Can Bee Venom Be Used as Anticancer Agent in Modern Medicine?
Abstract
Simple Summary
Abstract
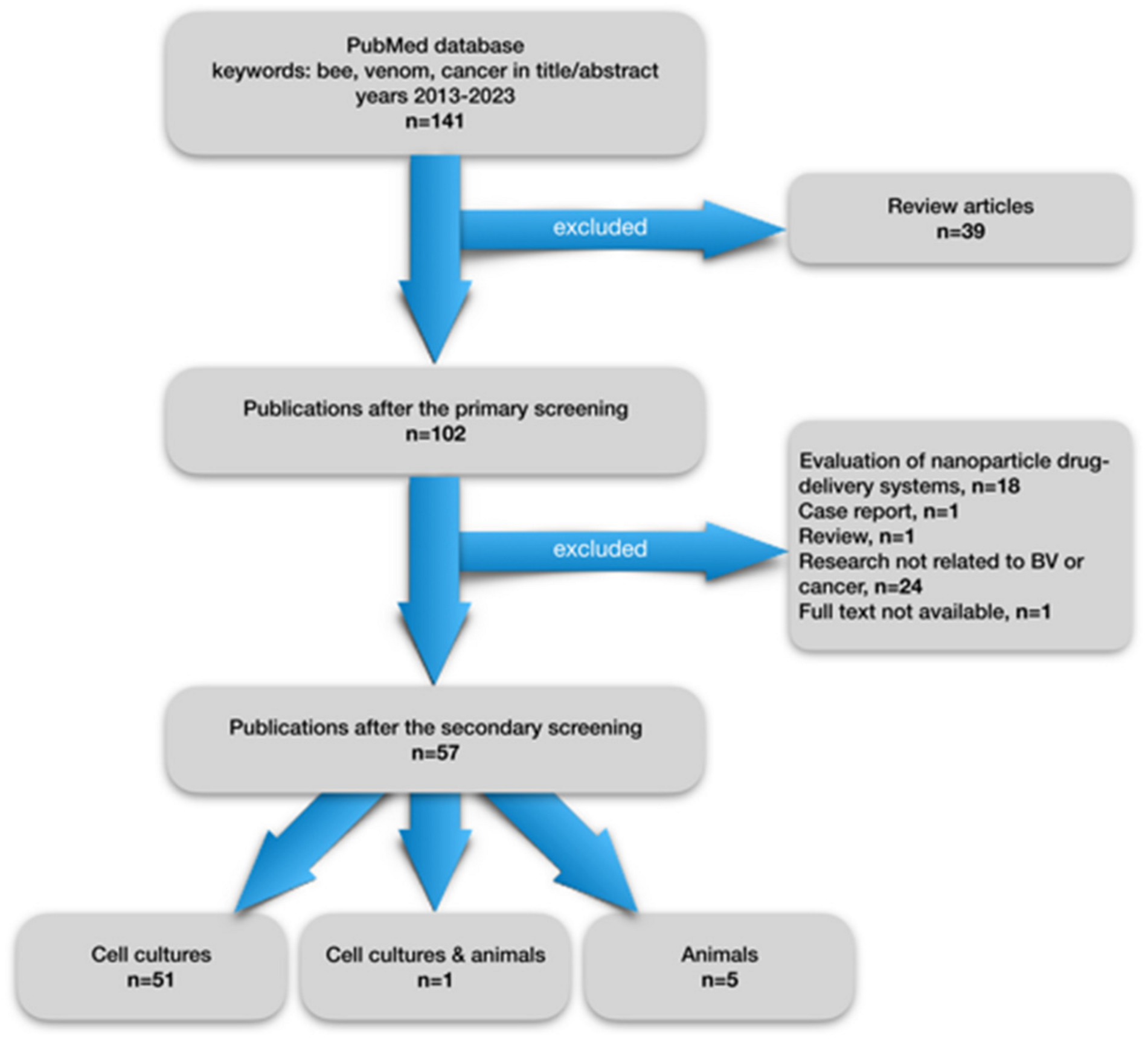
1. Introduction
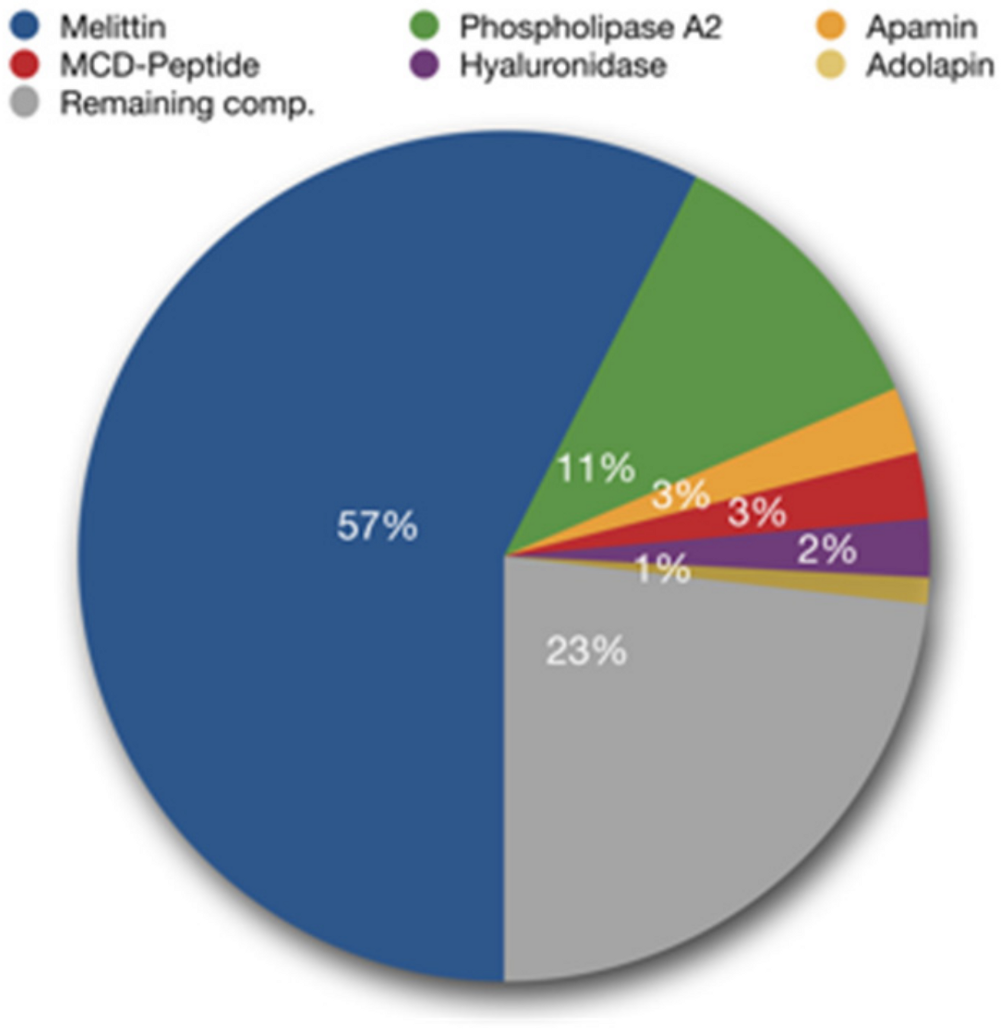
1.1. What Are the Components of Bee Venom?
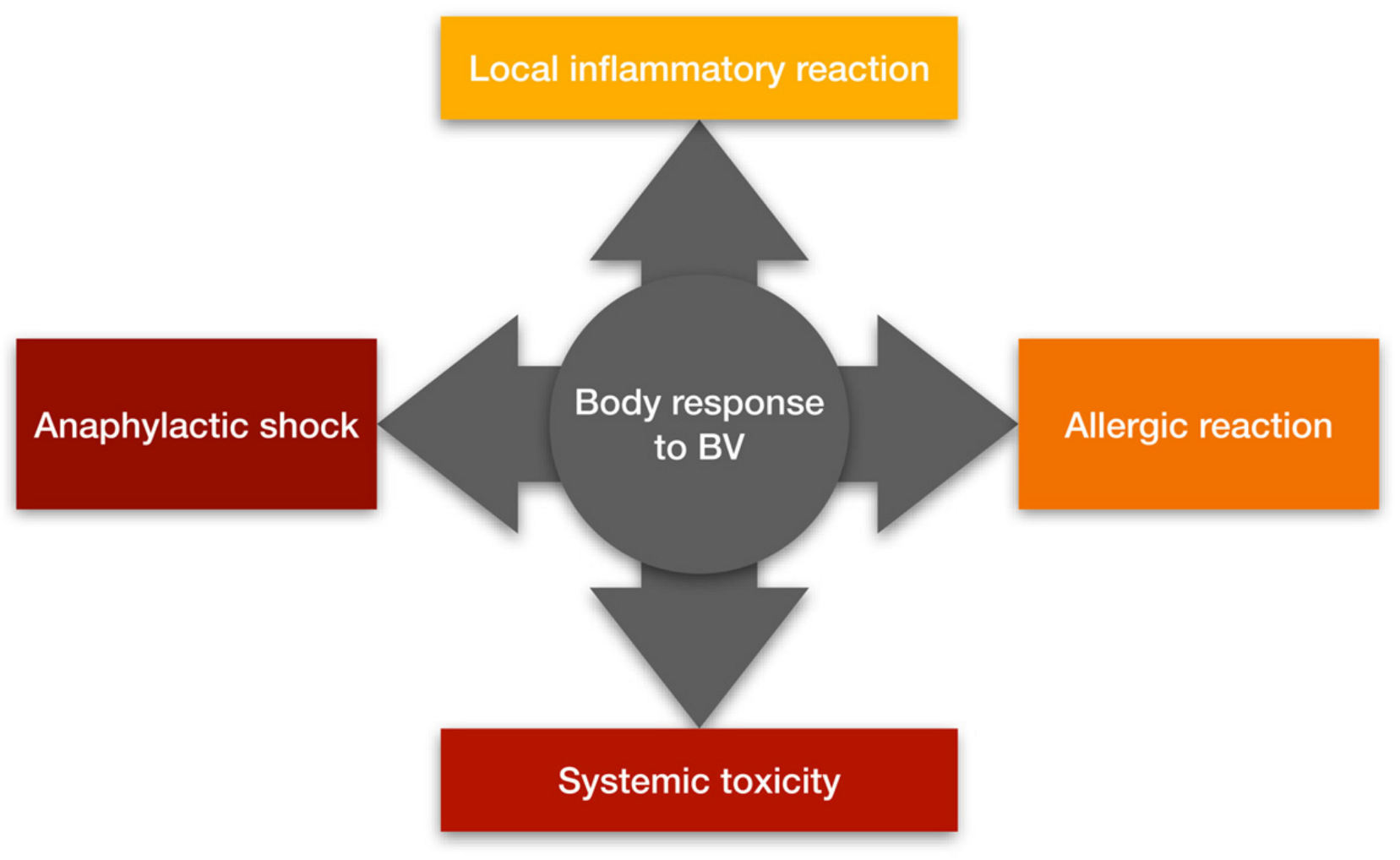
1.2. How Does the Body React to Bee Venom?
2. Can Bee Venom Cure Cancer?
Anti-Inflammatory, Antioxidative and Antimicrobial Effects of Bee Venom
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hardstone, M.C.; Scott, J.G. Is Apis mellifera more sensitive to insecticides than other insects? Pest. Manag. Sci. 2010, 66, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2021, 35, 743–750. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Bellik, Y. Bee Venom: Its Potential Use in Alternative Medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Pawlak, M.; Klupczynska, A.; Kokot, Z.J.; Matysiak, J. Extending Metabolomic Studies of Apis mellifera Venom: LC-MS-Based Targeted Analysis of Organic Acids. Toxins 2020, 12, 14. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. Stud. Nat. Prod. Chem. 2019, 60, 459–484. [Google Scholar] [CrossRef]
- Obeidat, M.; Al-Khraisat, I.F.; Jaradat, D.M.M.; Ghanim, B.Y.; Abdallah, Q.M.; Arqoub, D.A.; Sabbah, D.; Al-Sanabra, O.M.; Arafat, T.; Qinna, N.A. Melittin peptide quantification in seasonally collected crude bee venom and its anticancer effects on myelogenous K562 human leukaemia cell line. BMC Complement. Med. Ther. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukht, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Badawi, J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins 2021, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Samel, M.; Vija, H.; Kurvet, I.; Künnis-Beres, K.; Trummal, K.; Subbi, J.; Kahru, A.; Siigur, J. Interactions of PLA2-s from Vipera lebetina, Vipera berus berus and Naja naja oxiana Venom with Platelets, Bacterial and Cancer Cells. Toxins 2013, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Bee Venom: Composition, Health, Medicine: A Review. 2017. Available online: www.bee-hexagon.net (accessed on 5 June 2023).
