Programmed Cell Death Pathways in Cholangiocarcinoma: Opportunities for Targeted Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
Signaling Pathways and Therapeutical Approaches
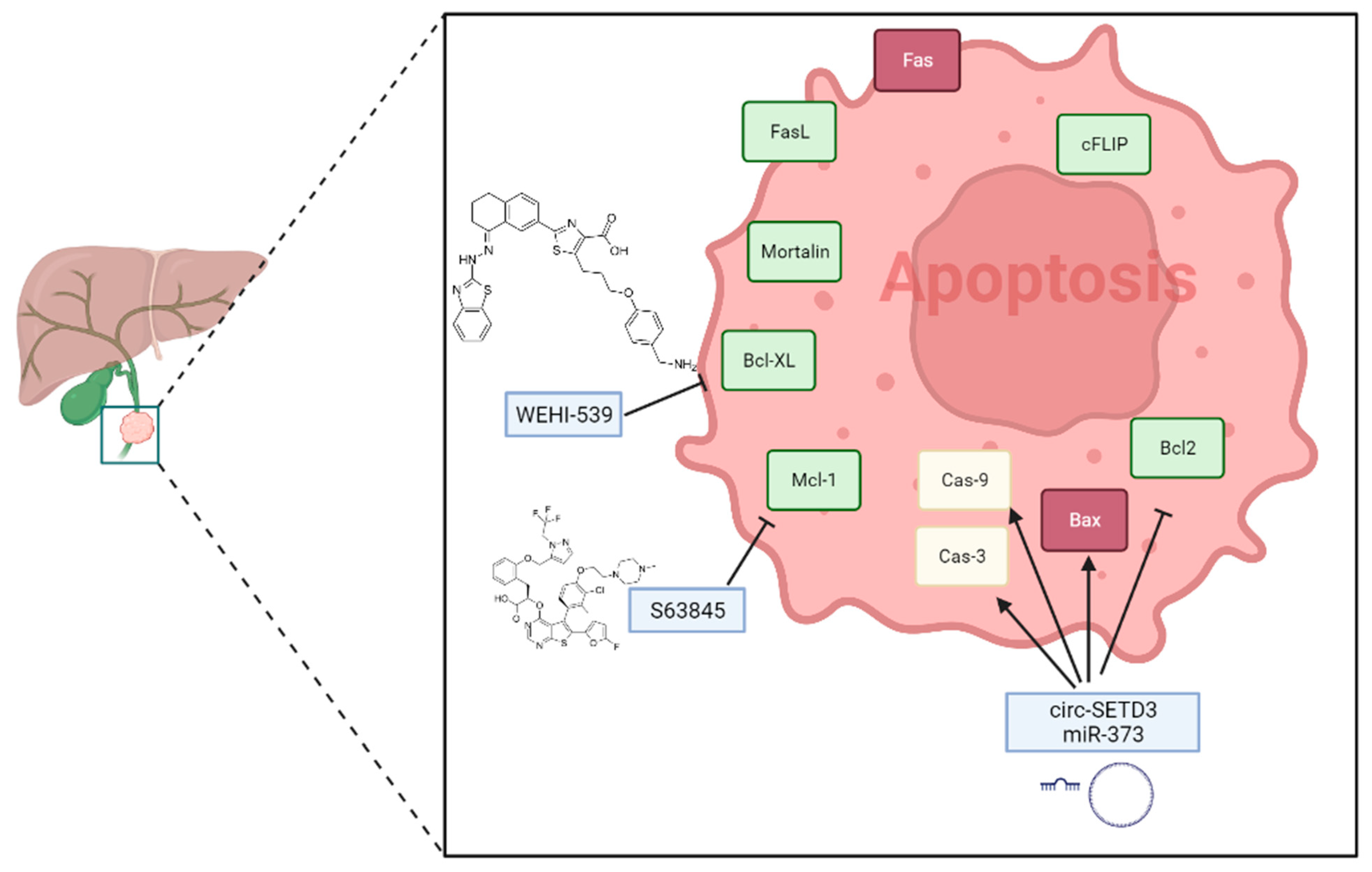
2. Apoptosis Regulation in Cholangiocarcinoma
- Apoptosis is a genetically controlled cell death program that regulates the balance between the proliferation and elimination of old/damaged cells—[29]
- Apoptosis is morphologically characterized by a reduction in cell dimensions, nuclear and chromatin fragmentation, vacuolization, and the formation of apoptotic bodies—[32]
- Several pathological stimuli activate apoptotic processes mainly in the presence of damaged cells, although cancer cells frequently undergo apoptosis—[33]
2.1. Main Signaling Pathways
| Molecules | Biological Function | Type of Cell Death | Pathway | References |
|---|---|---|---|---|
| Fas | TNF receptor family of proteins, containing a cytoplasmatic protein-binding regions called Death Domain (DD). After its binding with FasL, trimerization of activated receptors and FADD recruitment occurs. | Apoptosis | Extrinsic | [36] |
| FasL | Transmembrane protein of the TNF family. Induce apoptosis following the interaction with its receptor Fas. | Apoptosis | Extrinsic | [36] |
| FADD | Adaptor molecule that interacts with various cell surface receptors such as Fas or TNFR1 and mediates cell apoptotic signaling. It recruits and activates pro-caspase8. | Apoptosis | Extrinsic | [36] |
| TNFα | Extracellular cytokine able to activate inflammation, proliferation, and apoptosis. It interacts with TNFR1 to activate apoptotic signaling. | Apoptosis | Extrinsic | [37] |
| TNFR1 | Cell membrane receptor, which binds soluble TNF. Its intracellular region contains a DD, involved in homo- and hetero-typic interactions with other DD-containing proteins. | Apoptosis | Extrinsic | [37] |
| TRADD | Adaptor molecule that interacts with DDs of Fas or TNFR1, forming a complex with RIP. | Apoptosis | Extrinsic | [37] |
| RIP | This protein interacts with TRADD or FADD on cytoplasmatic DDs of activated receptors to form a complex that activates caspase-activating cleavage. | Apoptosis | Extrinsic | [37] |
| Caspase-8 | Pro-caspase8 is recruited by FADD and activated by lytic cleavage. Fas, FADD and caspase-8 interact and form the so called DISC (death-inducing signaling complex). | Apoptosis | Extrinsic | [37] |
| Caspase-3 | Effector of apoptotic pathway, activated by cleavage after Cas-8 activation. | Apoptosis | Extrinsic and intrinsic | [37] |
| Bid | Activated by caspase-8 cleavage, with the release of c-Bid or t-Bid. It interacts with Bax, promoting the MOMP and apoptosis. | Apoptosis | Intrinsic | [44] |
| Bax | Pro-apoptotic mediator. It promotes cytochrome c release from mitochondria through permeabilization of the outer mitochondrial membrane. | Apoptosis | Intrinsic | [44] |
| Bak | Pro-apoptotic mediator. It promotes cytochrome c release from mitochondria through permeabilization of the outer mitochondrial membrane. | Apoptosis | Intrinsic | [44] |
| Bcl-XL | Anti-apoptotic mediator. It inhibits the activator Bid or other BH3-only proteins and Bax/Bak, by mutual sequestration. | Apoptosis | Intrinsic | [44] |
| Mcl-1 | Anti-apoptotic mediator. It inhibits BH3-only proteins and Bax/Bak pore formation. | Apoptosis | Intrinsic | [44] |
| Bcl-2 | Anti-apoptotic mediator. It inhibits BH3-only proteins and Bax/Bak pore formation. | Apoptosis | Intrinsic | [44] |
| cytochrome c | Cytochrome c is released by mitochondria as an apoptotic signal. It interacts with Apaf-1 and pro-caspase 9, resulting in the formation of the apoptosome complex. | Apoptosis | Intrinsic | [32] |
| Smac/DIABLO | These proteins are released from the intermembrane space of mitochondria into the cytosol. Their interactors are multiple IAPs (inhibitor apoptosis proteins), which are removed to activate both initiator and effector caspases. | Apoptosis | Intrinsic | [45] |
| Apaf-1 | This protein contains caspase recruitment domain (CARD) in its N-terminal. It interacts with cytochrome c and recruits pro-caspase 9, forming the apoptosome complex and promoting activated caspase-9 formation by cleavage. | Apoptosis | Intrinsic | [32] |
| Caspase-9 | Activated caspase-9 promotes the cleavage and activation of apoptotic caspase effector, caspase-3. | Apoptosis | Intrinsic | [32] |
| Caspase-6 | Apoptotic caspase effector. | Apoptosis | Intrinsic | [32] |
| Caspase-7 | Apoptotic caspase effector. | Apoptosis | Intrinsic | [32] |
| Granzyme B | Serine protease characterized by a perforin-dependent pro-apoptotic function triggered in infected or cancer cells by cytotoxic immune cells. Activates apoptosis by interacting directly with caspases or by cleaving Bid. | Apoptosis | Immune cells activated apoptosis | [58] |
| Granzyme A | Granzyme A cleaves proteins at sites after basic amino acids. | Apoptosis | Immune cells activated apoptosis | [58] |
| Perforin | Granule protein released by cytotoxic cells, which forms membrane pores on targeted cells. | Apoptosis | Immune cells activated apoptosis | [58] |
2.2. Potential Therapeutical Approaches
| Molecule | Function | References |
|---|---|---|
| circSETD3 | It is a circular RNA containing multiple miRNA binding sites. circSETD3 has been implicated in CCA progression. | [79] |
| miR-421 | It promotes cell proliferation in human gastric cancer and represents a promising therapeutic target for CCA treatment. | [80,81] |
| miR-373 | Its overexpression promotes apoptosis in CCA cells by targeting ULK1. | [82] |
| miR-191 | It is involved in the initiation and progression of CCA. | [83] |
3. Ferroptosis in Cholangiocarcinoma
- Ferroptosis is a form of cell death characterized by the accumulation of reactive oxygen species (ROS) that result from iron-dependent lipid peroxidation—[87]
- During ferroptosis, the mitochondria undergo morphological changes, such as shrinkage, increased membrane density, and decreased mitochondrial crests. These changes are thought to be a result of lipid peroxidation and damage to the mitochondrial membranes, leading to the loss of membrane potential and mitochondrial dysfunction—[29]
- Rsl3 and Rsl5 are small molecules that have been identified as potent inducers of ferroptosis. They act by inhibiting the activity of glutathione peroxidase 4 (GPX4), a key regulator of lipid peroxidation, and lead to the increased accumulation of lipid reactive oxygen species (ROS) and subsequent cell death. The inhibition of GPX4 activity by Rsl3 or Rsl5 can also lead to a decrease in glutathione levels, which further enhances lipid peroxidation and ferroptosis. Therefore, these mediators are considered as negative regulators of GPX4 and promote iron-dependent oxidative cell death—[89]
- Slc7a11 has been shown to increase the resistance of cancer cells to ferroptosis, leading to tumor progression and therapy resistance in CCA—[89]
3.1. Main Signaling Pathways
| Molecules | Biological Function | Type of Cell Death | References |
|---|---|---|---|
| glutathione peroxidase 4 | GPX4 is an antioxidant enzyme that plays a crucial role in repairing oxidative damage to lipids and is a key inhibitor of ferroptosis. | Ferroptosis | [87] |
