with Cancer. Clin. Cancer Res. 2023, 29, 635–646. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Hagemann, K.; Riecken, K.; Jung, J.M.; Hildebrandt, H.; Menzel, S.; Bunders, M.J.; Fehse, B.; Koch-Nolte, F.; Heinrich, F.; Peine, S.; et al. Natural killer cell-mediated ADCC in SARS-CoV-2-infected individuals and vaccine recipients. Eur. J. Immunol. 2022, 52, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Morikawa, M.; Adachi, Y.; Kabasawa, K.; Sax, N.; Moriyama, S.; Sun, L.; Isogawa, M.; Nishiyama, A.; Onodera, T.; et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell. Rep. Med. 2022, 3, 100631. [Google Scholar] [CrossRef]
- La Sala, L.; Gandini, S.; Bruno, A.; Allevi, R.; Gallazzi, M.; Senesi, P.; Palano, M.T.; Meregalli, P.; Longhi, E.; Sommese, C.; et al. SARS-CoV-2 Immunization Orchestrates the Amplification of IFNγ-Producing T Cell and NK Cell Persistence. Front. Immunol. 2022, 13, 798813. [Google Scholar] [CrossRef]
- Ruggeri, E.M.; Nelli, F.; Giannarelli, D.; Fabbri, A.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Mazzotta, M.; Schirripa, M.; Signorelli, C.; et al. Dynamic changes in peripheral lymphocytes and antibody response following a third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients. Sci. Rep. 2022, 12, 21908. [Google Scholar] [CrossRef]
- Brest, P.; Mograbi, B.; Hofman, P.; Milano, G. COVID-19 vaccination and cancer immunotherapy: Should they stick together? Br. J. Cancer 2022, 126, 1–3. [Google Scholar] [CrossRef]
- Spitzer, M.H.; Carmi, Y.; Reticker-Flynn, N.E.; Kwek, S.S.; Madhireddy, D.; Martins, M.M.; Gherardini, P.F.; Prestwood, T.R.; Chabon, J.; Bendall, S.C.; et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017, 168, 487–502.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Chon, H.J.; Kim, C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 9414. [Google Scholar] [CrossRef] [PubMed]
- Gascón, M.; Isla, D.; Cruellas, M.; Gálvez, E.M.; Lastra, R.; Ocáriz, M.; Paño, J.R.; Ramírez, A.; Sesma, A.; Torres-Ramón, I.; et al. Intratumoral Versus Circulating Lymphoid Cells as Predictive Biomarkers in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: Is the Easiest Path the Best One? Cells 2020, 9, 1525. [Google Scholar] [CrossRef] [PubMed]
- AdviseDx SARS-CoV-2 IgG II. Package Insert. Abbott Laboratories. 2021. Available online: https://www.fda.gov/media/146371/download (accessed on 4 June 2023).
- Saker, K.; Escuret, V.; Pitiot, V.; Massardier-Pilonchéry, A.; Paul, S.; Mokdad, B.; Langlois-Jacques, C.; Rabilloud, M.; Goncalves, D.; Fabien, N.; et al. Evaluation of Commercial Anti-SARS-CoV-2 Antibody Assays and Comparison of Standardized Titers in Vaccinated Health Care Workers. J. Clin. Microbiol. 2022, 60, e0174621. [Google Scholar] [CrossRef]
- BD FACSCanto™ Software. Available online: https://www.bdbiosciences.com/en-eu/products/software/instrument-software/bd-facscanto-clinical-software (accessed on 4 June 2023).
