Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
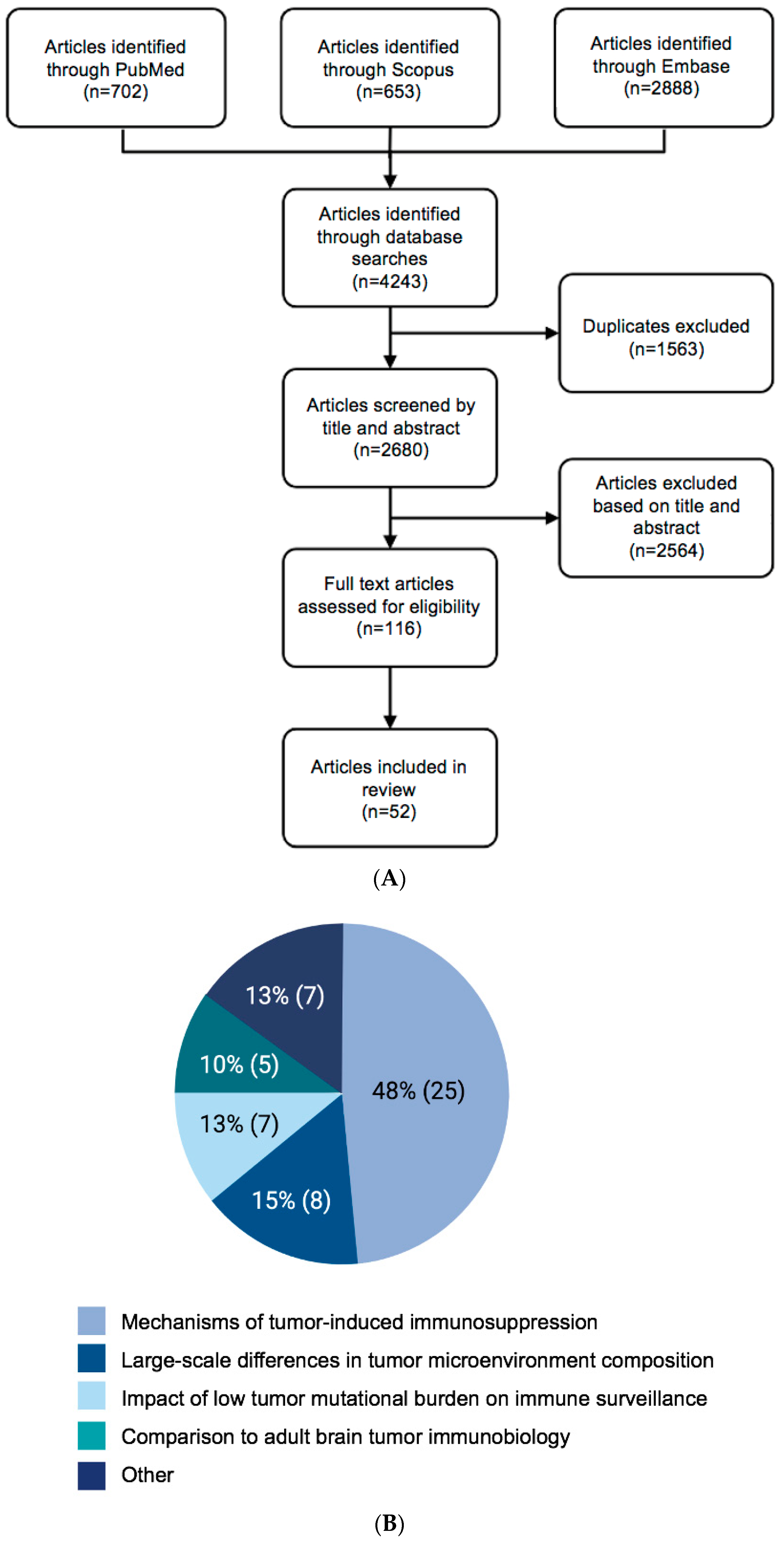
2. Methods
Search Strategy
3. Results
4. Discussion
4.1. General Themes of Pediatric Brain Tumor Immunobiology
4.2. Differential Immune Surveillance within Pediatric Brain Tumors
4.3. The Role of Immune Suppression in Pediatric Brain Tumors
4.4. Pediatric Brain Tumors Typically Have a Very Low Mutational Burden
4.5. Immunoediting as a Framework for Pediatric Brain Tumor Immunology
4.6. Evolution of Pediatric Brain Tumor Classification
4.7. The Paucity of Pre-Clinical Pediatric Brain Tumor Models
5. Conclusions
5.1. Overview of Findings, Future Perspectives, and Implementations
5.2. Key Areas for Future Investigation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtin, S.C.; Warner, M.; Hedegaard, H. Increase in Suicide in the United States, 1999–2014; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2016.