| RSL3 | RSL3 is a small molecule compound that inhibits the activity of GPX4, an antioxidant defense enzyme. This inhibition leads to the accumulation of lipid peroxides and the induction of ferroptosis, a form of regulated cell death. | Ferroptosis | [89] |
| RSL5 | It is a transcription factor that increases the expression of iron metabolism inhibitors such as ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1). | Ferroptosis | [89] |
| p53 | The role of p53 in ferroptosis is paradoxical, as it can have both pro- and anti-ferroptotic effects. On one hand, p53 can induce ferroptosis by inhibiting Solute carrier family 7 member 11 (SLC7A11), a component of the glutamate-cystine antiporter, which reduces intracellular cysteine levels and impairs glutathione synthesis, a crucial antioxidant. Additionally, p53 can induce ferroptosis by upregulating spermidine/spermine N1-acetyltransferase 1 (SAT1) or glutaminase 2 (GLS2), which leads to an increase in lipid peroxidation and reactive oxygen species (ROS) production. | Ferroptosis, apoptosis | [141] |
| BNIP3 | It is a mitochondrial autophagy receptor involved in the production of ROS. | Ferroptosis | [125,126] |
| nicotinamide adenine dinucleotide phosphate (NADPH) Oxidase 4 (NOX4) | (NADPH) Oxidase 4 (NOX4) is an enzyme complex consisting of multiple subunits that use nicotinamide adenine dinucleotide phosphate (NADPH) as a substrate to generate reactive oxygen species (ROS), including superoxide anions. The excessive production of ROS by NOX4 has been shown to promote ferroptosis, a type of cell death characterized by the accumulation of lipid peroxides. | Ferroptosis, apoptosis | [150,151,152] |
| ALOX5 | ALOX5 gene encodes a family of lipases that are essential for the production of leukotrienes and involved in several activities, including ferroptosis, inflammation and tumors. | Ferroptosis | [113] |
| STMN1 | The primary regulator of microtubule dynamics STMN1 is essential for controlling the cell cycle, which is strongly associated with the division and proliferation of tumor cells. | Ferroptosis | [120,121] |
| TNFSF13B | TNFSF13B facilitates the transfer of Fe3+ by interacting with the TFRC receptor on cluster 4. | Ferroptosis | [137] |
| BH4 | BH4 regulates cellular responses to ferroptosis following GPX4 inhibition. | Ferroptosis | [140] |
3.2. Possible Therapeutical Approaches
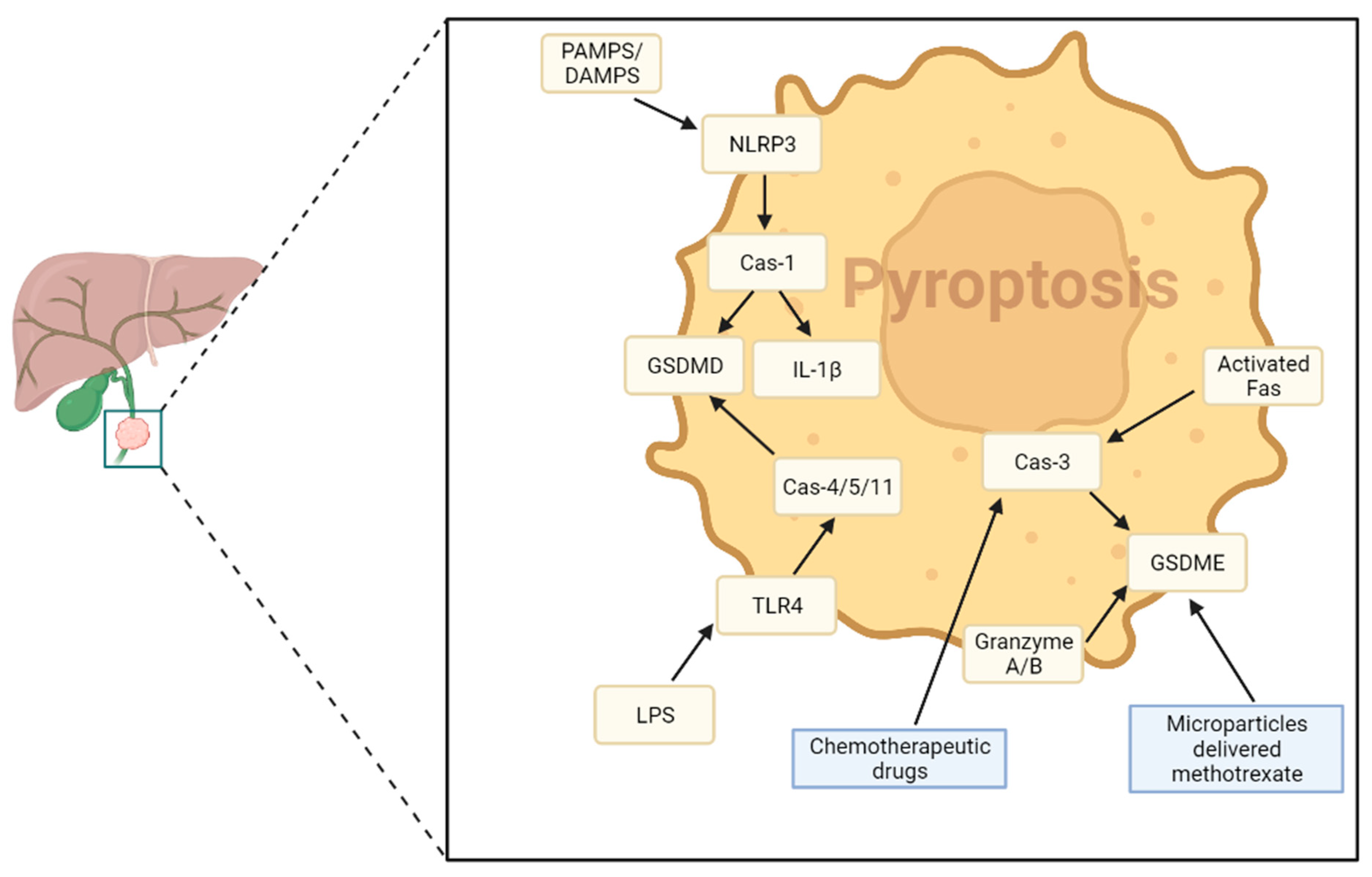
4. Pyroptosis in Cholangiocarcinoma
- Pyroptosis is a form of lytic and inflammatory cell death, which leads to inflammasomes activation and pro-inflammatory cytokines efflux—[157]
- Pyroptosis may modulate the TME and activate immune cells against cancer cells—[161]
- The delivery of chemotherapeutic drug methotrexate within CCA cells increased pyroptosis and triggered antitumor activity and tumor disruption—[161]
4.1. Main Signaling Pathways
| Molecules | Biological Function | Type of Cell Death | Pathway | References |
|---|---|---|---|---|
| ASC | It is a component of the inflammasome complex, promotes Cas-1 activation. | Pyroptosis | Canonical | [159,165] |
| Caspase-1 | Caspase-1 induces pyroptosis through activating cleavage of GSDMD, IL-1β and IL-18. | Pyroptosis | Canonical | [159,165] |
| IL-1β | It is an effector of inflammatory response pyroptotic cell lysis. | Pyroptosis | Canonical | [159,165] |
| IL-18 | It is an effector of inflammatory response pyroptotic cell lysis. | Pyroptosis | Canonical | [159,165] |
| NLRP3 | NLRP3 triggers the formation of the inflammasome complex after interaction with DAMPS, PAMPs and LPS. | Pyroptosis | Canonical | [159,165] |
| GSDMD | It forms cell membrane pores after N-terminal lytic activation and induces pyroptotic inflammatory lysis. | Pyroptosis | Canonical and non-canonical | [159] |
| Caspase-4 | Caspase-4induces pyroptosis through GSDMD cleavage. | Pyroptosis | Non-canonical | [159] |
| Caspase-5 | Caspase-5 induces pyroptosis through GSDMD cleavage. | Pyroptosis | Non-canonical | [159] |
| Caspase-11 | Caspase-11 induces pyroptosis through GSDMD cleavage. | Pyroptosis | Non-canonical | [150] |
| GSDME | It forms a cell membrane pore. | Pyroptosis | Inflammasome non-dependent | [166] |
| Caspase-3 | Caspase-3 mediates the switching from apoptosis to pyroptosis and promotes GSDME cleavage and activation | Pyroptosis | Inflammasome non-dependent | [166] |
| granzyme A | It catalyzes proteolytic activation of GSDMB. | Pyroptosis | Inflammasome non-dependent | [166] |
| granzyme B | It catalyzes proteolytic activation of GSDME. | Pyroptosis | Inflammasome non-dependent | [166] |
4.2. Possible Therapeutical Approaches
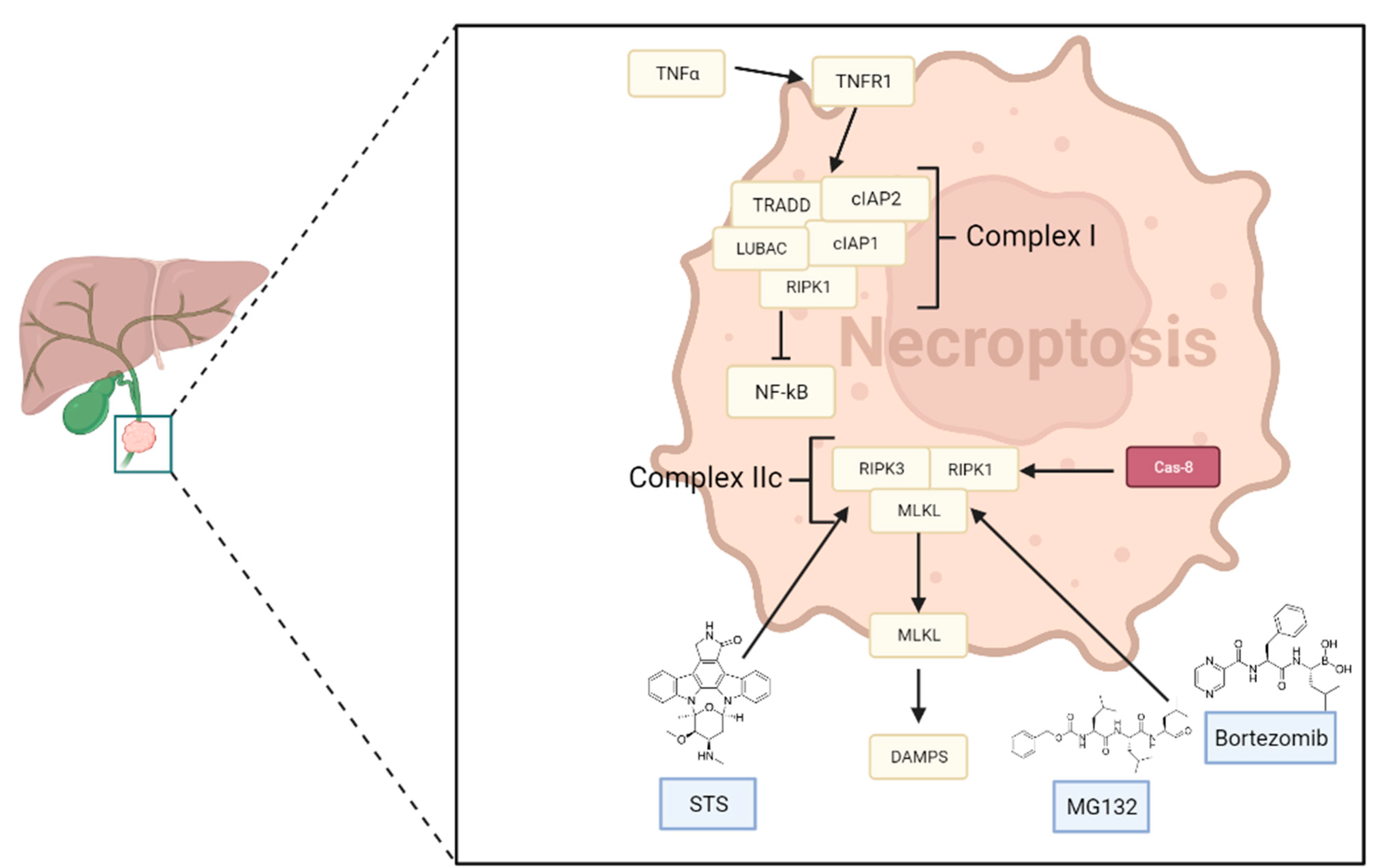
5. Necroptosis in Cholangiocarcinoma
- Necroptosis is characterized by morphological features of necrosis, such as increased cytoplasmic translucency, organelle swelling, lysosomal membrane permeabilization, increased cell volume, and intact nucleus, unlike apoptosis—[172]
- The most studied necroptosis pathway is the TNF-α/TNFR1 axis—[172]
- Ripk3, ripk1, and mlkl are responsible for the crucial event of necrosome formation—[175]
- Promoting necroptosis or manipulating its pathways has emerged as a promising therapeutic strategy for cancer treatment, as it offers an alternative mechanism for eliminating cancer cells that are resistant to other forms of cell death—[175]
5.1. Main Signaling Pathways
- cIAPs, after the polyubiquitination of RIPK1, promote the activation of the NF-κB pathway, which stimulates cell survival by activating genes encoding molecules that have cytoprotective functions [176];
- If NF-κB or its regulators are blocked, RIPK1 undergoes deubiquitination, facilitating necroptosis. Additionally, several drugs can directly enhance the deubiquitination of RIPK1 [177].
| Molecules | Biological Functions | Type of Cell Death | References |
|---|---|---|---|
| IFN-γ | Molecule capable of inducing necroptosis | Necroptosis | [174] |
| TNFα | Molecule capable of inducing necroptosis | Necroptosis | [174] |
| Fas ligand | Molecule capable of inducing necroptosis | Necroptosis | [174] |
| LPS | Molecule capable of inducing necroptosis | Necroptosis | [174] |
| RIPK1 | It is crucial for the formation of complex IIb (RIPK1-RIPK3-MLKL) | Necroptosis | [178] |
| RIPK3 | RIPK3 is a serine/threonine–protein kinase that activates necroptosis | Necroptosis | [178] |
| MLKL | MLKL is a pseudokinase that is involved in TNF-induced necroptosis | Necroptosis | [175] |
| TNFR1 | When it becomes active, it enables the recruitment of three specific necroptosis related proteins known as TRADD, RIP1, and TRAF2 | Necroptosis | [174] |
| TRAF2 | It can protect cells, inhibiting necroptotic cell death by TNF-induced NF-kB activation | Necroptosis | [174,175] |
| NF-kβ | If NF-κB is blocked, RIPK1 undergoes deubiquitination and is released, which leads to the formation of molecular complexes that facilitate necroptosis | Necroptosis | [177] |
| TRADD | It is a target protein for TNF-induced necroptosis in the absence of RIPK1 | Necroptosis | [174,175] |
