Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Endoscopic Ultrasound-Guided HybridTherm Probe Ablation
2.3. Flow-Cytometry
2.4. Multiplex Immunoassays
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patients and Clinical Outcomes
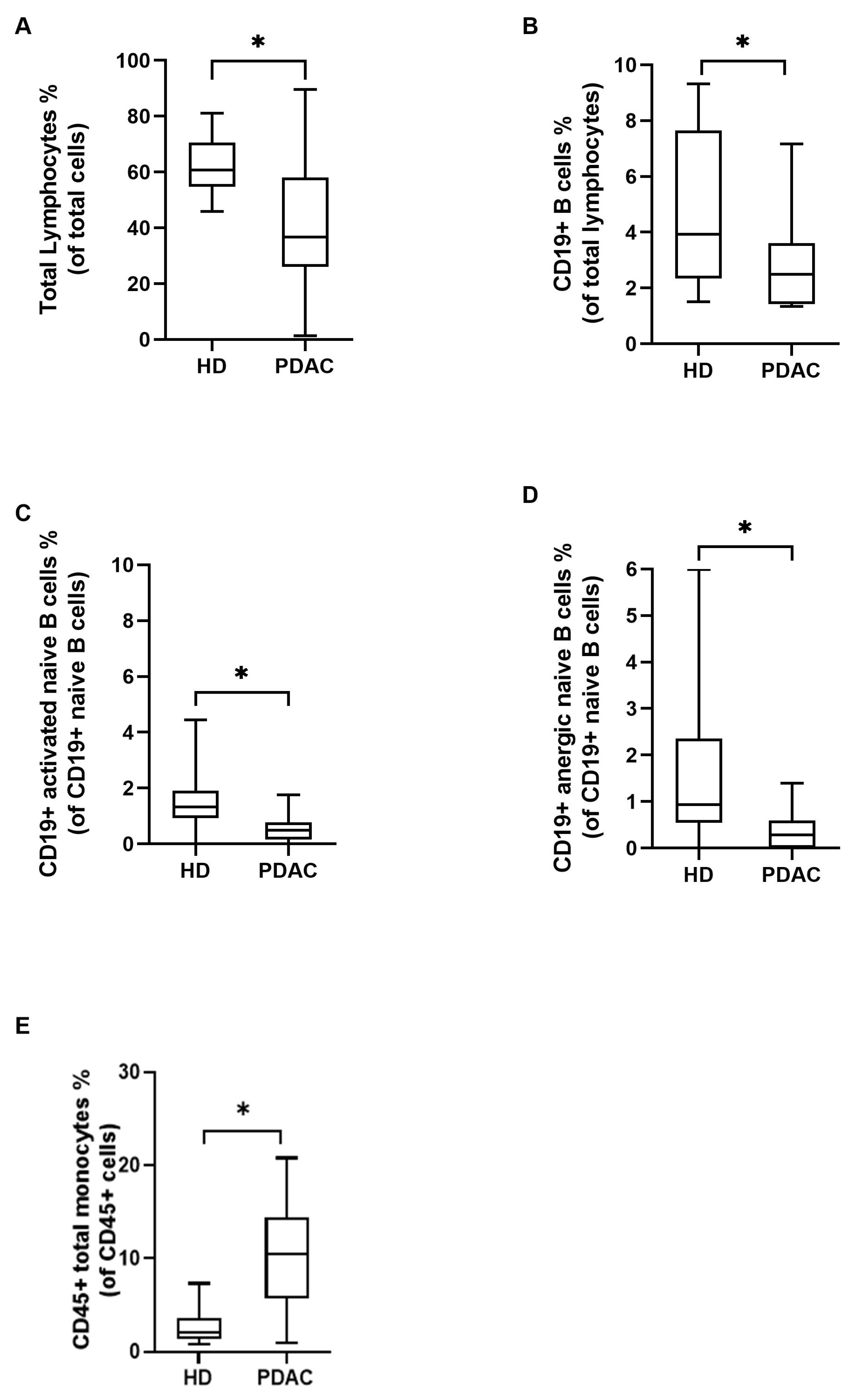
3.2. Immunological Landscape of PDAC Patients at Baseline Compared to Healthy Donors