- Mushti, S.L.; Mulkey, F.; Sridhara, R. Evaluation of Overall Response Rate and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Immunotherapy Trials. Clin. Cancer Res. 2018, 24, 2268–2275. [Google Scholar] [CrossRef] [Green Version]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef]
- Bakouny, Z.; Labaki, C.; Grover, P.; Awosika, J.; Gulati, S.; Hsu, C.Y.; Alimohamed, S.I.; Bashir, B.; Berg, S.; Bilen, M.A.; et al. Interplay of Immunosuppression and Immunotherapy Among Patients with Cancer and COVID-19. JAMA Oncol. 2023, 9, 128–134. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Walle, T.; Bajaj, S.; Kraske, J.A.; Rösner, T.; Cussigh, C.S.; Kälber, K.A.; Müller, L.J.; Strobel, S.B.; Burghaus, J.; Kallenberger, S.M.; et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat. Cancer 2022, 3, 1039–1051. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.; Hu, J.; Medina, T.; Kessler, E.R.; Lam, E.T. Safety of COVID-19 vaccines in subjects with solid tumor cancers receiving immune checkpoint inhibitors. Hum. VaccinImmunother. 2023, 9, 2207438. [Google Scholar] [CrossRef] [PubMed]
- Widman, A.J.; Cohen, B.; Park, V.; McClure, T.; Wolchok, J.; Kamboj, M. Immune-Related Adverse Events Among COVID-19-Vaccinated Patients With Cancer Receiving Immune Checkpoint Blockade. J. Natl. ComprCancNetw. 2022, 20, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Uryu, K.; Takeda, T.; Kunimatsu, Y.; Shiotsu, S.; Uchino, J.; Hirai, S.; Yamada, T.; Okada, A.; Hasegawa, Y.; et al. Safety and Immunogenicity of mRNA Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 in Patients with Lung Cancer Receiving Immune Checkpoint Inhibitors: A Multicenter Observational Study in Japan. J. Thorac. Oncol. 2022, 17, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Donahue, R.N.; Marté, J.L.; Goswami, M.; Toney, N.J.; Tsai, Y.T.; Gulley, J.L.; Schlom, J. Interrogation of the cellular immunome of cancer patients with regard to the COVID-19 pandemic. J. Immunother. Cancer 2021, 9, e002087. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R.; et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell. Discov. 2021, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, J.; Hou, X.; Yang, Q.; Zhou, Y.; Ye, J.; Wu, X.; Feng, Y.; Hu, T.; Xu, Z.; et al. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 2021, 17, 3477–3484. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Upadhyaya, B.; Tuballes, K.; Kappes, K.; Gleason, C.R.; Beach, K.; Agte, S.; Srivastava, K.; Van Oekelen, O.; et al.; PVI/Seronet Study Group Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell 2021, 39, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Canzoniero, J.V.; Rosner, S.; Zhang, G.; White, J.R.; Belcaid, Z.; Cherry, C.; Balan, A.; Pereira, G.; Curry, A.; et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J. Immunother. Cancer 2022, 10, e004688. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuapio, A.; Boulouis, C.; Filipovic, I.; Wullimann, D.; Kammann, T.; Parrot, T.; Chen, P.; Akber, M.; Gao, Y.; Hammer, Q.; et al. NK cell frequencies, function and correlates to vaccine outcome in BNT162b2 mRNA anti-SARS-CoV-2 vaccinated healthy and immunocompromised individuals. Mol. Med. 2022, 28, 20. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xiang, T.; Xu, F.; Jiang, Y.; Zhong, L.; Peng, Y.; Le, A.; Zhang, W.; Liu, Y. Redistribution and Activation of CD16brightCD56dim NK Cell Subset to Fight against Omicron Subvariant BA.2 after COVID-19 Vaccination. Microorganisms 2023, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Yu, Y. The Function of NK Cells in Tumor Metastasis and NK Cell-Based Immunotherapy. Cancers 2023, 15, 2323. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal. Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural killer cells: A promising immunotherapy for cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.I.; Park, S.M.; Park, S.; Kim, G.; Lee, H.J.; Son, J.; Hong, M.H.; Ghaderpour, A.; Baik, B.; Islam, J.; et al. Peripheral natural killer cells and myeloid-derived suppressor cells correlate with anti-PD-1 responses in non-small cell lung cancer. Sci. Rep. 2020, 10, 9050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.G.; Donaubauer, A.J.; Frey, B.; Becker, I.; Rutzner, S.; Eckstein, M.; Sun, R.; Ma, H.; Schubert, P.; Schweizer, C.; et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001845. [Google Scholar] [CrossRef]
- Kim, H.R.; Kang, J.H.; Kim, S.H.; Kim, S.T.; Kim, I.; Min, Y.J.; Shin, S.H.; Oh, S.Y.; Lee, G.W.; Lee, J.H.; et al. Changes of Immune Cell Fractions in Patients Treated with Immune Checkpoint Inhibitors. Cancers 2022, 14, 3440. [Google Scholar] [CrossRef]
- Tewari, K.; Nakayama, Y.; Suresh, M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J. Immunol. 2007, 179, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Sercan, O.; Stoycheva, D.; Hämmerling, G.J.; Arnold, B.; Schüler, T. IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation. J. Immunol. 2010, 184, 2855–2862. [Google Scholar] [CrossRef] [Green Version]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5, eabd6832. [Google Scholar] [CrossRef]
- Bersanelli, M.; Giannarelli, D.; Castrignanò, P.; Fornarini, G.; Panni, S.; Mazzoni, F.; Tiseo, M.; Rossetti, S.; Gambale, E.; Rossi, E.; et al. Influenza vaccine indication during therapy with immune checkpoint inhibitors: A transversal challenge. The INVIDIa study. Immunotherapy 2018, 10, 1229–1239. [Google Scholar] [CrossRef]