| FADD | It recruits the initiator caspase-8, forming death-inducing signaling complex (DISC) | Necroptosis | [174,175] |
| cIAP1 | cIAP1 adds ubiquitin molecules to NF-κB, which triggers the activation of kinase NIK and the suppression of non-canonical NF-κB signaling | Necroptosis | [151] |
| cIAP2 | It ubiquitinates NF-kB, inducing kinase (NIK) to suppress non-canonical NF-kB signaling and RIPK1 to promote cell survival | Necroptosis | [175] |
| TAK1 | This is a serine/threonine kinase that modifies RIPK1 through phosphorylation, which controls its association with RIPK3 and facilitates necroptosis. It is a part of the TAK1 complex | Necroptosis | [175] |
5.2. Possible Therapeutical Approaches
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, D.; Low, A.; Koh, Y.; Goh, B.; Cheow, P.C.; Kam, J.H.; Teo, J.Y.; Tan, E.K.; Chung, A.; Ooi, L.L.; et al. A retrospective review of correlative radiological assessment and surgical exploration for hilar cholangiocarcinoma. Ann. Hepatobiliary Pancreat. Surg. 2018, 22, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Emadossadaty, S.; Ladep, N.G.; Thomas, H.C.; Elliott, P.; Taylor-Robinson, S.D.; Toledano, M.B. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J. Hepatol. 2012, 56, 848–854. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Lindnér, P.; Rizell, M.; Hafström, L. The impact of changed strategies for patients with cholangiocarcinoma in this millenium. HPB Surg. 2015, 2015, 736049. [Google Scholar] [CrossRef] [Green Version]
- Kamsa-Ard, S.; Luvira, V.; Suwanrungruang, K.; Kamsa-Ard, S.; Luvira, V.; Santong, C.; Srisuk, T.; Pugkhem, A.; Bhudhisawasdi, V.; Pairojkul, C. Cholangiocarcinoma Trends, Incidence, and Relative Survival in Khon Kaen, Thailand from 1989 Through 2013: A Population-Based Cancer Registry Study. J. Epidemiol. 2019, 29, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Strijker, M.; Belkouz, A.; van der Geest, L.G.; van Gulik, T.M.; van Hooft, J.E.; de Meijer, V.E.; Haj Mohammad, N.; de Reuver, P.R.; Verheij, J.; de Vos-Geelen, J.; et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: A nationwide study. Acta Oncol. 2019, 58, 1048–1055. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Guedj, N. Pathology of Cholangiocarcinomas. Curr. Oncol. 2022, 30, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.M.; Graubard, B.I.; Zeuzem, S.; El-Serag, H.B.; Davila, J.A.; McGlynn, K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-Medicare database. Hepatology 2011, 54, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ren, G.; Li, K.; Liu, Z.; Wang, Y.; Chen, T.; Mu, W.; Yang, X.; Li, X.; Shi, A.; et al. The Smad4-MYO18A-PP1A complex regulates β-catenin phosphorylation and pemigatinib resistance by inhibiting PAK1 in cholangiocarcinoma. Cell Death Differ. 2022, 29, 818–831. [Google Scholar] [CrossRef]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [CrossRef]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef]
- Pan, Y.R.; Wu, C.E.; Jung, S.M.; Huang, S.C.; Lin, S.H.; Chou, W.C.; Chang, Y.C.; Chen, M.H.; Hung, T.H.; Yu, A.L.; et al. Mucin 4 Confers Gemcitabine Resistance and an Unfavorable Prognosis in Patients with Cholangiocarcinoma via AKT Activation. Int. J. Biol. Sci. 2023, 19, 2772–2786. [Google Scholar] [CrossRef]
- Loeuillard, E.; Li, B.; Stumpf, H.E.; Yang, J.; Tomlinson, J.L.; Graham, R.P.; Smoot, R.L.; Dong, H.; Ilyas, S.I. Noncanonical TRAIL Signaling Facilitates Tumor Immunosuppression and Cholangiocarcinoma Growth via Myeloid-Derived Suppressor Cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Du, L.; Zhao, J.; Pan, L.; Zhang, G.; Wang, F.; Liu, R. Case Report: Persistent response to combination therapy of pemigatinib, chemotherapy, and immune checkpoint inhibitor in a patient with advanced intrahepatic cholangiocarcinoma. Front. Immunol. 2023, 14, 1124482. [Google Scholar] [CrossRef] [PubMed]
- Gandhy, S.U.; Casak, S.J.; Mushti, S.L.; Cheng, J.; Subramaniam, S.; Zhao, H.; Zhao, M.; Bi, Y.; Liu, G.; Fan, J.; et al. FDA Approval Summary: Futibatinib for Unresectable Advanced or Metastatic, Chemotherapy Refractory Intrahepatic Cholangiocarcinoma with FGFR2 Fusions or Other Rearrangements. Clin. Cancer Res. 2023, CCR-23-1042. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015, 35 (Suppl. S0), S78–S103. [Google Scholar] [CrossRef] [PubMed]
- Labib, P.L.; Goodchild, G.; Pereira, S.P. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer 2019, 19, 185. [Google Scholar] [CrossRef] [Green Version]
- Ganini, C.; Montanaro, M.; Scimeca, M.; Palmieri, G.; Anemona, L.; Concetti, L.; Melino, G.; Bove, P.; Amelio, I.; Candi, E.; et al. No Time to Die: How Kidney Cancer Evades Cell Death. Int. J. Mol. Sci. 2022, 23, 6198. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, S.; Rossi, A.; Di Daniele, N.; Melino, G.; Annicchiarico-Petruzzelli, M.; Raschellà, G. DNA repair and aging: The impact of the p53 family. Aging 2015, 7, 1050–1065. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour. Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, M.P.; Branco, L.M.; Strasser, A.; Dixit, V.M.; Bortoluci, K.R. Paradise revealed III: Why so many ways to die? Apoptosis, necroptosis, pyroptosis, and beyond. Cell Death Differ. 2020, 27, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Song, J.; Liu, S.; Tang, B.; Shen, L.; Zhu, J.; Fang, S.; Wu, F.; Zheng, L.; Qiu, R.; et al. USP9X promotes apoptosis in cholangiocarcinoma by modulation expression of KIF1Bβ via deubiquitinating EGLN3. J. Biomed. Sci. 2021, 28, 44. [Google Scholar] [CrossRef] [PubMed]
- Celli, A.; Que, F.G. Dysregulation of apoptosis in the cholangiopathies and cholangiocarcinoma. Semin Liver Dis. 1998, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Poulaki, V.; Mitsiades, C.S.; Mitsiades, N. The role of Fas and FasL as mediators of anticancer chemotherapy. Drug Resist. Updat. 2001, 4, 233–242. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Quarato, G.; Llambi, F.; Guy, C.S.; Min, J.; Actis, M.; Sun, H.; Narina, S.; Pruett-Miller, S.M.; Peng, J.; Rankovic, Z.; et al. Ca2+-mediated mitochondrial inner membrane permeabilization induces cell death independently of Bax and Bak. Cell Death Differ. 2022, 29, 1318–1334. [Google Scholar] [CrossRef]
- Dörflinger, B.; Badr, M.T.; Haimovici, A.; Fischer, L.; Vier, J.; Metz, A.; Eisele, B.; Bronsert, P.; Aumann, K.; Höppner, J.; et al. Mitochondria supply sub-lethal signals for cytokine secretion and DNA-damage in H. pylori infection. Cell Death Differ. 2022, 29, 2218–2232. [Google Scholar] [CrossRef]
- Koessinger, A.L.; Cloix, C.; Koessinger, D.; Heiland, D.H.; Bock, F.J.; Strathdee, K.; Kinch, K.; Martínez-Escardó, L.; Paul, N.R.; Nixon, C.; et al. Increased apoptotic sensitivity of glioblastoma enables therapeutic targeting by BH3-mimetics. Cell Death Differ. 2022, 29, 2089–2104. [Google Scholar] [CrossRef]