- Turner, C.D.; Rey-Casserly, C.; Liptak, C.C.; Chordas, C. Late effects of therapy for pediatric brain tumor survivors. J. Child Neurol. 2009, 24, 1455–1463. [Google Scholar] [CrossRef]
- Foster, J.B.; Madsen, P.J.; Hegde, M.; Ahmed, N.; Cole, K.A.; Maris, J.M.; Resnick, A.C.; Storm, P.B.; Waanders, A.J. Immunotherapy for pediatric brain tumors: Past and present. Neuro-Oncology 2019, 21, 1226–1238. [Google Scholar] [CrossRef]
- Sayour, E.J.; Mitchell, D.A. Immunotherapy for pediatric brain tumors. Brain Sci. 2017, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, M.R. Towards immunotherapy for pediatric brain tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.L.; Cheung, N.-K.V. Immunotherapy of pediatric solid tumors: Treatments at a crossroads, with an emphasis on antibodies. Cancer Immunol. Res. 2020, 8, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Jha, P.; Manjunath, N.; Singh, J.; Mani, K.; Garg, A.; Kaur, K.; Sharma, M.C.; Raheja, A.; Suri, A.; Sarkar, C. Analysis of PD-L1 expression and T cell infiltration in different molecular subgroups of diffuse midline gliomas. Neuropathology 2019, 39, 413–424. [Google Scholar] [CrossRef]
- Haberthur, K.; Brennan, K.; Hoglund, V.; Balcaitis, S.; Chinn, H.; Davis, A.; Kreuser, S.; Winter, C.; Leary, S.E.; Deutsch, G.H. NKG2D ligand expression in pediatric brain tumors. Cancer Biol. Ther. 2016, 17, 1253–1265. [Google Scholar] [CrossRef]
- Bockmayr, M.; Klauschen, F.; Maire, C.L.; Rutkowski, S.; Westphal, M.; Lamszus, K.; Schüller, U.; Mohme, M. Immunologic Profiling of Mutational and Transcriptional Subgroups in Pediatric and Adult High-Grade GliomasImmune Profiling of Pediatric and Adult Gliomas. Cancer Immunol. Res. 2019, 7, 1401–1411. [Google Scholar] [CrossRef]
- Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell 2020, 183, 1962–1985.e1931. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Birks, D.K.; Donson, A.M.; Amani, V.; Hoffman, L.M.; Waziri, A.; Wang, M.; Handler, M.H.; Foreman, N.K. Characterization of distinct immunophenotypes across pediatric brain tumor types. J. Immunol. 2013, 191, 4880–4888. [Google Scholar] [CrossRef]
- Robinson, M.H.; Vasquez, J.; Kaushal, A.; MacDonald, T.J.; Vega, J.E.V.; Schniederjan, M.; Dhodapkar, K. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J. Immunother. Cancer 2020, 8, e001066. [Google Scholar] [CrossRef]
- Tang, K.; Kurland, D.; Vasudevaraja, V.; Serrano, J.; Delorenzo, M.; Radmanesh, A.; Thomas, C.; Spino, M.; Gardner, S.; Allen, J.C. Exploring DNA methylation for prognosis and analyzing the tumor microenvironment in pleomorphic xanthoastrocytoma. J. Neuropathol. Exp. Neurol. 2020, 79, 880–890. [Google Scholar] [CrossRef]
- Diao, S.; Gu, C.; Zhang, H.; Yu, C. Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma. Oncol. Lett. 2020, 20, 397. [Google Scholar] [CrossRef]
- Najem, H.; Ott, M.; Kassab, C.; Rao, A.; Rao, G.; Marisetty, A.; Sonabend, A.M.; Horbinski, C.; Verhaak, R.; Shankar, A. Central nervous system immune interactome is a function of cancer lineage, tumor microenvironment, and STAT3 expression. JCI Insight 2022, 7, e157612. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. The second touch hypothesis: T cell activation, homing and polarization. F1000Research 2014, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Bodey, B.; Bodey, B., Jr.; Siegel, S.E.; Kaiser, H.E. Immunocytochemical detection of leukocyte-associated and apoptosis-related antigen expression in childhood brain tumors. Crit. Rev. Oncol. Hematol. 2001, 39, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Xue, Y.; Liu, Y. Decreased natural killer cells in diffuse intrinsic pontine glioma patients. Child’s Nerv. Syst. 2020, 36, 1345–1346. [Google Scholar] [CrossRef]