- Valachis, A.; Rosén, C.; Koliadi, A.; Digkas, E.; Gustavsson, A.; Nearchou, A.; Ullenhag, G.J. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology 2021, 10, 1886725. [Google Scholar] [CrossRef]
- Mei, Q.; Hu, G.; Yang, Y.; Liu, B.; Yin, J.; Li, M.; Huang, Q.; Tang, X.; Böhner, A.; Bryant, A.; et al. Impact of COVID-19 vaccination on the use of PD-1 inhibitor in treating patients with cancer: A real-world study. J. Immunother. Cancer 2022, 10, e004157. [Google Scholar] [CrossRef] [PubMed]
- Gleiss, A.; Oberbauer, R.; Heinze, G. An unjustified benefit: Immortal time bias in the analysis of time-dependent events. Transpl. Int. 2018, 31, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.K.; Heo, M.H.; Lee, H.S.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer ChemotherPharmacol 2017, 80, 591–598. [Google Scholar] [CrossRef] [PubMed]
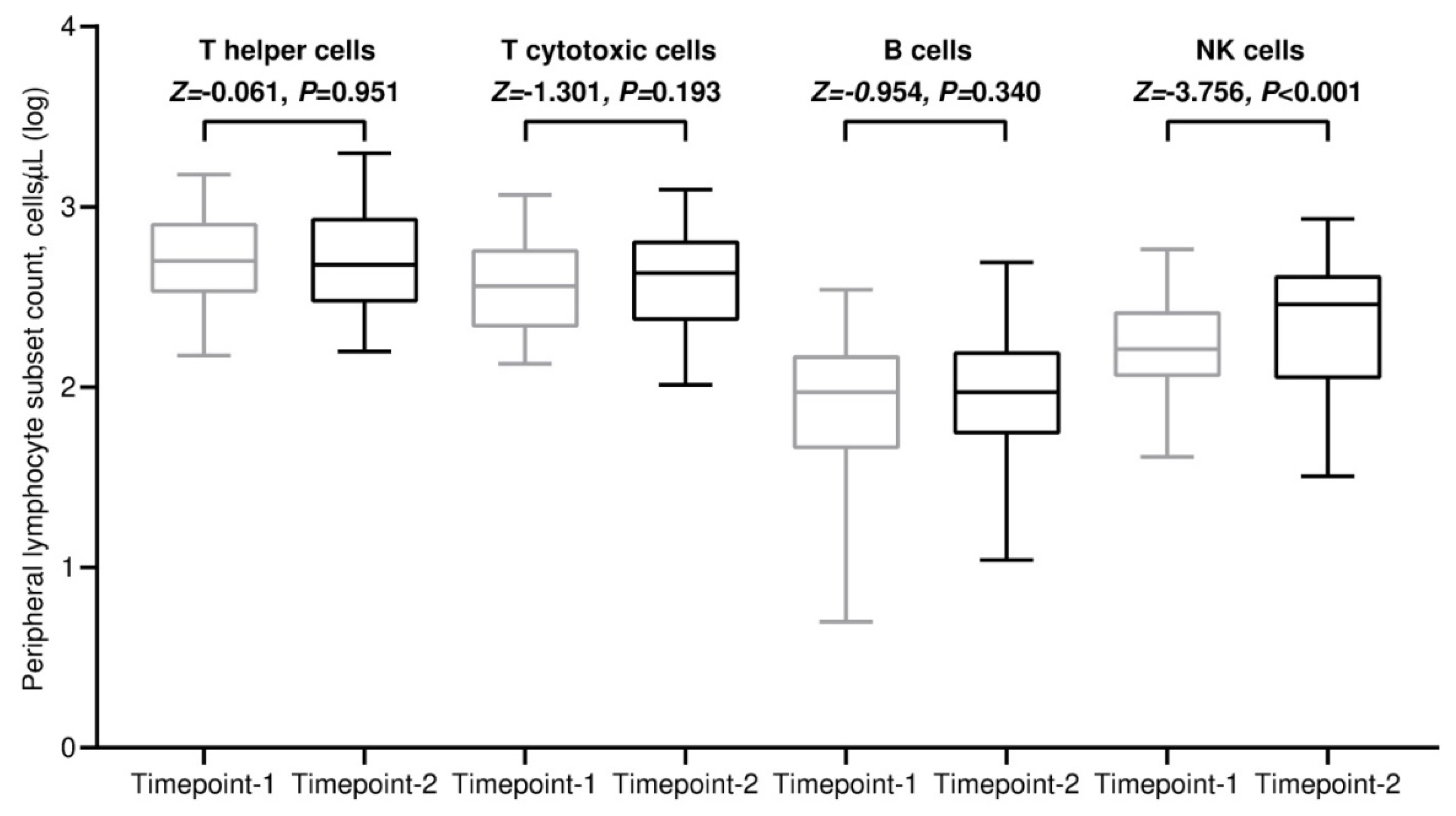

| Characteristic | General Population, N = 56 (100%) |
|---|---|
| Mean age, years (SD) | 65.9 (10.0) |
| Sex | |
| - female | 16 (28.6%) |
| - male | 40 (71.4%) |
| ECOG PS | |
| - 0 | 17 (30.4%) |
| - 1 | 39 (69.6%) |
| Cancer type | |
| - non-small-cell lung cancer | 31 (55.3%) |
| - small-cell lung cancer | 6 (10.7%) |
| - kidney | 7 (12.5%) |
| - dermal (melanoma, Merkel-cell, squamous cell carcinoma) | 7 (12.5%) |
| - bladder | 4 (7.1%) |
| - esophageal | 1 (1.8%) |
| Disease extent | |
| - metastatic | 56 (100%) |
| Treatment setting | |
| - metastatic, first line | 36 (64.3%) |
| - metastatic, second or later line | 20 (35.7%) |
| Number of metastatic sites | |
| - 1 | 23 (41.1%) |
| - ≥2 | 33 (58.9%) |
| Brain metastases | |
| - not present | 44 (78.6%) |
| - any | 12 (21.4%) |
| Liver metastases | |
| - not present | 50 (89.3%) |
| - any | 6 (10.7%) |
| Bone metastases | |
| - not present | 39 (69.6%) |
| - any | 17 (30.4%) |
| Weight loss a | |
| - <10% | 39 (69.6%) |
| - ≥10% | 17 (30.4%) |
| PD-L1 TPS | |
| - ≥1% | 35 (62.5%) |
| - <1% | 16 (28.5%) |
| - unknown | 5 (8.9%) |
| Corticosteroid therapy b | 8 (14.3%) |
| ICI-based treatment | |
| - pembrolizumab | 25 (44.6%) |
| - nivolumab | 21 (37.5%) |
| - atezolizumab | 6 (10.7%) |
| - durvalumab | 2 (3.6%) |
| - avelumab | 1 (1.8%) |
| - cemiplimab | 1 (1.8%) |
| Length (months) of ICI treatment, median (IQR) | 19.8 (10.5–26.9) |
| Length (months) of ICI treatment before third vaccine dose, median (IQR) | 8.4 (5.1–16.1) |
| Length (months) of ICI treatment after third vaccine dose, median (IQR) | 4.6 (1.5–18.8) |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| NCB N = 30 (100%) | DCB N = 26 (100%) | p Value | OR (95% CI) | p Value | |