- Basu-Shrivastava, M.; Mojsa, B.; Mora, S.; Robbins, I.; Bossis, G.; Lassot, I.; Desagher, S. Trim39 regulates neuronal apoptosis by acting as a SUMO-targeted E3 ubiquitin-ligase for the transcription factor NFATc3. Cell Death Differ. 2022, 29, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Ahn, E.Y.; Chen, Y.; Feng, G.; Reddy, V.; Jhala, N.C.; McDonald, J.M. Reciprocal co-expression of Fas and Fas ligand in human cholangiocarcinoma. Int. J. Oncol. 2007, 31, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, G.; Carpino, G.; Cardinale, V.; Pisciotta, A.; Riccio, M.; Bertoni, L.; Gibellini, L.; De Biasi, S.; Nevi, L.; Costantini, D.; et al. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Sci. Rep. 2017, 7, 14419. [Google Scholar] [CrossRef] [Green Version]
- Utaisincharoen, P.; Tangthawornchaikul, N.; Ubol, S.; Chaisuriya, P.; Sirisinha, S. TNF-alpha induces caspase 3 (CPP 32) dependent apoptosis in human cholangiocarcinoma cell line. Southeast Asian J. Trop. Med. Public Health 2000, 31 (Suppl. S1), 167–170. [Google Scholar]
- Chen, J.H.; Xiang, J.Y.; Ding, G.P.; Cao, L.P. Cholangiocarcinoma-derived exosomes inhibit the antitumor activity of cytokine-induced killer cells by down-regulating the secretion of tumor necrosis factor-α and perforin. J. Zhejiang Univ. Sci. B 2016, 17, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Okaro, A.C.; Deery, A.R.; Hutchins, R.R.; Davidson, B.R. The expression of antiapoptotic proteins Bcl-2, Bcl-X(L), and Mcl-1 in benign, dysplastic, and malignant biliary epithelium. J. Clin. Pathol. 2001, 54, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Hoffmeister-Wittmann, P.; Mock, A.; Nichetti, F.; Korell, F.; Heilig, C.E.; Scherr, A.L.; Günther, M.; Albrecht, T.; Kelmendi, E.; Xu, K.; et al. Bcl-xL as prognostic marker and potential therapeutic target in cholangiocarcinoma. Liver Int. 2022, 42, 2855–2870. [Google Scholar] [CrossRef]
- Kang, Q.; Zou, H.; Yang, X.; Cai, J.B.; Liu, L.X.; Xie, N.; Wang, L.M.; Li, Y.H.; Zhang, X.W. Characterization and prognostic significance of mortalin, Bcl-2 and Bax in intrahepatic cholangiocarcinoma. Oncol. Lett. 2018, 15, 2161–2168. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Morine, Y.; Imura, S.; Ikemoto, T.; Saito, Y.; Takasu, C.; Yamada, S.; Tokuda, K.; Okikawa, S.; Miyazaki, K.; et al. A new pathological classification of intrahepatic cholangiocarcinoma according to protein expression of SSTR2 and Bcl2. World J. Surg. Oncol. 2021, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; Nardozi, D.; Scimeca, M.; Cerroni, C.; Mauriello, A.; Bonanno, E. PD-L1 in immune-escape of breast and prostate cancers: From biology to therapy. Future Oncol. 2017, 13, 2129–2131. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Tentori, L.; Atzori, M.G.; Lacal, P.M.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; de Martino, M.G.; Vespasiani, G.; et al. Ellagic Acid Inhibits Bladder Cancer Invasiveness and In Vivo Tumor Growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimeca, M.; Bonfiglio, R.; Urbano, N.; Cerroni, C.; Anemona, L.; Montanaro, M.; Fazi, S.; Schillaci, O.; Mauriello, A.; Bonanno, E. Programmed death ligand 1 expression in prostate cancer cells is associated with deep changes of the tumor inflammatory infiltrate composition. Urol. Oncol. 2019, 37, e19–e297. [Google Scholar] [CrossRef]
- Lacal, P.M.; Atzori, M.G.; Ruffini, F.; Scimeca, M.; Bonanno, E.; Cicconi, R.; Mattei, M.; Bernardini, R.; D’Atri, S.; Tentori, L.; et al. Targeting the vascular endothelial growth factor receptor-1 by the monoclonal antibody D16F7 to increase the activity of immune checkpoint inhibitors against cutaneous melanoma. Pharmacol. Res. 2020, 159, 104957. [Google Scholar] [CrossRef]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Fan, S.; Ge, Y.; Liu, J.; Liu, H.; Yan, R.; Gao, T.; Fan, X.; Xiao, Z.; An, G. Combination of anlotinib and gemcitabine promotes the G0/G1 cell cycle arrest and apoptosis of intrahepatic cholangiocarcinoma in vitro. J. Clin. Lab. Anal. 2021, 35, e23986. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Gradilone, S.A.; Masyuk, T.V.; Tabibian, J.H.; LaRusso, N.F. MicroRNAs in Cholangiopathies. Curr. Pathobiol. Rep. 2014, 2, 133–142. [Google Scholar] [CrossRef]
- Melino, G.; Bernassola, F.; Ranalli, M.; Yee, K.; Zong, W.X.; Corazzari, M.; Knight, R.A.; Green, D.R.; Thompson, C.; Vousden, K.H. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J. Biol. Chem. 2004, 279, 8076–8083. [Google Scholar] [CrossRef] [Green Version]
- Pitolli, C.; Wang, Y.; Candi, E.; Shi, Y.; Melino, G.; Amelio, I. p53-Mediated Tumor Suppression: DNA-Damage Response and Alternative Mechanisms. Cancers 2019, 11, 1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimeca, M.; Montanaro, M.; Bonfiglio, R.; Anemona, L.; Agrò, E.F.; Asimakopoulos, A.D.; Bei, R.; Manzari, V.; Urbano, N.; Giacobbi, E.; et al. The ETS Homologous Factor (EHF) Represents a Useful Immunohistochemical Marker for Predicting Prostate Cancer Metastasis. Diagnostics 2022, 12, 800. [Google Scholar] [CrossRef]
- Scimeca, M.; Trivigno, D.; Bonfiglio, R.; Ciuffa, S.; Urbano, N.; Schillaci, O.; Bonanno, E. Breast cancer metastasis to bone: From epithelial to mesenchymal transition to breast osteoblast-like cells. Semin Cancer Biol. 2021, 72, 155–164. [Google Scholar] [CrossRef]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Duggento, A.; Toschi, N.; Schillaci, O.; Bonanno, E. Novel insights into breast cancer progression and metastasis: A multidisciplinary opportunity to transition from biology to clinical oncology. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Mapelli, S.N.; Catapano, C.V.; Carbone, G.M.; Ciuffa, S.; Tavolozza, M.; Schillaci, O.; Mauriello, A.; et al. Prostate Osteoblast-Like Cells: A Reliable Prognostic Marker of Bone Metastasis in Prostate Cancer Patients. Contrast Media Mol. Imaging 2018, 2018, 9840962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimeca, M.; Antonacci, C.; Toschi, N.; Giannini, E.; Bonfiglio, R.; Buonomo, C.O.; Pistolese, C.A.; Tarantino, U.; Bonanno, E. Breast Osteoblast-like Cells: A Reliable Early Marker for Bone Metastases From Breast Cancer. Clin. Breast Cancer 2018, 18, e659–e669. [Google Scholar] [CrossRef] [PubMed]
- Urbano, N.; Scimeca, M.; Di Russo, C.; Mauriello, A.; Bonanno, E.; Schillaci, O. [99mTc]Sestamibi SPECT Can Predict Proliferation Index, Angiogenesis, and Vascular Invasion in Parathyroid Patients: A Retrospective Study. J. Clin. Med. 2020, 9, 2213. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.G.; Tentori, L.; Ruffini, F.; Ceci, C.; Bonanno, E.; Scimeca, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor-1 Monoclonal Antibody D16F7 Inhibits Glioma Growth and Angiogenesis In Vivo. J. Pharmacol. Exp. Ther. 2018, 364, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.G.; Ceci, C.; Ruffini, F.; Scimeca, M.; Cicconi, R.; Mattei, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor 1 (VEGFR-1) D16F7 Monoclonal Antibody Inhibits Melanoma Adhesion to Soluble VEGFR-1 and Tissue Invasion in Response to Placenta Growth Factor. Cancers 2022, 14, 5578. [Google Scholar] [CrossRef]