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef]
- De Toledo, L.F.M.; Moore, D.C.B.C.; da Caixeta, D.M.L.; Salú, M.D.S.; Farias, C.V.B.; de Azevedo, Z.M.A. Multiple bee stings, multiple organs involved: A case report. Rev. Soc. Bras. Med. Trop. 2018, 51, 560–562. [Google Scholar] [CrossRef]
- Ediger, D.; Terzioglu, K.; Ozturk, R.T. Venom allergy, risk factors for systemic reactions and the knowledge levels among Turkish beekeepers. Asia Pac. Allergy 2018, 8, e15. [Google Scholar] [CrossRef]
- Hymenoptera Allergy Committee of the SEAIC; Alfaya Arias, T.; Soriano Gómis, V.; Soto Mera, T.; Vega Castro, A.; Vega Gutiérrez, J.; Dominguez Noche, C.; Gutierrez Fernandez, D.; Marques Amat, L.; Martinez Arcediano, A.; et al. Key issuesin hymenoptera venom allergy: An update. J. Investig. Allergol. Clin. Immunol. 2017, 27, 19–31. [Google Scholar] [CrossRef]
- Fitzgerald, K.T.; Flood, A.A. Hymenoptera Stings. Clin. Tech. Small Anim. Pract. 2006, 21, 194–204. [Google Scholar] [CrossRef]
- Fan, H.W.; Kalil, J. Massive bee envenomation. In Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 2627–2636. [Google Scholar]
- Nisahan, B.; Selvaratnam, G.; Kumanan, T. Myocardial injury following multiple bee stings. Trop. Doct. 2014, 44, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Revuelta, P.; Madrigal-Burgaleta, R. Death due to Live Bee Acupuncture Apitherapy. J. Investig. Allergol. Clin. Immunol. 2018, 28, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenom Da-Hyun ng Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Havas, L.J. Effect of bee venom on colchicine-induced tumours. Nature 1950, 166, 567–568. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.A.; Li, F.P.; Mehta, C.R. Cancer mortality among beekeepers. J. Occup. Med. 1979, 21, 811–813. [Google Scholar]
- Tetikoğlu, S.; Çelik-Uzuner, S. Bee Venom Induces the Interaction between Phosphorylated Histone Variant, H2AX, and the Intracellular Site of beta-Actin in Liver and Breast Cancer Cells. Chem. Biodivers. 2023, 25, e202300401. [Google Scholar] [CrossRef]
- Sevin, S.; Deveci Ozkan, A.; Tutun, H.; Kivrak, I.; Turna, O.; Guney Eskiler, G. Determination of the Effects of Bee Venom on Triple Negative Breast Cancer Cells in Vitro. Chem. Biodivers. 2023, 20, e202201263. [Google Scholar] [CrossRef]
- Hwang, Y.N.; Kwon, I.S.; Na, H.H.; Park, J.S.; Kim, K.C. Dual Cytotoxic Responses Induced by Treatment of A549 Human Lung Cancer Cells with Sweet Bee Venom in a Dose-Dependent Manner. J. Pharmacopunct. 2022, 25, 390–395. [Google Scholar] [CrossRef]
- Yu, J.E.; Kim, Y.; Hong, D.E.; Lee, D.W.; Chang, J.Y.; Yoo, S.S.; Kim, M.J.; Son, D.J.; Yun, J.; Han, S.B.; et al. Bee Venom Triggers Autophagy-Induced Apoptosis in Human Lung Cancer Cells via the mTOR Signaling Pathway. J. Oncol. 2022, 2022, 8916464. [Google Scholar] [CrossRef]
- Sevin, S.; Kivrak, İ.; Tutun, H.; Uyar, R.; Ayaz, F. Apis mellifera anatoliaca Venom Exerted Anti-Inflammatory Activity on LPS-Stimulated Mammalian Macrophages by Reducing the Production of the Inflammatory Cytokines. Appl. Biochem. Biotechnol. 2023, 195, 3194–3205. [Google Scholar] [CrossRef]
- Ertilav, K.; Nazıroğlu, M. Honey bee venom melittin increases the oxidant activity of cisplatin and kills human glioblastoma cells by stimulating the TRPM2 channel. Toxicon 2023, 222, 106993. [Google Scholar] [CrossRef]