| Age | 0.99 | - | - | ||
| - ≤70 years (N = 32) | 17 (56.7%) | 15 (57.7%) | |||
| - >70 years (N = 24) | 13 (43.3%) | 11 (42.3%) | |||
| Sex | 0.55 | - | - | ||
| - female (N = 16) | 10 (33.3%) | 6 (23.1%) | |||
| - male (N = 40) | 20 (66.7%) | 20 (76.9%) | |||
| ECOG PS | 0.25 | - | - | ||
| - 0 (N = 17) | 7 (23.3%) | 10 (38.5%) | |||
| - 1 (N = 39) | 23 (76.7%) | 16 (61.5%) | |||
| Cancer type | 0.005 † | 0.16 | |||
| - any other (N = 19) | 5 (16.7%) | 14 (53.8%) | 1.00 | ||
| - lung (N = 37) | 25 (83.3%) | 12 (46.2%) | 0.25 (0.03–1.71) | ||
| Number of metastatic sites | <0.001 † | 0.026 | |||
| - 1 (N = 23) | 4 (13.3%) | 19 (73.1%) | 1.00 | ||
| - ≥2 (N = 33) | 26 (86.7%) | 7 (26.9%) | 0.10 (0.01–0.76) | ||
| Brain metastases | 0.34 | - | - | ||
| - not present (N = 44) | 22 (73.3%) | 22 (84.6%) | |||
| - any (N = 12) | 8 (26.7%) | 4 (15.4%) | |||
| Bone metastases | 0.008 † | 0.66 | |||
| - not present (N = 39) | 16 (53.3%) | 23 (88.5%) | 1.00 | ||
| - any (N = 17) | 14 (46.7%) | 3 (11.5%) | 0.68 (0.12–3.85) | ||
| Liver metastases | 0.025 | - | - | ||
| - not present (N = 50) | 24 (80.0%) | 26 (100%) | |||
| - any (N = 6) | 6 (20.0%) | - | |||
| Weight loss a | 0.001 † | 0.28 | |||
| - <10% (N = 39) | 15 (50.0%) | 24 (92.3%) | 1.00 | ||
| - ≥10% (N = 17) | 15 (50.0%) | 2 (7.7%) | 0.31 (0.03–2.65) | ||
| PD-L1 TPS | <0.001 † | 0.010 | |||
| - <1% or unknown (N = 22) | 20 (66.7%) | 2 (7.7%) | 1.00 | ||
| - >1% (N = 34) | 10 (33.3%) | 24 (92.3%) | 13.29 (1.86–94) | ||
| Treatment setting | 0.26 | - | - | ||
| - first line (N = 36) | 17 (56.7%) | 19 (73.1%) | |||
| - second or later line (N = 20) | 13 (43.3%) | 7 (26.9%) | |||
| Corticosteroid therapy b | 0.34 | - | - | ||
| - no (N = 44) | 22 (73.3%) | 22 (84.6%) | |||
| - yes (N = 12) | 8 (26.7%) | 4 (15.4%) | |||
| ICI therapy | 0.48 | - | - | ||
| - anti-PD-1 (N = 47) | 24 (80.0%) | 23 (88.5%) | |||
| - anti-PD-L1 (N = 9) | 6 (20.0%) | 3 (11.5%) | |||
| Treatment type | 0.14 | - | - | ||
| - ICI monotherapy | 18 (60.0%) | 21 (80.8%) | |||
| - ICI and chemotherapy | 12 (40.0%) | 5 (19.2%) | |||
| NK cell level c | <0.001 † | 0.020 | |||
| - low-responders (N = 24) | 21 (70.0%) | 3 (11.5%) | 1.00 | ||
| - high-responders (N = 32) | 9 (30.0%) | 23 (88.5%) | 12.31 (1.48–102) | ||
| Variable | Vaccine-Related Time-to-Treatment Failure | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Median V-TTF (95% CI), Months | p Value | HR (95% CI) | p Value | Median OS (95% CI), Months | p Value | HR (95% CI) | p Value | |
| Cancer type | 0.002 | 0.091 | <0.001 | 0.019 | ||||