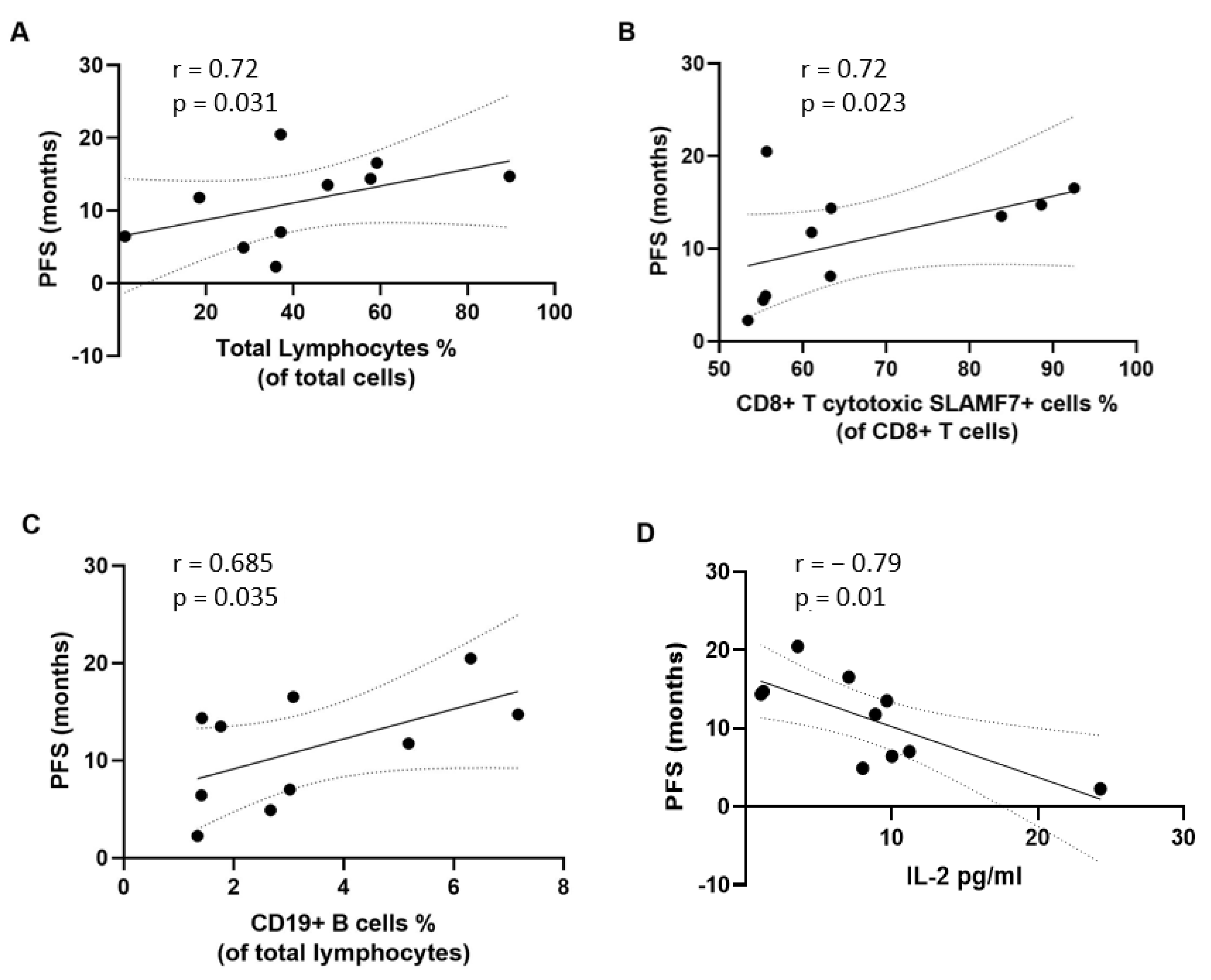
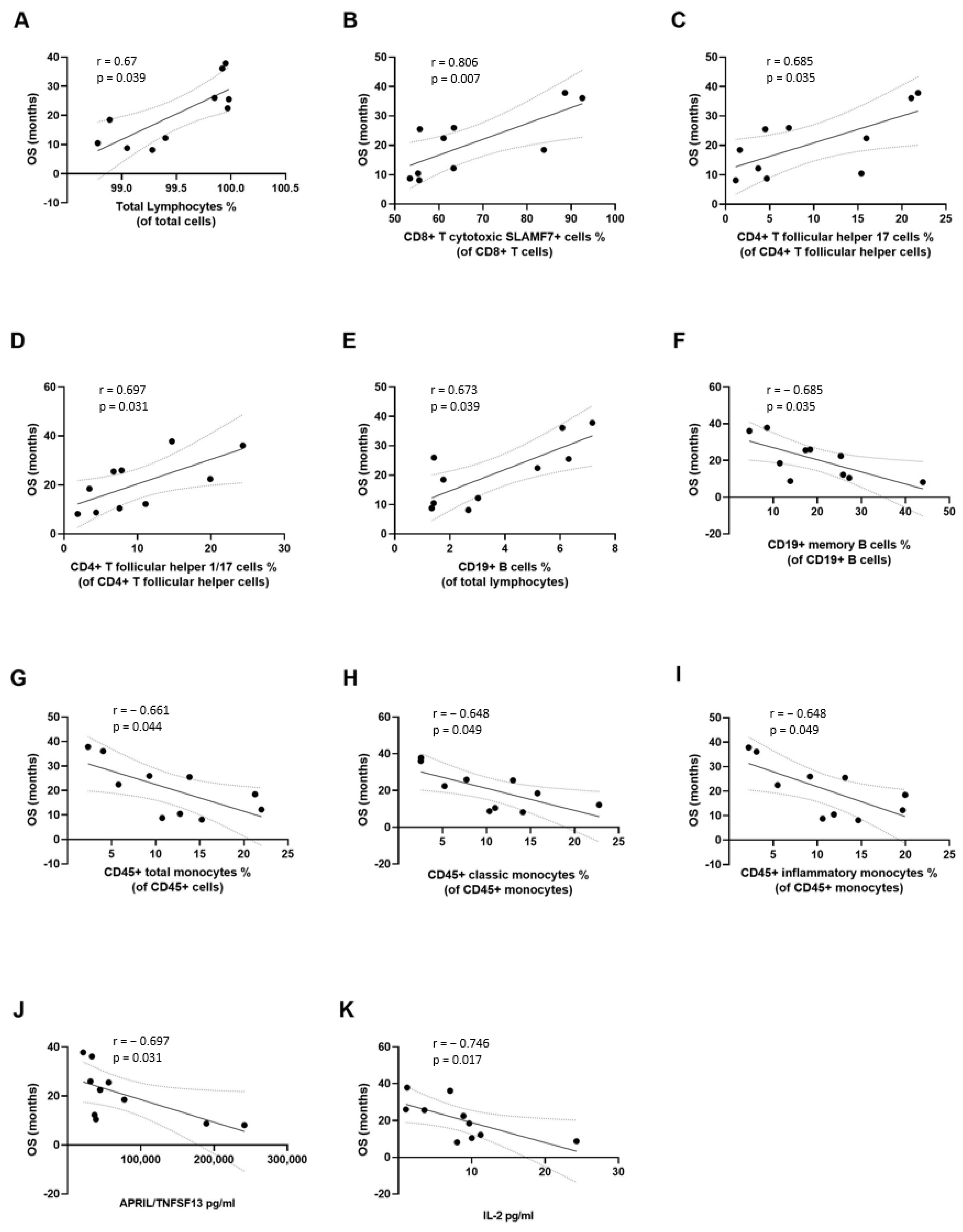
3.3. Correlation of Immunological Variables with Clinical Outcomes
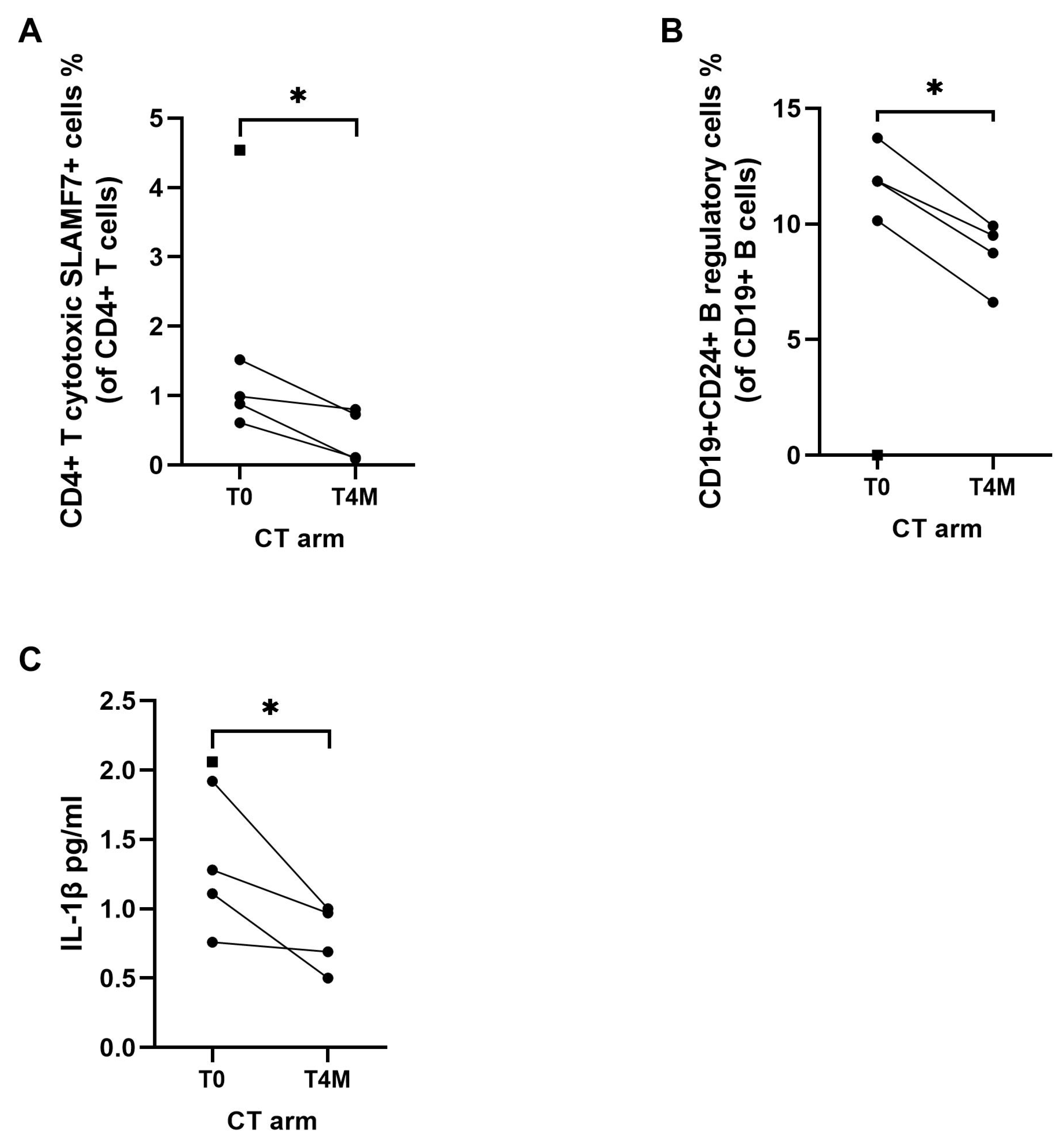
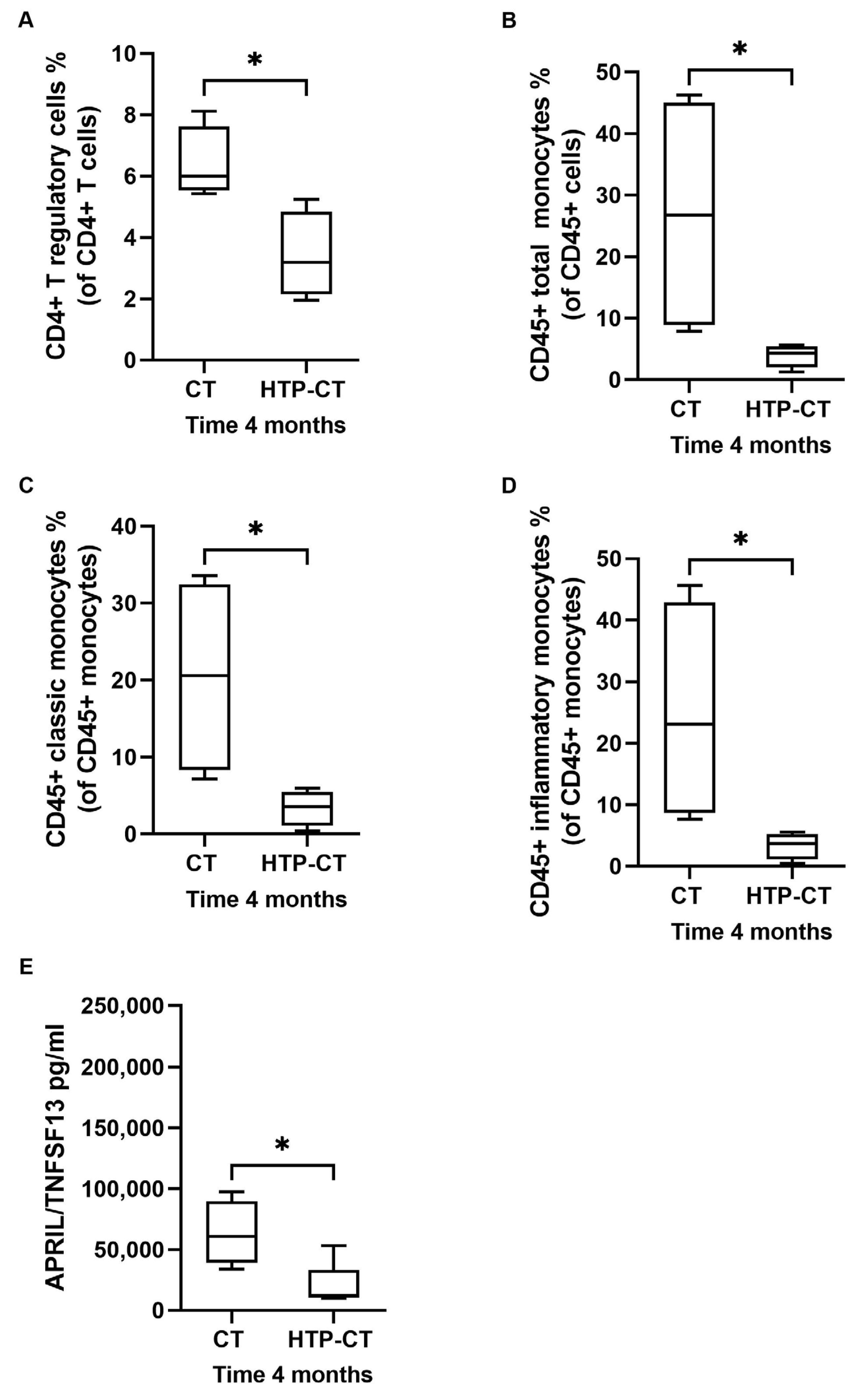
3.4. Effects of EUS-Guided Thermal Ablation on Circulating Immune Cells and Inflammatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; Del Chiaro, M.; Van Lienden, K.P.; Meijerink, M.R.; Van Tienhoven, G.; et al. Locally advanced pancreatic cancer: Work-up, staging, and local intervention strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Usui, M.; Isaji, S.; Nagakawa, T.; Wada, K.; Unno, M.; Nakao, A.; Miyakawa, S.; Ohta, T. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: A multi-institutional survey by the Japanese Society of Pancreatic Surgery. J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 601–610. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Guidelines Guidelines Ver. 2.2016 on Pancreatic Adenocarcinoma. Available online: http://www.nccn.org (accessed on 1 March 2016).