- Amelio, I.; Antonov, A.A.; Catani, M.V.; Massoud, R.; Bernassola, F.; Knight, R.A.; Melino, G.; Rufini, A. TAp73 promotes anabolism. Oncotarget 2014, 5, 12820–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palombo, R.; Caporali, S.; Falconi, M.; Iacovelli, F.; Morozzo Della Rocca, B.; Lo Surdo, A.; Campione, E.; Candi, E.; Melino, G.; Bernardini, S.; et al. Luteolin-7-O-β-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes. Int. J. Mol. Sci. 2019, 20, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klionsky, D.J.; Abdalla, F.C.; Abeliovich, H.; Abraham, R.T.; Acevedo-Arozena, A.; Adeli, K.; Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-Ghiso, J.A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimeca, M.; Giocondo, R.; Montanaro, M.; Granaglia, A.; Bonfiglio, R.; Tancredi, V.; Mauriello, A.; Urbano, N.; Schillaci, O.; Bonanno, E. BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin. Cells 2020, 9, 1381. [Google Scholar] [CrossRef]
- Scimeca, M.; Bonfiglio, R.; Menichini, E.; Albonici, L.; Urbano, N.; De Caro, M.T.; Mauriello, A.; Schillaci, O.; Gambacurta, A.; Bonanno, E. Microcalcifications Drive Breast Cancer Occurrence and Development by Macrophage-Mediated Epithelial to Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 5633. [Google Scholar] [CrossRef] [Green Version]
- Urbano, N.; Scimeca, M.; Crocco, A.; Mauriello, A.; Bonanno, E.; Schillaci, O. 18F-Choline PET/CT Identifies High-Grade Prostate Cancer Lesions Expressing Bone Biomarkers. J. Clin. Med. 2019, 8, 1657. [Google Scholar] [CrossRef] [Green Version]
- Scimeca, M.; Bonfiglio, R.; Varone, F.; Ciuffa, S.; Mauriello, A.; Bonanno, E. Calcifications in prostate cancer: An active phenomenon mediated by epithelial cells with osteoblast-phenotype. Microsc. Res. Tech. 2018, 81, 745–748. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, A.; Xiao, X.; Liu, W. CircSETD3 (hsa_circ_0000567) inhibits proliferation and induces apoptosis in cholangiocarcinoma cells via downregulation of microRNA-421 expression. Bioengineered 2022, 13, 10191–10201. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, S.; Liu, L. Circular RNA circ_C16orf62 Suppresses Cell Growth in Gastric Cancer by miR-421/Tubulin beta-2A Chain (TUBB2A) Axis. Med. Sci. Monit. 2020, 26, e924343. [Google Scholar] [CrossRef]
- Xue, L.; Yang, D. MiR-421 inhibited proliferation and metastasis of colorectal cancer by targeting MTA1. J. BUON 2018, 23, 1633–1639. [Google Scholar] [PubMed]
- Lv, P.; Luo, Y.F.; Zhou, W.Y.; Liu, B.; Zhou, Z.; Shi, Y.Z.; Huang, R.; Peng, C.; He, Z.L.; Wang, J.; et al. miR-373 inhibits autophagy and further promotes apoptosis of cholangiocarcinoma cells by targeting ULK1. Kaohsiung J. Med. Sci. 2020, 36, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, P.C.; Leng, K.M.; Liu, Y.P.; Liu, Y.; Xu, Y.; Qin, W.; Gao, J.J.; Wang, Z.D.; Tai, S.; Zhong, X.Y.; et al. miR-191 Inhibition Induces Apoptosis Through Reactivating Secreted Frizzled-Related Protein-1 in Cholangiocarcinoma. Cell Physiol. Biochem. 2018, 49, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Lane, D.J.R.; Balez, R.; Anastacio, H.T.D.; Zeng, Z.; Ganio, K.; McDevitt, C.A.; Acevedo, K.; Belaidi, A.A.; Koistinaho, J.; et al. Selective ferroptosis vulnerability due to familial Alzheimer’s disease presenilin mutations. Cell Death Differ. 2022, 29, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Balihodzic, A.; Prinz, F.; Dengler, M.A.; Calin, G.A.; Jost, P.J.; Pichler, M. Non-coding RNAs and ferroptosis: Potential implications for cancer therapy. Cell Death Differ. 2022, 29, 1094–1106. [Google Scholar] [CrossRef]
- Pentimalli, F.; Grelli, S.; Di Daniele, N.; Melino, G.; Amelio, I. Cell death pathologies: Targeting death pathways and the immune system for cancer therapy. Genes Immun. 2019, 20, 539–554. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef]
- Vučković, A.M.; Bosello Travain, V.; Bordin, L.; Cozza, G.; Miotto, G.; Rossetto, M.; Toppo, S.; Venerando, R.; Zaccarin, M.; Maiorino, M.; et al. Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14-3-3ε. FEBS Lett. 2020, 594, 611–624. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, M.; Cui, X.; O’Connell, D.; Yang, Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022, 29, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Southon, A.G.; Saleh, E.; Brinkmann, K.; Ke, F.; Iliopoulos, M.; Cross, R.S.; Jenkins, M.R.; Nhu, D.; Wang, Z.; et al. BH3 mimetic drugs cooperate with Temozolomide, JQ1 and inducers of ferroptosis in killing glioblastoma multiforme cells. Cell Death Differ. 2022, 29, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Gammella, E.; Correnti, M.; Buratti, P.; Forti, E.; Andersen, J.B.; Alpini, G.; Glaser, S.; Alvaro, D.; Invernizzi, P.; et al. Dysregulation of Iron Metabolism in Cholangiocarcinoma Stem-like Cells. Sci. Rep. 2017, 7, 17667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, J.; Matye, D.; Zhang, Y.; Dennis, K.; Ding, W.X.; Li, T. Bile acids regulate cysteine catabolism and glutathione regeneration to modulate hepatic sensitivity to oxidative injury. JCI Insight. 2018, 3, e99676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, K.S.; O’Brien, D.; Kang, Y.N.; Mounajjed, T.; Kim, Y.H.; Kim, T.S.; Kocher, J.A.; Allotey, L.K.; Borad, M.J.; Roberts, L.R.; et al. Prognostic subclass of intrahepatic cholangiocarcinoma by integrative molecular-clinical analysis and potential targeted approach. Hepatol. Int. 2019, 13, 490–500. [Google Scholar] [CrossRef]
- Thomas, A.F.; Kelly, G.L.; Strasser, A. Of the many cellular responses activated by TP53, which ones are critical for tumour suppression? Cell Death Differ. 2022, 29, 961–971. [Google Scholar] [CrossRef]
- Hoyos, D.; Greenbaum, B.; Levine, A.J. The genotypes and phenotypes of missense mutations in the proline domain of the p53 protein. Cell Death Differ. 2022, 29, 938–945. [Google Scholar] [CrossRef]
- El-Saafin, F.; Bergamasco, M.I.; Chen, Y.; May, R.E.; Esakky, P.; Hediyeh-Zadeh, S.; Dixon, M.; Wilcox, S.; Davis, M.J.; Strasser, A.; et al. Loss of TAF8 causes TFIID dysfunction and p53-mediated apoptotic neuronal cell death. Cell Death Differ. 2022, 29, 1013–1027. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Srdanovic, S.; Gao, Y.H.; Chen, D.Y.; Yan, Y.J.; Margetic, D.; Chen, Z.L. The photodynamic activity of 13(1)-[20-(2-pyridyl)ethylamine] chlorin e6 photosensitizer in human esophageal cancer. Bioorg. Med. Chem. Lett. 2018, 28, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Galluzzi, L. BAX and BAK dynamics control mitochondrial DNA release during apoptosis. Cell Death Differ. 2022, 29, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Murakami, G.; Nanashima, A.; Nonaka, T.; Tominaga, T.; Wakata, K.; Sumida, Y.; Akashi, H.; Okazaki, S.; Kataoka, H.; Nagayasu, T. Photodynamic Therapy Using Novel Glucose-conjugated Chlorin Increases Apoptosis of Cholangiocellular Carcinoma in Comparison with Talaporfin Sodium. Anticancer Res. 2016, 36, 4493–4501. [Google Scholar] [CrossRef]
- He, C.; Xia, J.; Gao, Y.; Chen, Z.; Wan, X. Chlorin A-mediated photodynamic therapy induced apoptosis in human cholangiocarcinoma cells via impaired autophagy flux. Am. J. Transl. Res. 2020, 12, 5080–5094. [Google Scholar]