- Aichmüller, C.F.; Iskar, M.; Jones, D.T.; Korshunov, A.; Radlwimmer, B.; Kool, M.; Ernst, A.; Pfister, S.M.; Lichter, P.; Zapatka, M. Pilocytic astrocytoma demethylation and transcriptional landscapes link bZIP transcription factors to immune response. Neuro-Oncology 2020, 22, 1327–1338. [Google Scholar] [CrossRef]
- Rackaityte, E.; Halkias, J. Mechanisms of fetal T cell tolerance and immune regulation. Front. Immunol. 2020, 11, 588. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Schwarz, J.M. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Pierre, W.C.; Smith, P.L.; Londono, I.; Chemtob, S.; Mallard, C.; Lodygensky, G.A. Neonatal microglia: The cornerstone of brain fate. Brain Behav. Immun. 2017, 59, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Freiberger, V.; Ventura, L.; Bragagnolo, D.; Dutra, M.L.; Horewicz, V.V.; Comim, C.M. Late Brain Involvement after Neonatal Immune Activation. BioMed Res. Int. 2019, 2019, 9573248. [Google Scholar] [CrossRef]
- Schwarz, J.; Scheckenbach, V.; Kugel, H.; Spring, B.; Pagel, J.; Härtel, C.; Pauluschke-Fröhlich, J.; Peter, A.; Poets, C.; Gille, C. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 2018, 191, 328–337. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Wendler, C.C. Adverse and protective influences of adenosine on the newborn and embryo: Implications for preterm white matter injury and embryo protection. Pediatr. Res. 2011, 69, 271–278. [Google Scholar] [CrossRef]
- Nör, C.; Ramaswamy, V. Piecing together the Pediatric Brain Tumor Puzzle. Trends Genet. 2021, 37, 204–206. [Google Scholar] [CrossRef]
- Ott, M.; Tomaszowski, K.H.; Marisetty, A.; Kong, L.Y.; Wei, J.; Duna, M.; Blumberg, K.; Ji, X.; Jacobs, C.; Fuller, G.N.; et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight 2020, 5, e134386. [Google Scholar] [CrossRef]
- Sandén, E.; Enríquez Pérez, J.; Visse, E.; Kool, M.; Carén, H.; Siesjö, P.; Darabi, A. Preoperative systemic levels of VEGFA, IL-7, IL-17A, and TNF-β delineate two distinct groups of children with brain tumors. Pediatr. Blood Cancer 2016, 63, 2112–2122. [Google Scholar] [CrossRef]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the developing nervous system. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef]
- Hattori, Y.; Miyata, T. Microglia extensively survey the developing cortex via the CXCL12/CXCR4 system to help neural progenitors to acquire differentiated properties. Genes Cells 2018, 23, 915–922. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Kettenmann, H. Microglia/brain macrophages as central drivers of brain tumor pathobiology. Neuron 2019, 104, 442–449. [Google Scholar] [CrossRef]
- Navarro, V.; Sanchez-Mejias, E.; Jimenez, S.; Muñoz-Castro, C.; Sanchez-Varo, R.; Davila, J.C.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Microglia in Alzheimer’s disease: Activated, dysfunctional or degenerative. Front. Aging Neurosci. 2018, 10, 140. [Google Scholar] [CrossRef]
- Parisi, R.; Patel, R.R.; Rood, G.; Bowden, A.; Turco, G.; Korones, D.N.; Andolina, J.R.; Comito, M.; Barth, M.; Weintraub, L. Multi-institution analysis of tumor mutational burden and outcomes in pediatric central nervous system tumor patients. Pediatr. Blood Cancer 2023, 70, e30139. [Google Scholar] [CrossRef] [PubMed]
- Noskova, H.; Kyr, M.; Pal, K.; Merta, T.; Mudry, P.; Polaskova, K.; Ivkovic, T.C.; Adamcova, S.; Hornakova, T.; Jezova, M. Assessment of tumor mutational burden in pediatric tumors by real-life whole-exome sequencing and in silico simulation of targeted gene panels: How the choice of method could affect the clinical decision? Cancers 2020, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Ramkissoon, S.H.; Ross, J.; Weintraub, L. Tumor mutational burden and driver mutations: Characterizing the genomic landscape of pediatric brain tumors. Pediatr. Blood Cancer 2020, 67, e28338. [Google Scholar] [CrossRef]