- Tempero, A.M.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Guidelines Insights: Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Hammel, P.; Huguet, F.F.; Van Laethem, J.-L.; Goldstein, D.D.; Glimelius, B.; Artru, P.P.; Borbath, I.; Bouché, O.; Shannon, J.J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Assifi, M.M.; Lu, X.; Eibl, G.; Reber, H.A.; Li, G.; Hines, O.J. Neoadjuvant therapy in pancreatic adenocarcinoma: A meta-analysis of phase II trials. Surgery 2011, 150, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; E Faris, J.; A Mellon, E.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-B.; Yang, Z.-H.; Guo, Q.-Q. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: Limitations and prospects: A systematic review. Cell Commun. Signal. 2021, 19, 117. [Google Scholar] [CrossRef]
- Timmer, F.E.F.; Geboers, B.; Nieuwenhuizen, S.; Dijkstra, M.; Schouten, E.A.C.; Puijk, R.S.; de Vries, J.J.J.; van den Tol, M.P.; Bruynzeel, A.M.E.; Streppel, M.M.; et al. Pancreatic Cancer and Immunotherapy: A Clinical Overview. Cancers 2021, 13, 4138. [Google Scholar] [CrossRef]
- Geboers, B.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; Scheffer, H.J.; de Gruijl, T.D.; Meijerink, M.R. Needle-guided ablation of locally advanced pancreatic cancer: Cytoreduction or immunomodulation by in vivo vaccination? Chin. Clin. Oncol. 2019, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Ostapoff, K.T.; Kuvshinoff, B.; Hochwald, S.N. Ablative Therapies for Locally Advanced Pancreatic Cancer. Pancreas 2018, 47, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.J.; Curtis, L.T.; Wu, M.; Ho, J.C.; Corr, S.J.; Curley, S.A.; Godin, B.; Frieboes, H.B. Pancreatic adenocarcinoma response to chemotherapy enhanced with non-invasive radio frequency evaluated via an integrated experimental/computational approach. Sci. Rep. 2017, 7, 3437. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bastianpillai, C.; Petrides, N.; Shah, T.; Guillaumier, S.; Ahmed, H.U.; Arya, M. Harnessing the immunomodulatory effect of thermal and non-thermal ablative therapies for cancer treatment. Tumor Biol. 2015, 36, 9137–9146. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Dietrich, F.C.; Testoni, S.G.G.; Healey, A.J.; Arcidiacono, P.G. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc. Ultrasound 2020, 9, 83–100. [Google Scholar] [CrossRef]
- Paiella, S.; Salvia, R.; Ramera, M.; Girelli, R.; Frigerio, I.; Giardino, A.; Allegrini, V.; Bassi, C. Local Ablative Strategies for Ductal Pancreatic Cancer (Radiofrequency Ablation, Irreversible Electroporation): A Review. Gastroenterol. Res. Pract. 2016, 2016, 4508376. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef]
- He, K.; Liu, P.; Xu, L.X. The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response. Cell Death Dis. 2017, 8, e2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Lou, Y.; Liu, P.; Xu, L.X. Tumor-related HSP70 released after cryo-thermal therapy targeted innate immune initiation in the antitumor immune response. Int. J. Hyperth. 2020, 37, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Hu, H.; Liu, P.; Xu, L.X. Neoantigen-specific CD4+ T-cell response is critical for the therapeutic efficacy of cryo-thermal therapy. J. Immunother. Cancer 2020, 8, e000421. [Google Scholar] [CrossRef] [PubMed]
- Testoni, S.G.G.; Petrone, M.C.; Reni, M.; Rossi, G.; Barbera, M.; Nicoletti, V.; Gusmini, S.; Balzano, G.; Linzenbold, W.; Enderle, M.; et al. Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers 2021, 13, 4512. [Google Scholar] [CrossRef] [PubMed]