- Zhang, Z.J.; Huang, Y.P.; Li, X.X.; Liu, Z.T.; Liu, K.; Deng, X.F.; Xiong, L.; Zou, H.; Wen, Y. A Novel Ferroptosis-Related 4-Gene Prognostic Signature for Cholangiocarcinoma and Photodynamic Therapy. Front. Oncol. 2021, 11, 747445. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, X.; Huang, J.; Li, J.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.; Duan, H.; et al. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat. Commun. 2020, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, X.; Jiang, Z. Immune infiltration and a ferroptosis-related gene signature for predicting the prognosis of patients with cholangiocarcinoma. Am. J. Transl. Res. 2022, 14, 1204–1219. [Google Scholar]
- Vitali, A.; Botta, B.; Delle Monache, G.; Zappitelli, S.; Ricciardi, P.; Melino, S.; Petruzzelli, R.; Giardina, B. Purification and partial characterization of a peroxidase from plant cell cultures of Cassia didymobotrya and biotransformation studies. Biochem. J. 1998, 331, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Fazi, B.; Melino, S.; De Rubeis, S.; Bagni, C.; Paci, M.; Piacentini, M.; Di Sano, F. Acetylation of RTN-1C regulates the induction of ER stress by the inhibition of HDAC activity in neuroectodermal tumors. Oncogene 2009, 28, 3814–3824. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, S.; Sacchetta, P.; Moio, P.; Melino, S.; Petruzzelli, R.; Gervasi, P.; Di Ilio, C. Purification and characterization of glutathione transferases from the sea bass (Dicentrarchus labrax) liver. Arch. Biochem. Biophys. 2000, 373, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Otto, H.F.; Gebbers, J.O. Epitheldysplasien bei Colitis ulcerosa. Histologische Möglichkeiten zur (Früh-)Erfassung der sog. “Colitis”-Carcinome [Epithelial dysplasia in ulcerative colitis. Histological possibility for the early diagnosis of “colitis”-carcinoma (author’s transl)]. Langenbecks Arch. Chir. 1976, 341, 99–110, In German. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. New perspectives in cancer: Modulation of lipid metabolism and inflammation resolution. Pharmacol. Res. 2018, 128, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, A.H.; Macleod, K.F. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy 2015, 11, 1937–1938. [Google Scholar] [CrossRef] [Green Version]
- Güngör, N.; Knaapen, A.M.; Munnia, A.; Peluso, M.; Haenen, G.R.; Chiu, R.K.; Godschalk, R.W.; van Schooten, F.J. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 2010, 25, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Karuppagounder, S.S.; Alin, L.; Chen, Y.; Brand, D.; Bourassa, M.W.; Dietrich, K.; Wilkinson, C.M.; Nadeau, C.A.; Kumar, A.; Perry, S.; et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 2018, 84, 854–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melino, S.; Nepravishta, R.; Bellomaria, A.; Di Marco, S.; Paci, M. Nucleic acid binding of the RTN1-C C-terminal region: Toward the functional role of a reticulon protein. Biochemistry 2009, 48, 242–253. [Google Scholar] [CrossRef]
- Gallo, M.; Paludi, D.; Cicero, D.O.; Chiovitti, K.; Millo, E.; Salis, A.; Damonte, G.; Corsaro, A.; Thellung, S.; Schettini, G.; et al. Identification of a conserved N-capping box important for the structural autonomy of the prion alpha 3-helix: The disease associated D202N mutation destabilizes the helical conformation. Int. J. Immunopathol. Pharmacol. 2005, 18, 95–112. [Google Scholar] [CrossRef]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef] [Green Version]
- Belletti, B.; Baldassarre, G. Stathmin: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin. Targets 2011, 15, 1249–1266. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Guo, X.; Guo, S.Q.; Wang, Q.; Chen, X.Q.; Li, X.N.; Guo, L.S. STMN1 in colon cancer: Expression and prognosis in Chinese patients. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2038–2044. [Google Scholar]
- Li, M.; Yang, J.; Zhou, W.; Ren, Y.; Wang, X.; Chen, H.; Zhang, J.; Chen, J.; Sun, Y.; Cui, L.; et al. Activation of an AKT/FOXM1/STMN1 pathway drives resistance to tyrosine kinase inhibitors in lung cancer. Br. J. Cancer 2017, 117, 974–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Yan, J.; Zhao, W.; Dou, P.; Sun, N.; Wang, Y.; Huang, X. BNIP3 Upregulation Characterizes Cancer Cell Subpopulation With Increased Fitness and Proliferation. Front. Oncol. 2022, 12, 923890. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Jonges, L.E.; Albertsson, P.; van Vlierberghe, R.L.; Ensink, N.G.; Johansson, B.R.; van de Velde, C.J.; Fleuren, G.J.; Nannmark, U.; Kuppen, P.J. The phenotypic heterogeneity of human natural killer cells: Presence of at least 48 different subsets in the peripheral blood. Scand. J. Immunol. 2001, 53, 103–110. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016, 1, e89829. [Google Scholar] [CrossRef] [Green Version]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer. 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pagès, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Loose, D.; Van de Wiele, C. The immune system and cancer. Cancer Biother. Radiopharm. 2009, 24, 369–376. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, R.V.; Mahindroo, N. Recent advances in cancer immunology and immunology-based anticancer therapies. Biomed. Pharmacother. 2017, 96, 1491–1500. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Shen, S.; Yan, Y.; Ji, W.; Wang, J.; Qian, H.; Jiang, X.; Li, Z.; Wu, M.; et al. OCT4 increases BIRC5 and CCND1 expression and promotes cancer progression in hepatocellular carcinoma. BMC Cancer 2013, 13, 82. [Google Scholar] [CrossRef] [Green Version]
- Ozen, M.; Ittmann, M. Increased expression and activity of CDC25C phosphatase and an alternatively spliced variant in prostate cancer. Clin. Cancer Res. 2005, 11, 4701–4706. [Google Scholar] [CrossRef] [Green Version]
- Law, C.T.; Wei, L.; Tsang, F.H.; Chan, C.Y.; Xu, I.M.; Lai, R.K.; Ho, D.W.; Lee, J.M.; Wong, C.C.; Ng, I.O.; et al. HELLS Regulates Chromatin Remodeling and Epigenetic Silencing of Multiple Tumor Suppressor Genes in Human Hepatocellular Carcinoma. Hepatology 2019, 69, 2013–2030. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, X.; He, Y.; Tian, M.; Lu, S.; Wang, Q.; Zheng, Y.; Lv, Z.; Hao, C.; Xue, D.; et al. ScRNA-seq and bulk RNA-seq reveal the characteristics of ferroptosis and establish a risk signature in cholangiocarcinoma. Mol. Ther. Oncolytics. 2022, 27, 48–60. [Google Scholar] [CrossRef]
- Lei, S.; Cao, W.; Zeng, Z.; Zhang, Z.; Jin, B.; Tian, Q.; Wu, Y.; Zhang, T.; Li, D.; Hu, C.; et al. JUND/linc00976 promotes cholangiocarcinoma progression and metastasis, inhibits ferroptosis by regulating the miR-3202/GPX4 axis. Cell Death Dis. 2022, 13, 967. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, R.; Sun, H.; Long, D.; Li, Z.; Liao, H.; Xue, T.; Zhang, Z.; Kang, Y.; Mao, G. Long Non-coding RNAs LOC100126784 and POM121L9P Derived From Bone Marrow Mesenchymal Stem Cells Enhance Osteogenic Differentiation via the miR-503-5p/SORBS1 Axis. Front. Cell Dev. Biol. 2021, 9, 723759. [Google Scholar] [CrossRef]