- Abro, B.; Kaushal, M.; Chen, L.; Wu, R.; Dehner, L.P.; Pfeifer, J.D.; He, M. Tumor mutation burden, DNA mismatch repair status and checkpoint immunotherapy markers in primary and relapsed malignant rhabdoid tumors. Pathol.-Res. Pract. 2019, 215, 152395. [Google Scholar] [CrossRef]
- Johnson, A.; Severson, E.; Gay, L.; Vergilio, J.A.; Elvin, J.; Suh, J.; Daniel, S.; Covert, M.; Frampton, G.M.; Hsu, S. Comprehensive genomic profiling of 282 pediatric low-and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist 2017, 22, 1478–1490. [Google Scholar] [CrossRef]
- Richman, L.P.; Vonderheide, R.H.; Rech, A.J. Neoantigen dissimilarity to the self-proteome predicts immunogenicity and response to immune checkpoint blockade. Cell Syst. 2019, 9, 375–382.e374. [Google Scholar] [CrossRef]
- Zhang, J.G.; Kruse, C.A.; Driggers, L.; Hoa, N.; Wisoff, J.; Allen, J.C.; Zagzag, D.; Newcomb, E.W.; Jadus, M.R. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J. Neuro-Oncol. 2008, 88, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.H.; Chang, C.C.; Wu, K.S.; Yu, A.L.; Sung, S.Y.; Lee, Y.Y.; Liang, M.-L.; Chen, H.H.; Fen, J.J.; Chao, M.E.; et al. Notch signaling and natural killer cell infiltration in tumor tissues underlie medulloblastoma prognosis. Sci. Rep. 2021, 1, 23282. [Google Scholar] [CrossRef]
- Vasquez, J.C.; Huttner, A.; Zhang, L.; Marks, A.; Chan, A.; Baehring, J.M.; Kahle, K.T.; Dhodapkar, K.M. SOX2 immunity and tissue resident memory in children and young adults with glioma. J. Neuro-Oncol. 2017, 134, 41–53. [Google Scholar] [CrossRef]
- Blaeschke, F.; Paul, M.C.; Schuhmann, M.U.; Rabsteyn, A.; Schroeder, C.; Casadei, N.; Matthes, J.; Mohr, C.; Lotfi, R.; Wagner, B.; et al. Low mutational load in pediatric medulloblastoma still translates into neoantigens as targets for specific T-cell immunotherapy. Cytotherapy 2019, 21, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Brown, M.C.; Zhang, G.; Lin, X.; Chen, Y.; Wei, Z.; Beaubier, N.; Yan, H.; He, Y.; Desjardins, A.; et al. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat. Commun. 2021, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Yanai, H.; Hangai, S.; Taniguchi, T. Damage-associated molecular patterns and Toll-like receptors in the tumor immune microenvironment. Int. Immunol. 2021, 33, 841–846. [Google Scholar] [CrossRef]
- Mohme, M.; Neidert, M.C. Tumor-specific T cell activation in malignant brain tumors. Front. Immunol. 2020, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Arellano, L.; Eguía-Aguilar, P.; Piña-Sánchez, P.; González-García, N.; Palma-Guzman, A.; Perezpeña-Diazconti, M.; Maldonado-Bernal, C. High expression of Toll-like receptor 7 is a survival factor in pediatric medulloblastoma. Child’s Nerv. Syst. 2021, 37, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Engler, J.R.; Robinson, A.E.; Smirnov, I.; Hodgson, J.G.; Berger, M.S.; Gupta, N.; James, C.D.; Molinaro, A.; Phillips, J.J. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS ONE 2012, 7, e43339. [Google Scholar] [CrossRef] [PubMed]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like receptor stimulation in cancer: A pro-and anti-tumor double-edged sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef]
- Xun, Y.; Yang, H.; Kaminska, B.; You, H. Toll-like receptors and toll-like receptor-targeted immunotherapy against glioma. J. Hematol. Oncol. 2021, 14, 176. [Google Scholar] [CrossRef]
- Romerio, A.; Peri, F. Increasing the chemical variety of small-molecule-based TLR4 modulators: An overview. Front. Immunol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Ha, W.; Sevim-Nalkiran, H.; Zaman, A.M.; Matsuda, K.; Khasraw, M.; Nowak, A.K.; Chung, L.; Baxter, R.C.; McDonald, K.L. Ibudilast sensitizes glioblastoma to temozolomide by targeting Macrophage Migration Inhibitory Factor (MIF). Sci. Rep. 2019, 9, 2905. [Google Scholar] [CrossRef]