- Testoni, S.G.G.; Capurso, G.; Petrone, M.C.; Barbera, M.; Linzenbold, W.; Enderle, M.; Gusmini, S.; Nicoletti, R.; Della Torre, E.; Mariani, A.; et al. Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer. Endosc. Int. Open 2020, 8, E1511–E1519. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, C.S.; Macapinlac, H.A.; Burgess, A.M.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of Computed Tomography and Positron Emission Tomography in Patients with Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Carrara, S.; Arcidiacono, P.G.; Albarello, L.; Addis, A.; Enderle, M.D.; Boemo, C.; Campagnol, M.; Ambrosi, A.; Doglioni, C.; Testoni, P.A. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: A preliminary study. Endoscopy 2008, 40, 321–326. [Google Scholar] [CrossRef]
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Petrone, M.C.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151. [Google Scholar] [CrossRef]
- Petrone, M.C.; Arcidiacono, P.G.; Carrara, S.; Albarello, L.; Enderle, M.D.; Neugebauer, A.; Boemo, C.; Doglioni, C.; Testoni, P.A. US-guided application of a new hybrid probe in human pancreatic adenocarcinoma: An ex vivo study. Gastrointest. Endosc. 2010, 71, 1294–1297. [Google Scholar] [CrossRef]
- Mattoo, H.; Mahajan, V.S.; Maehara, T.; Deshpande, V.; Della-Torre, E.; Wallace, Z.S.; Kulikova, M.; Drijvers, J.M.; Daccache, J.; Carruthers, M.N.; et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J. Allergy Clin. Immunol. 2016, 138, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Della-Torre, E.; Bozzalla-Cassione, E.; Sciorati, C.; Ruggiero, E.; Lanzillotta, M.; Bonfiglio, S.; Mattoo, H.; Perugino, C.A.; Bozzolo, E.; Rovati, L.; et al. A CD8α− Subset of CD4+SLAMF7+ Cytotoxic T Cells Is Expanded in Patients with IgG4-Related Disease and Decreases Following Glucocorticoid Treatment. Arthritis Rheumatol. 2018, 70, 1133–1143. [Google Scholar] [CrossRef]
- Lambin, T.; Lafon, C.; Drainville, R.A.; Pioche, M.; Prat, F. Locoregional therapies and their effects on the tumoral microenvironment of pancreatic ductal adenocarcinoma. World J. Gastroenterol. 2022, 28, 1288–1303. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, L.; Lu, J.; Wang, Y.; Liu, C.; You, L.; Guo, J. Stroma-Targeting Therapy in Pancreatic Cancer: One Coin with Two Sides? Front. Oncol. 2020, 10, 576399. [Google Scholar] [CrossRef]
- Skelton, R.A.; Javed, A.; Zheng, L.; He, J. Overcoming the resistance of pancreatic cancer to immune checkpoint inhibitors. J. Surg. Oncol. 2017, 116, 55–62. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kubota, K.; Kato, S.; Watanabe, S.; Shimamura, T.; Kirikoshi, H.; Saito, S.; Ueda, M.; Endo, I.; Lnayama, Y.; et al. FOXP3+ Regulatory T Cells and Tumoral Indoleamine 2,3-Dioxygenase Expression Predicts the Carcinogenesis of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreatology 2010, 10, 631–640. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yanagimoto, H.; Satoi, S.; Toyokawa, H.; Hirooka, S.; Yamaki, S.; Yui, R.; Yamao, J.; Kim, S.; Kwon, A.-H. Circulating CD4+CD25+ Regulatory T Cells in Patients with Pancreatic Cancer. Pancreas 2012, 41, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, M.; Feng, Y.; He, R.; Xu, X.; Xie, Y.; Shi, X.; Zhou, M.; Pan, S.; Wang, M.; et al. Regulatory B cells induced by pancreatic cancer cell-derived interleukin-18 promote immune tolerance via the PD-1/PD-L1 pathway. Oncotarget 2017, 9, 14803–14814. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Hiroshima, Y.; Matsuyama, R.; Homma, Y.; Hoffman, M.R.; Endo, I. Role of the tumor microenvironment in pancreatic cancer. Ann. Gastroenterol. Surg. 2019, 3, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.-Q.; Yang, X.; Cai, M.-H.; Yao, J.-Y.; Jin, W.-W.; Mou, Y.-P.; Ma, Y.-Y. Role of Treg/Th17 Imbalance, Microbiota and miRNAs in Pancreatic Cancer: Therapeutic Options. Crit. Rev. Immunol. 2020, 40, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Minici, C.; Testoni, S.; Della-Torre, E. B-Lymphocytes in the Pathophysiology of Pancreatic Adenocarcinoma. Front. Immunol. 2022, 13, 867902. [Google Scholar] [CrossRef]