| - others | 19.0 (10.5–27.5) | 1.00 | 38.0 (27.5-NR) | 1.00 | ||||
| - lung | 2.7 (1.8–3.6) | 1.96 (0.89–4.29) | 14.9 (11.4–18.3) | 4.18 (1.26–13.86) | ||||
| Number of metastatic sites | <0.001 | 0.007 | <0.001 | 0.14 | ||||
| - 1 | 19.4 (10.5–28.2) | 1.00 | 26.2 (23.8-NR) | 1.00 | ||||
| - ≥2 | 2.7 (1.7–3.7) | 3.08 (1.36–6.99) | 14.7 (12.4–17.0) | 2.37 (0.73–7.63) | ||||
| Bone metastases | 0.001 | 0.47 | 0.002 | 0.50 | ||||
| - not present | 9.4 (0.1–9.3) | 1.00 | 56.2 (21.8–90.6) | 1.00 | ||||
| - any | 2.3 (0.4–4.2) | 1.30 (0.63–2.68) | 14.8 (11.5–18.1) | 1.31 (0.58–2.93) | ||||
| Weight loss a | <0.001 | 0.47 | <0.001 | 0.83 | ||||
| - <10% | 9.4 (3.7–15.1) | 1.00 | 53.8 (27.2–80.4) | 1.00 | ||||
| - ≥10% | 2.0 (1.0–3.1) | 1.34 (0.60–2.98) | 13.7 (10.7–16.7) | 1.09 (0.46–2.58) | ||||
| PD-L1 TPS | 0.001 | 0.47 | <0.001 | 0.015 | ||||
| - <1% or unknown | 2.5 (1.0–3.9) | 1.00 | 12.4 (10.3–14.5) | 1.00 | ||||
| - ≥1% | 9.8 (2.3–17.3) | 0.76 (0.37–1.59) | 56.2 (31.7–80.7) | 0.34 (0.14–0.81) | ||||
| NK cell level b | <0.001 | 0.014 | <0.001 | 0.027 | ||||
| - low-responders | 2.4 (1.8–2.9) | 1.00 | 12.0 (8.1–15.8) | 1.00 | ||||
| - high-responders | 14.0 (1.8–26.2) | 0.34 (0.14–0.80) | 56.8 (29.6–78.0) | 0.36 (0.15–0.89) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, F.; Signorelli, C.; Fabbri, A.; Giannarelli, D.; Virtuoso, A.; Giron Berrios, J.R.; Marrucci, E.; Fiore, C.; Schirripa, M.; Chilelli, M.G.; et al. Changes in Peripheral Immune Cells after the Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine and Disease Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Prospective Analysis of the Vax-on-Third-Profile Study. Cancers 2023, 15, 3625. https://doi.org/10.3390/cancers15143625
Nelli F, Signorelli C, Fabbri A, Giannarelli D, Virtuoso A, Giron Berrios JR, Marrucci E, Fiore C, Schirripa M, Chilelli MG, et al. Changes in Peripheral Immune Cells after the Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine and Disease Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Prospective Analysis of the Vax-on-Third-Profile Study. Cancers. 2023; 15(14):3625. https://doi.org/10.3390/cancers15143625
Chicago/Turabian StyleNelli, Fabrizio, Carlo Signorelli, Agnese Fabbri, Diana Giannarelli, Antonella Virtuoso, Julio Rodrigo Giron Berrios, Eleonora Marrucci, Cristina Fiore, Marta Schirripa, Mario Giovanni Chilelli, and et al. 2023. "Changes in Peripheral Immune Cells after the Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine and Disease Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Prospective Analysis of the Vax-on-Third-Profile Study" Cancers 15, no. 14: 3625. https://doi.org/10.3390/cancers15143625