- Wang, Y.; Su, L.; Morin, M.D.; Jones, B.T.; Mifune, Y.; Shi, H.; Wang, K.-W.; Zhan, X.; Liu, A.; Wang, J. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti–PD-L1 to eliminate melanoma in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E8698–E8706. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef]
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E., 2nd; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729. [Google Scholar] [CrossRef]
- Greiner, J.W. Modulation of antigen expression in human tumor cell populations. Cancer Investig. 1986, 4, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Akbasak, A.; Oldfield, E.H.; Saris, S.C. Expression and modulation of major histocompatibility antigens on murine primary brain tumor in vitro. J. Neurosurg. 1991, 75, 922–929. [Google Scholar] [CrossRef]
- Bubeník, J. Tumour MHC class I downregulation and immunotherapy. Oncol. Rep. 2003, 10, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.-G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC class I downregulation in cancer: Underlying mechanisms and potential targets for cancer immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Low, J.T.; Chandramohan, V.; Bowie, M.L.; Brown, M.C.; Waitkus, M.S.; Briley, A.; Stevenson, K.; Fuller, R.; Reitman, Z.J.; Muscat, A.M.; et al. Epigenetic STING silencing is developmentally conserved in gliomas and can be rescued by methyltransferase inhibition. Cancer Cell 2022, 40, 439–440. [Google Scholar] [CrossRef]
- Vermeulen, J.F.; van Hecke, W.; Spliet, W.G.; Villacorta Hidalgo, J.; Fisch, P.; Broekhuizen, R.; Bovenschen, N. Pediatric primitive neuroectodermal tumors of the central nervous system differentially express granzyme inhibitors. PLoS ONE 2016, 11, e0151465. [Google Scholar] [CrossRef]
- Vermeulen, J.F.; Van Hecke, W.; Adriaansen, E.J.; Jansen, M.K.; Bouma, R.G.; Villacorta Hidalgo, J.; Fisch, P.; Broekhuizen, R.; Spliet, W.G.; Kool, M. Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology 2018, 7, e1398877. [Google Scholar] [CrossRef]
- Folgiero, V.; Miele, E.; Carai, A.; Ferretti, E.; Alfano, V.; Po, A.; Bertaina, V.; Goffredo, B.M.; Benedetti, M.C.; Camassei, F.D. IDO1 involvement in mTOR pathway: A molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget 2016, 7, 52900. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Vacchelli, E.; Aranda, F.; Eggermont, A.; Sautes-Fridman, C.; Tartour, E.; Kennedy, E.P.; Platten, M.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014, 3, e957994. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, J.Y.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar]
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.-M.; Oh, M.-H.; Sun, I.-H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366, 1013–1021. [Google Scholar] [CrossRef]
- Silva, C.G.; Métin, C.; Fazeli, W.; Machado, N.J.; Darmopil, S.; Launay, P.-S.; Ghestem, A.; Nesa, M.-P.; Bassot, E.; Szabó, E. Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med. 2013, 5, ra104–ra197. [Google Scholar] [CrossRef]
- Ohta, A. A metabolic immune checkpoint: Adenosine in tumor microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef]
- Gate, D.; Danielpour, M.; Rodriguez, J., Jr.; Kim, G.-B.; Levy, R.; Bannykh, S.; Breunig, J.J.; Kaech, S.M.; Flavell, R.A.; Town, T. T-cell TGF-β signaling abrogation restricts medulloblastoma progression. Proc. Natl. Acad. Sci. USA 2014, 111, E3458–E3466. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Powell, A.B.; Yadavilli, S.; Saunders, D.; Van Pelt, S.; Chorvinsky, E.; Burga, R.A.; Albihani, S.; Hanley, P.J.; Xu, Z.; Pei, Y. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J. Transl. Med. 2019, 17, 321. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, 8–11. [Google Scholar] [CrossRef]