- Minici, C.; Rigamonti, E.; Lanzillotta, M.; Monno, A.; Rovati, L.; Maehara, T.; Kaneko, N.; Deshpande, V.; Protti, M.P.; De Monte, L.; et al. B lymphocytes contribute to stromal reaction in pancreatic ductal adenocarcinoma. Oncoimmunology 2020, 9, 1794359. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, M.; Della-Torre, E.; Milani, R.; Bozzolo, E.; Bozzalla-Cassione, E.; Rovati, L.; Arcidiacono, P.G.; Partelli, S.; Falconi, M.; Ciceri, F.; et al. Increase of circulating memory B cells after glucocorticoid-induced remission identifies patients at risk of IgG4-related disease relapse. Thromb. Haemost. 2018, 20, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Brok, M.H.M.G.M.D.; Sutmuller, R.P.M.; van der Voort, R.; Bennink, E.J.; Figdor, G.C.; Ruers, T.J.M.; Adema, G.J. In Situ Tumor Ablation Creates an Antigen Source for the Generation of Antitumor Immunity. Cancer Res 2004, 64, 4024–4029. [Google Scholar] [CrossRef] [Green Version]
- Fei, Q.; Pan, Y.; Lin, W.; Zhou, Y.; Yu, X.; Hou, Z.; Yu, X.; Lin, X.; Lin, R.; Lu, F.; et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis. 2020, 11, 589. [Google Scholar] [CrossRef]
- Faraoni, E.Y.; O’Brien, B.J.; Strickland, L.N.; Osborn, B.K.; Mota, V.; Chaney, J.; Atkins, C.L.; Cen, P.; Rowe, J.; Cardenas, J.; et al. Radiofrequency Ablation Remodels the Tumor Microenvironment and Promotes Neutrophil-Mediated Abscopal Immunomodulation in Pancreatic Cancer. Cancer Immunol. Res. 2022, 11, 4–12. [Google Scholar] [CrossRef]
- Mirlekar, B.; Wang, Y.; Li, S.; Zhou, M.; Entwistle, S.; De Buysscher, T.; Morrison, A.; Herrera, G.; Harris, C.; Vincent, B.G.; et al. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep. Med. 2022, 3, 100744. [Google Scholar] [CrossRef]
- Barber, M.D.; Powell, J.J.; Lynch, S.F.; Fearon, K.C.H.; A Ross, J. A polymorphism of the interleukin-1 β gene influences survival in pancreatic cancer. Br. J. Cancer 2000, 83, 1443–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Di Xiao, D.; Jin, F.; Sun, X.; Yu, J.; Wang, H.; Liu, J.; Cai, W.; Huang, C.; Wang, X.; et al. ESE3-positive PSCs drive pancreatic cancer fibrosis, chemoresistance and poor prognosis via tumour–stromal IL-1β/NF–κB/ESE3 signalling axis. Br. J. Cancer 2022, 127, 1461–1472. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Ni, Q.; Yu, X. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int. J. Oncol. 2017, 51, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, D.B.; et al. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chen, L.; Ding, W.; Wang, G.; Wu, Y.; Wang, J.; Luo, L.; Cong, H.; Wang, Y.; Ju, S.; et al. Serum APRIL, a potential tumor marker in pancreatic cancer. Clin. Chem. Lab. Med. 2011, 49, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Von Bernstorff, W.; Voss, M.; Freichel, S.; Schmid, A.; Vogel, I.; Jöhnk, C.; Henne-Bruns, D.; Kremer, B.; Kalthoff, H. Systemic and local immunosuppression in pancreatic cancer patients. Clin. Cancer Res. 2001, 7 (Suppl. S3), 925s–932s. [Google Scholar] [PubMed]
- Chen, S.; Huang, F.; He, C.; Li, J.; Chen, S.; Li, Y.; Chen, Y.; Lian, G.; Huang, K. Peripheral blood monocytes predict clinical prognosis and support tumor invasiveness through NF-κB-dependent upregulation of Snail in pancreatic cancer. Transl. Cancer Res. 2021, 10, 4773–4785. [Google Scholar] [CrossRef]
- Tsou, C.-L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Xu, J.; Long, J.; Liu, L.; Yu, X.; Liu, C. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 2016, 16, 1080–1084. [Google Scholar] [CrossRef]