- Zhao, X.; Su, L.; He, X.; Zhao, B.; Miao, J. Long noncoding RNA CA7-4 promotes autophagy and apoptosis via sponging MIR877-3P and MIR5680 in high glucose-induced vascular endothelial cells. Autophagy 2020, 16, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, B.; Wang, M.; Zhang, S.; He, B.; Shi, Z.; Deng, T.; Bao, W.; Wang, Y.; Chen, G.; et al. Exploration and validation of a novel ferroptosis-related gene signature predicting the prognosis of intrahepatic cholangiocarcinoma. Acta Biochim. Biophys Sin. 2022, 54, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Panatta, E.; Butera, A.; Celardo, I.; Leist, M.; Melino, G.; Amelio, I. p53 regulates expression of nuclear envelope components in cancer cells. Biol. Direct. 2022, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Liu, M.; Hu, L.; Guo, D.; Wang, D.; Qi, B.; Ren, G.; Hu, C.; Zhang, F.; Chun, H.J.; et al. LncRNA Airn alleviates diabetic cardiac fibrosis by inhibiting activation of cardiac fibroblasts via a m6A-IMP2-p53 axis. Biol. Direct. 2022, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Roy, M.; Zampieri, C.; Mammarella, E.; Panatta, E.; Melino, G.; D’Alessandro, A.; Amelio, I. p53-driven lipidome influences non-cell-autonomous lysophospholipids in pancreatic cancer. Biol. Direct. 2022, 17, 6. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef]
- Yi, K.; Liu, J.; Rong, Y.; Wang, C.; Tang, X.; Zhang, X.; Xiong, Y.; Wang, F. Biological Functions and Prognostic Value of Ferroptosis-Related Genes in Bladder Cancer. Front. Mol. Biosci. 2021, 8, 631152. [Google Scholar] [CrossRef]
- Sarcognato, S.; Sacchi, D.; Fabris, L.; Zanus, G.; Gringeri, E.; Niero, M.; Gallina, G.; Guido, M. Ferroptosis in Intrahepatic Cholangiocarcinoma: IDH1105GGT Single Nucleotide Polymorphism Is Associated With Its Activation and Better Prognosis. Front. Med. 2022, 9, 886229. [Google Scholar] [CrossRef]
- Acquaviva, G.; Visani, M.; de Biase, D.; Marucci, G.; Franceschi, E.; Tosoni, A.; Brandes, A.A.; Rhoden, K.J.; Pession, A.; Tallini, G. Prevalence of the single-nucleotide polymorphism rs11554137 (IDH1105GGT) in brain tumors of a cohort of Italian patients. Sci. Rep. 2018, 8, 4459. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.W.; Boisselier, B.; Rossetto, M.; Marie, Y.; Idbaih, A.; Mokhtari, K.; Gousias, K.; Hoang-Xuan, K.; Delattre, J.Y.; Simon, M.; et al. Prognostic impact of the isocitrate dehydrogenase 1 single-nucleotide polymorphism rs11554137 in malignant gliomas. Cancer 2013, 119, 806–813. [Google Scholar] [CrossRef]
- Wagner, K.; Damm, F.; Göhring, G.; Görlich, K.; Heuser, M.; Schäfer, I.; Ottmann, O.; Lübbert, M.; Heit, W.; Kanz, L.; et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J. Clin. Oncol. 2010, 28, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Monack, D.M.; Raupach, B.; Hromockyj, A.E.; Falkow, S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 9833–9838. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergsbaken, T.; Cookson, B.T. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007, 3, e161. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, K. Switching from Apoptosis to Pyroptosis: Gasdermin-Elicited Inflammation and Antitumor Immunity. Int. J. Mol. Sci. 2021, 22, 426. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Loveless, R.; Bloomquist, R.; Teng, Y. Pyroptosis at the forefront of anticancer immunity. J. Exp. Clin. Cancer Res. 2021, 40, 264. [Google Scholar] [CrossRef]
- Minton, K. Pyroptosis heats tumour immunity. Nat. Rev. Immunol. 2020, 20, 274–275. [Google Scholar] [CrossRef]
- Baatarjav, C.; Komada, T.; Karasawa, T.; Yamada, N.; Sampilvanjil, A.; Matsumura, T.; Takahashi, M. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022, 29, 2487–2502. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. In Retrospect: The inflammasome turns 15. Nature 2017, 548, 534–535. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Maroni, L.; Agostinelli, L.; Saccomanno, S.; Pinto, C.; Giordano, D.M.; Rychlicki, C.; De Minicis, S.; Trozzi, L.; Banales, J.M.; Melum, E.; et al. Nlrp3 Activation Induces Il-18 Synthesis and Affects the Epithelial Barrier Function in Reactive Cholangiocytes. Am. J. Pathol. 2017, 187, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Yang, G.; Chen, H.Y.; Hsu, D.K.; Tomilov, A.; Olson, K.A.; Dehnad, A.; Fish, S.R.; Cortopassi, G.; Zhao, B.; et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016, 30, 4202–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Chen, P.; Cheng, B.; Liu, Y.; Zhang, X.; Xu, Q.; Huang, M.; Dai, X.; Huang, K.; Zhang, L.; et al. Pyroptosis predicts immunotherapy outcomes across multiple cancer types. Clin. Immunol. 2022, 245, 109163. [Google Scholar] [CrossRef]
- Wang, R.; Liu, P.; Li, F.; Qiao, H. Dexmedetomidine protects against Ropivacaine-induced neuronal pyroptosis via the Nrf2/HO-1 pathway. J. Toxicol. Sci. 2023, 48, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Zhou, N.; Xu, P.; Wang, J.; Gao, Y.; Jin, X.; Liang, X.; Lv, J.; Zhang, Y.; et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat. Biomed. Eng. 2020, 4, 743–753. [Google Scholar] [CrossRef]
- Sasaki, M.; Nakanuma, Y. Stress-induced cellular responses and cell death mechanisms during inflammatory cholangiopathies. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 129–138. [Google Scholar] [CrossRef]
- Laster, S.M.; Wood, J.G.; Gooding, L.R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 1988, 141, 2629–2634. [Google Scholar] [CrossRef]
- Vanlangenakker, N.; Vanden Berghe, T.; Vandenabeele, P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012, 19, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Verstegen, M.M.A.; Mezzanotte, L.; de Jonge, J.; Löwik, C.W.G.M.; van der Laan, L.J.W. Necroptotic Cell Death in Liver Transplantation and Underlying Diseases: Mechanisms and Clinical Perspective. Liver Transpl. 2019, 25, 1091–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Zhong, C.Q.; Zhang, D.W. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat. Immunol. 2011, 12, 1143–1149. [Google Scholar] [CrossRef]
- Lalaoui, N.; Brumatti, G. Relevance of necroptosis in cancer. Immunol. Cell Biol. 2017, 95, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, K.; Chan, F.K. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Ma, D.; Tan, Y.X.; Wang, H.Y.; Cai, Z. The role of necroptosis in cancer: A double-edged sword? Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 259–266. [Google Scholar] [CrossRef]
- Jiao, D.; Cai, Z.; Choksi, S.; Ma, D.; Choe, M.; Kwon, H.J.; Baik, J.Y.; Rowan, B.G.; Liu, C.; Liu, Z.G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870. [Google Scholar] [CrossRef] [Green Version]
- Squadroni, M.; Tondulli, L.; Gatta, G.; Mosconi, S.; Beretta, G.; Labianca, R. Cholangiocarcinoma. Crit. Rev. Oncol. Hematol. 2017, 116, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Bartella, I.; Dufour, J.F. Clinical Diagnosis and Staging of Intrahepatic Cholangiocarcinoma. J. Gastrointestin. Liver Dis. 2015, 24, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Saeed, W.K.; Jun, D.W. Viewpoint: Necroptosis influences the type of liver cancer via changes of hepatic microenvironment. Hepatobiliary Surg. Nutr. 2019, 8, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Seehawer, M.; Heinzmann, F.; D’Artista, L.; Harbig, J.; Roux, P.F.; Hoenicke, L.; Dang, H.; Klotz, S.; Robinson, L.; Doré, G.; et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018, 562, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.; Brar, G.; Tsukamoto, H. Cell Death and Liver Disease. Gut Liver 2020, 14, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Kayhanian, H.; Smyth, E.C.; Braconi, C. Emerging molecular targets and therapy for cholangiocarcinoma. World J. Gastrointest. Oncol. 2017, 9, 268–280. [Google Scholar] [CrossRef]