- Katakowski, M.; Chopp, M. Exosomes as tools to suppress primary brain tumor. Cell. Mol. Neurobiol. 2016, 36, 343–352. [Google Scholar] [CrossRef]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.T.; Graner, M.W. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. F1000Research 2012, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009, 23, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Sandén, E.; Dyberg, C.; Krona, C.; Visse, E.; Carén, H.; Northcott, P.A.; Kool, M.; Ståhl, N.; Persson, A.; Englund, E. Aberrant immunostaining pattern of the CD24 glycoprotein in clinical samples and experimental models of pediatric medulloblastomas. J. Neuro-Oncol. 2015, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, A.M.; Witt, D.A.; Grob, S.T.; Georgio Westover, S.R.; Donson, A.M.; Sanford, B.; Mulcahy Levy, J.M.; Wong, R.; Moreira, D.C.; DeSisto, J.A. NF-κB upregulation through epigenetic silencing of LDOC1 drives tumor biology and specific immunophenotype in Group A ependymoma. Neuro-Oncology 2017, 19, 1350–1360. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lewis, C.E. NF-KB as a central regulator of macrophage function in tumors. J. Leukoc. Biol. 2010, 88, 877–884. [Google Scholar] [CrossRef]
- Ross, J.L.; Chen, Z.; Herting, C.J.; Grabovska, Y.; Szulzewsky, F.; Puigdelloses, M.; Monterroza, L.; Switchenko, J.; Wadhwani, N.R.; Cimino, P.J. Platelet-derived growth factor beta is a potent inflammatory driver in paediatric high-grade glioma. Brain 2021, 144, 53–69. [Google Scholar] [CrossRef]
- Peng, J.; Yang, L.; Pan, J.; Wang, C.; Nie, J.; Liu, Y.; Fan, J.; Zhou, J.; Qi, S. Clinical features and prognosis of pediatric infradiaphragmatic craniopharyngioma relative to the tumor inflammatory response. Pediatr. Res. 2021, 89, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Ott, M.; Fang, D.; Heimberger, A.B. The Role and Therapeutic Targeting of JAK/STAT Signaling in Glioblastoma. Cancers 2021, 13, 437. [Google Scholar] [CrossRef]
- Pham, C.D.; Mitchell, D.A. Know your neighbors: Different tumor microenvironments have implications in immunotherapeutic targeting strategies across MB subgroups. Oncoimmunology 2016, 5, e1144002. [Google Scholar] [CrossRef]
- Maximov, V.; Chen, Z.; Wei, Y.; Robinson, M.H.; Herting, C.J.; Shanmugam, N.S.; Rudneva, V.A.; Goldsmith, K.C.; MacDonald, T.J.; Northcott, P.A. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat. Commun. 2019, 10, 2410. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, H.; Liang, X.; Ni, S. Targeting tumor-associated macrophages for the immunotherapy of glioblastoma: Navigating the clinical and translational landscape. Front. Immunol. 2022, 13, 1024921. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer ImmunotherapyPD-L1 IHC as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilie, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Koh, E.J.; Choi, E.J.; Kang, T.H.; Han, J.H.; Choe, G.; Park, S.-H.; Yearley, J.H.; Annamalai, L.; Sathe, M.; et al. PD-1/PD-L1 and immune-related gene expression pattern in pediatric malignant brain tumors: Clinical correlation with survival data in Korean population. J. Neuro-Oncol. 2018, 139, 281–291. [Google Scholar] [CrossRef]
- Martin, A.M.; Bell, W.R.; Yuan, M.; Harris, L.; Poore, B.; Arnold, A.; Engle, E.L.; Asnaghi, L.; Lim, M.; Raabe, E.H.; et al. PD-L1 expression in pediatric low-grade gliomas is independent of BRAF V600E mutational status. J. Neuropathol. Exp. Neurol. 2020, 79, 74–85. [Google Scholar] [CrossRef]
- Martin, A.M.; Nirschl, C.J.; Polanczyk, M.J.; Bell, W.R.; Nirschl, T.R.; Harris-Bookman, S.; Phallen, J.; Hicks, J.; Martinez, D.; Ogurtsova, A. PD-L1 expression in medulloblastoma: An evaluation by subgroup. Oncotarget 2018, 9, 19177. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Yan, C.; Yang, Y.; Li, Z.; Yang, Z. The role of clinical factors and immunocheckpoint molecules in the prognosis of patients with supratentorial extraventricular ependymoma: A single-center retrospective study. J. Cancer Res. Clin. Oncol. 2021, 147, 1259–1270. [Google Scholar] [CrossRef]