- Pombeiro, I.; Loosen, S.H.; Roy, S.; Schueller, F.; Niewenhuisen, L.; Luedde, M.; Vucur, M.; Tacke, F.; Binnebösel, M.; Schoening, W.; et al. Differential Roles of Tumor Necrosis Factor Ligand Superfamily Members as Biomarkers in Pancreatic Cancer. J. Clin. Med. 2018, 7, 175. [Google Scholar] [CrossRef] [Green Version]


| Features | HTP-CT Arm | CT Arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat. 1 | Pat. 2 | Pat. 3 | Pat. 4 | Pat. 5 | Median (Range) | Pat. 1 | Pat. 2 | Pat. 3 | Pat. 4 | Pat. 5 | Median (Range) | p-Value | |
| Sex, M/F, n (%) | M | M | F | F | F | - | M | M | F | M | F | - | 0.55 |
| Age (years) | 68 | 73 | 77 | 61 | 74 | 64 (61–68) | 67 | 54 | 57 | 65 | 59 | 59 (54–67) | 0.21 |
| Tumor site, H/B/T, n (%) | H | B | H | B | B | - | B | H | B | H | H | - | 0.55 |
| Tumor size (mm) at MDCT | |||||||||||||
| -short axis | 20.3 | 34.1 | 29.9 | 42.7 | 22.1 | 29.9 (20.3–42.7) | 64.5 | 21.9 | 29.7 | 34.7 | 33 | 33 (21.9–65.5) | 0.60 |
| -long axis | 33.8 | 58 | 44.7 | 50.1 | 32.3 | 44.7 (32.3–58) | 95.4 | 29.3 | 37.4 | 49.9 | 44.7 | 44.7 (29.3–95.4) | 1.00 |
| Tumor volume (cc) at MDCT | 12.14 | 31.34 | 25.29 | 35.97 | 10.15 | 25.3 (10.1–36) | 101.4 | 5.3 | 16.85 | 29.4 | 21.8 | 21.8 (5.3–101.4) | 0.92 |
| Tumor staging, BR/LA, n (%) | BR | LA | LA | LA | LA | - | LA | LA | LA | LA | BR | - | 1.00 |
| CA19.9 serum levels (U/mL) | 362 | 2495 | 43 | 602 | 281 | 362 (43–2495) | 3503 | 243.8 | 361 | 5192 | 20 | 361 (20–5192) | 0.92 |
| WBC serum levels (109/L) | 7.7 | 7.45 | 9.9 | 6.8 | 9.1 | 7.7 (6.8–9.9) | 11.5 | 7.46 | 6.7 | 9.9 | 7.6 | 7.9 (6.7–11.5) | 0.69 |
| PLT serum levels (109/L) | 316 | 215 | 253 | 259 | 361 | 259 (215–361) | 213 | 255 | 288 | 256 | 188 | 255 (188–325) | 0.23 |
| Neutrophils serum levels (109/L) | 4.9 | 3.99 | 6.5 | 4.6 | 6.7 | 4.9 (3.99–6.7) | 8.4 | 5.63 | 2 | 6.3 | 4.3 | 4.6 (2–8.4) | 0.99 |
| Lymphocytes serum levels (109/L) | 1.9 | 1.99 | 2.5 | 1.5 | 1.6 | 1.5 (1.9–2.5) | 2.1 | 1.2 | 3.2 | 2.3 | 2.1 | 2.15 (1.2–3.2) | 0.46 |
| Monocytes serum levels (109/L) | 0.6 | 0.83 | 0.8 | 0.6 | 0.7 | 0.7 (0.6–0.8) | 0.8 | 0.54 | 0.5 | 0.9 | 0.8 | 0.65 (0.5–0.9) | 0.98 |
| Neutrophil/Lymphocyte ratio | 2.58 | 2.01 | 2.6 | 3.07 | 4.19 | 2.6 (2–4.19) | 4 | 4.69 | 0.625 | 2.74 | 2.05 | 2.74 (0.62–4.69) | 0.92 |
| PLT/Lymphocyte ratio | 166.32 | 108.04 | 101.2 | 172.67 | 225.63 | 166.3 (101–226) | 101.43 | 212.5 | 90 | 111.3 | 89.52 | 101.43 (90–212.5) | 0.17 |
| Lymphocyte/Monocyte ratio | 3.17 | 2.397 | 3.13 | 2.5 | 2.29 | 2.5 (2.29–3.17) | 2.625 | 2.22 | 6.4 | 2.56 | 2.62 | 2.62 (2.22–6.4) | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testoni, S.G.G.; Minici, C.; Benetti, E.; Clemente, F.; Boselli, D.; Sciorati, C.; De Monte, L.; Petrone, M.C.; Enderle, M.; Linzenbold, W.; et al. Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 3704. https://doi.org/10.3390/cancers15143704
Testoni SGG, Minici C, Benetti E, Clemente F, Boselli D, Sciorati C, De Monte L, Petrone MC, Enderle M, Linzenbold W, et al. Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma. Cancers. 2023; 15(14):3704. https://doi.org/10.3390/cancers15143704
Chicago/Turabian StyleTestoni, Sabrina Gloria Giulia, Claudia Minici, Elisa Benetti, Francesca Clemente, Daniela Boselli, Clara Sciorati, Lucia De Monte, Maria Chiara Petrone, Markus Enderle, Walter Linzenbold, and et al. 2023. "Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma" Cancers 15, no. 14: 3704. https://doi.org/10.3390/cancers15143704
APA StyleTestoni, S. G. G., Minici, C., Benetti, E., Clemente, F., Boselli, D., Sciorati, C., De Monte, L., Petrone, M. C., Enderle, M., Linzenbold, W., Protti, M. P., Manfredi, A., De Cobelli, F., Reni, M., Falconi, M., Capurso, G., Arcidiacono, P. G., & Della-Torre, E. (2023). Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma. Cancers, 15(14), 3704. https://doi.org/10.3390/cancers15143704