- Han, W.; Li, L.; Qiu, S.; Lu, Q.; Pan, Q.; Gu, Y.; Luo, J.; Hu, X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol. Cancer Ther. 2007, 6, 1641–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunai, Z.A.; Imre, G.; Barna, G.; Korcsmaros, T.; Petak, I.; Bauer, P.I.; Mihalik, R. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS ONE 2012, 7, e41945. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Gong, Y.; Chen, J.; Zhao, X.; Qing, H.; Dong, Y.; Li, S.; Li, J.; Wang, Z. Identification of fatty acid metabolism-related lncRNAs in the prognosis and immune microenvironment of colon adenocarcinoma. Biol. Direct. 2022, 17, 19. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Zuo, Z.; Xu, C.; Lin, L.; Li, Y. Downregulation of amphiregulin improves cardiac hypertrophy via attenuating oxidative stress and apoptosis. Biol. Direct. 2022, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Deeraksa, A.; Pan, J.; Sha, Y.; Liu, X.D.; Eissa, N.T.; Lin, S.H.; Yu-Lee, L.Y. Plk1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis. Oncogene 2013, 32, 2973–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Labrada, A.; López-Royuela, N.; Jarauta, V.; Galán-Malo, P.; Azaceta, G.; Palomera, L.; Pardo, J.; Anel, A.; Marzo, I.; Naval, J. Two death pathways induced by sorafenib in myeloma cells: Puma-mediated apoptosis and necroptosis. Clin. Transl. Oncol. 2015, 17, 121–132. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Zhong, M.; Yang, M.; Sun, X.; Liu, J.; Kroemer, G.; Lotze, M.; Zeh, H.J., 3rd; Kang, R.; et al. Inhibition of Aurora Kinase A Induces Necroptosis in Pancreatic Carcinoma. Gastroenterology 2017, 153, 1429–1443.e5. [Google Scholar] [CrossRef] [Green Version]
- Geserick, P.; Hupe, M.; Moulin, M.; Wong, W.W.; Feoktistova, M.; Kellert, B.; Gollnick, H.; Silke, J.; Leverkus, M. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 2009, 187, 1037–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol. Ther. 2013, 14, 999–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, E.; Zauszkiewicz-Pawlak, A.; Wojcik, M.; Inkielewicz-Stepniak, I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget 2017, 9, 4675–4697. [Google Scholar] [CrossRef] [Green Version]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yu, J.; Zhang, L. Necroptosis: An alternative cell death program defending against cancer. Biochim. Biophys. Acta 2016, 1865, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimeca, M.; Urbano, N.; Toschi, N.; Bonanno, E.; Schillaci, O. Precision medicine in breast cancer: From biological imaging to artificial intelligence. Semin. Cancer Biol. 2021, 72, 1–3. [Google Scholar] [CrossRef]
- Schillaci, O.; Scimeca, M.; Trivigno, D.; Chiaravalloti, A.; Facchetti, S.; Anemona, L.; Bonfiglio, R.; Santeusanio, G.; Tancredi, V.; Bonanno, E.; et al. Prostate cancer and inflammation: A new molecular imaging challenge in the era of personalized medicine. Nucl. Med. Biol. 2019, 68–69, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Urbano, N.; Scimeca, M.; Bonanno, E.; Schillaci, O. Nuclear medicine and anatomic pathology in personalized medicine: A challenging alliance. Per Med. 2018, 15, 457–459. [Google Scholar] [CrossRef]
- Ganini, C.; Amelio, I.; Bertolo, R.; Bove, P.; Buonomo, O.C.; Candi, E.; Cipriani, C.; Di Daniele, N.; Juhl, H.; Mauriello, A.; et al. Global mapping of cancers: The Cancer Genome Atlas and beyond. Mol. Oncol. 2021, 15, 2823–2840. [Google Scholar] [CrossRef] [PubMed]
- Duggento, A.; Conti, A.; Mauriello, A.; Guerrisi, M.; Toschi, N. Deep computational pathology in breast cancer. Semin. Cancer Biol. 2021, 72, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Scimeca, M.; Toschi, N.; Bonfiglio, R.; Urbano, N.; Bonanno, E. Combining Diagnostic Imaging and Pathology for Improving Diagnosis and Prognosis of Cancer. Contrast Media Mol. Imaging. 2019, 2019, 9429761. [Google Scholar] [CrossRef] [Green Version]
- Bonfiglio, R.; Scimeca, M.; Urbano, N.; Bonanno, E.; Schillaci, O. Breast microcalcifications: Biological and diagnostic perspectives. Future Oncol. 2018, 14, 3097–3099. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Di Pietro, M.L. The impact of oral contraceptive use on breast cancer risk: State of the art and future perspectives in the era of 4P medicine. Semin Cancer Biol. 2021, 72, 11–18. [Google Scholar] [CrossRef]
- Scimeca, M.; Anemona, L.; Granaglia, A.; Bonfiglio, R.; Urbano, N.; Toschi, N.; Santeusanio, G.; Schiaroli, S.; Mauriello, S.; Tancredi, V.; et al. Plaque calcification is driven by different mechanisms of mineralization associated with specific cardiovascular risk factors. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1330–1336. [Google Scholar] [CrossRef]
- Zampieri, C.; Panatta, E.; Corbo, V.; Mauriello, A.; Melino, G.; Amelio, I. p53 mutations define the chromatin landscape to confer drug tolerance in pancreatic cancer. Mol. Oncol. 2022, 16, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Mammarella, E.; Zampieri, C.; Panatta, E.; Melino, G.; Amelio, I. NUAK2 and RCan2 participate in the p53 mutant pro-tumorigenic network. Biol. Direct. 2021, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Pitolli, C.; Wang, Y.; Mancini, M.; Shi, Y.; Melino, G.; Amelio, I. Do Mutations Turn p53 into an Oncogene? Int. J. Mol. Sci. 2019, 20, 6241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amelio, I.; Mancini, M.; Petrova, V.; Cairns, R.A.; Vikhreva, P.; Nicolai, S.; Marini, A.; Antonov, A.A.; Le Quesne, J.; Baena Acevedo, J.D.; et al. p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc. Natl. Acad. Sci. USA 2018, 115, E10869–E10878. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scimeca, M.; Rovella, V.; Palumbo, V.; Scioli, M.P.; Bonfiglio, R.; TOR CENTRE; Melino, G.; Piacentini, M.; Frati, L.; Agostini, M.; et al. Programmed Cell Death Pathways in Cholangiocarcinoma: Opportunities for Targeted Therapy. Cancers 2023, 15, 3638. https://doi.org/10.3390/cancers15143638
Scimeca M, Rovella V, Palumbo V, Scioli MP, Bonfiglio R, TOR CENTRE, Melino G, Piacentini M, Frati L, Agostini M, et al. Programmed Cell Death Pathways in Cholangiocarcinoma: Opportunities for Targeted Therapy. Cancers. 2023; 15(14):3638. https://doi.org/10.3390/cancers15143638
Chicago/Turabian StyleScimeca, Manuel, Valentina Rovella, Valeria Palumbo, Maria Paola Scioli, Rita Bonfiglio, TOR CENTRE, Gerry Melino, Mauro Piacentini, Luigi Frati, Massimiliano Agostini, and et al. 2023. "Programmed Cell Death Pathways in Cholangiocarcinoma: Opportunities for Targeted Therapy" Cancers 15, no. 14: 3638. https://doi.org/10.3390/cancers15143638
APA StyleScimeca, M., Rovella, V., Palumbo, V., Scioli, M. P., Bonfiglio, R., TOR CENTRE, Melino, G., Piacentini, M., Frati, L., Agostini, M., Candi, E., & Mauriello, A. (2023). Programmed Cell Death Pathways in Cholangiocarcinoma: Opportunities for Targeted Therapy. Cancers, 15(14), 3638. https://doi.org/10.3390/cancers15143638