- Witt, D.A.; Donson, A.M.; Amani, V.; Moreira, D.C.; Sanford, B.; Hoffman, L.M.; Handler, M.H.; Levy, J.M.M.; Jones, K.L.; Nellan, A. Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: Implications for PD-1-targeted therapy. Pediatr. Blood Cancer 2018, 65, e26960. [Google Scholar] [CrossRef]
- Liu, B.; Arakawa, Y.; Yokogawa, R.; Tokunaga, S.; Terada, Y.; Murata, D.; Matsui, Y.; Fujimoto, K.; Fukui, N.; Mineharu, Y.; et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS ONE 2018, 13, e0194594. [Google Scholar] [CrossRef]
- Majzner, R.G.; Simon, J.S.; Grosso, J.F.; Martinez, D.; Pawel, B.R.; Santi, M.; Merchant, M.S.; Geoerger, B.; Hezam, I.; Marty, V.; et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer 2017, 123, 3807–3815. [Google Scholar] [CrossRef]
- Özören, N.; El-Deiry, W.S. Cell surface death receptor signaling in normal and cancer cells. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 135–147. [Google Scholar]
- French, L.E.; Tschopp, J. Defective death receptor signaling as a cause of tumor immune escape. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 51–55. [Google Scholar]
- Irmler, M.; Thome, M.; Hahne, M.; Schneider, P.; Hofmann, K.; Steiner, V.; Bodmer, J.-L.; Schröter, M.; Burns, K.; Mattmann, C. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190–195. [Google Scholar] [CrossRef]
- Thorburn, A. Death receptor-induced cell killing. Cell. Signal. 2004, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Iaccarino, L.; Ghirardello, A.; Bassi, N.; Pontisso, P.; Punzi, L.; Shoenfeld, Y.; Doria, A. Serpins, immunity and autoimmunity: Old molecules, new functions. Clin. Rev. Allergy Immunol. 2013, 45, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Gessi, M.; Gielen, G.H.; Hammes, J.; Dorner, E.; Muhlen, A.Z.; Waha, A.; Pietsch, T. H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: Possible diagnostic and therapeutic implications? J. Neurooncol. 2013, 112, 67–72. [Google Scholar] [CrossRef]
- Korshunov, A.; Capper, D.; Reuss, D.; Schrimpf, D.; Ryzhova, M.; Hovestadt, V.; Sturm, D.; Meyer, J.; Jones, C.; Zheludkova, O.; et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 2016, 131, 137–146. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours [Internet], 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Venneti, S.; et al. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Levy, R.; Antonuk, C.D.; Molina, J.; Dutra-Clarke, M.; Park, H.; Akhtar, A.A.; Kim, G.B.; Town, T.; Hu, X. Ets factors regulate neural stem cell depletion and gliogenesis in Ras pathway glioma. Cell Rep. 2015, 12, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Langhans, S.A. In vivo and ex vivo pediatric brain tumor models: An overview. Front. Oncol. 2021, 11, 620831. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budhiraja, S.; Najem, H.; Tripathi, S.; Wadhawani, N.R.; Horbinski, C.; McCord, M.; Lenzen, A.C.; Heimberger, A.B.; DeCuypere, M. Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review. Cancers 2023, 15, 3655. https://doi.org/10.3390/cancers15143655
Budhiraja S, Najem H, Tripathi S, Wadhawani NR, Horbinski C, McCord M, Lenzen AC, Heimberger AB, DeCuypere M. Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review. Cancers. 2023; 15(14):3655. https://doi.org/10.3390/cancers15143655
Chicago/Turabian StyleBudhiraja, Shreya, Hinda Najem, Shashwat Tripathi, Nitin R. Wadhawani, Craig Horbinski, Matthew McCord, Alicia C. Lenzen, Amy B. Heimberger, and Michael DeCuypere. 2023. "Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review" Cancers 15, no. 14: 3655. https://doi.org/10.3390/cancers15143655
APA StyleBudhiraja, S., Najem, H., Tripathi, S., Wadhawani, N. R., Horbinski, C., McCord, M., Lenzen, A. C., Heimberger, A. B., & DeCuypere, M. (2023). Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review. Cancers, 15(14), 3655. https://doi.org/10.3390/cancers15143655