- Erkoc, P.; von Reumont, B.M.; Lüddecke, T.; Henke, M.; Ulshöfer, T.; Vilcinskas, A.; Fürst, R.; Schiffmann, S. The Pharmacological Potential of Novel Melittin Variants from the Honeybee and Solitary Bees against Inflammation and Cancer. Toxins 2022, 14, 818. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Li, Z.; Meng, Y.Q.; Huang, S.J.; Yu, Q.Y.; Li, B. Melittin induces ferroptosis and ER stress-CHOP mediated apoptosis in A549 cells. Free Radic. Res. 2022, 56, 398–410. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, W.; Zhang, Z.; Zhou, Z.; Duan, J.; Dong, Z.; Liu, H.; Yan, C. Bee venom protects against pancreatic cancer via inducing cell cycle arrest and apoptosis with suppression of cell migration. J. Gastrointest. Oncol. 2022, 13, 847–858. [Google Scholar] [CrossRef]
- Duarte, D.; Falcão, S.I.; El Mehdi, I.; Vilas-Boas, M.; Vale, N. Honeybee Venom Synergistically Enhances the Cytotoxic Effect of CNS Drugs in HT-29 Colon and MCF-7 Breast Cancer Cell Lines. Pharmaceutics 2022, 14, 511. [Google Scholar] [CrossRef]
- Małek, A.; Kocot, J.; Mitrowska, K.; Posyniak, A.; Jacek Kurzepa, J. Bee Venom Effect on Glioblastoma Cells Viability and Gelatinase Secretion. Front. Neurosci. 2022, 16, 792970. [Google Scholar] [CrossRef]
- Yaacoub, C.; Wehbe, R.; Salma, Y.; El-Obeid, D.; El Bersaoui, R.; Coutard, B.; Fajloun, Z. Apis mellifera syriaca Venom: Evaluation of Its Anticoagulant Effect, Proteolytic Activity, and Cytotoxicity along with Its Two Main Compounds-MEL and PLA2-On HeLa Cancer Cells. Molecules 2022, 27, 1653. [Google Scholar] [CrossRef]
- Lischer, K.; Sitorus, S.R.A.; Guslianto, B.W.; Avila, F.; Khayrani, A.C.; Sahlan, M. Anti- Breast Cancer Activity on MCF-7 Cells of Melittin from Indonesia’s Apis cerana: An In Vitro Study. Asian Pac. J. Cancer Prev. 2021, 22, 3913–3919. [Google Scholar] [CrossRef]
- Gasanoff, E.; Liu, Y.; Li, F.; Hanlon, P.; Garab, G. Bee Venom Melittin Disintegrates the Respiration of Mitochondria in Healthy Cells and Lymphoblasts, and Induces the Formation of Non-Bilayer Structures in Model Inner Mitochondrial Membranes. Int. J. Mol. Sci. 2021, 22, 11122. [Google Scholar] [CrossRef]
- Mansour, G.H.; El-Magd, M.A.; Mahfouz, D.H.; Abdelhamid, I.A.; Mohamed, M.F.; Ibrahim, N.S.; Hady AAbdel Wahab, A.; Elzayat, E.M. Bee venom and its active component Melittin synergistically potentiate the anticancer effect of Sorafenib against HepG2 cells. Bioorganic Chem. 2021, 116, 105329. [Google Scholar] [CrossRef]
- Huang, J.Y.; Peng, S.F.; Chueh, F.S.; Chen, P.Y.; Huang, Y.P.; Huang, W.W.; Chung, J.G. Melittin suppresses epithelial-mesenchymal transition and metastasis in human gastric cancer AGS cells via regulating Wnt/BMP associated pathway. Biosci. Biotechnol. Biochem. 2021, 85, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Lebel, A.A.; Kisembo, M.V.; Soucy, M.N.; Hébert, M.P.A.; Morin, P.J.; Boudreau, L.H. Molecular characterization of the anticancer properties associated with bee venom and its components in glioblastoma multiforme. Chem. Biol. Interact. 2021, 347, 109622. [Google Scholar] [CrossRef] [PubMed]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.M.; Coutard, B.; Fajloun, Z. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components-Melittin and PLA2-On Colon Cancer HCT116 Cell Lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef]
- Borojeni, S.K.; Zolfagharian, H.; Babaie, M.; Javadi, I. Cytotoxic Effect of Bee (A. mellifera) Venom on Cancer Cell Lines. J. Pharmacopunct. 2020, 23, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kreinest, T.; Volkmer, I.; Staege, M.S. Melittin Increases Cisplatin Sensitivity and Kills KM-H2 and L-428 Hodgkin Lymphoma Cells. Int. J. Mol. Sci. 2020, 22, 343. [Google Scholar] [CrossRef]
- Grawish, M.E.; Mourad, M.I.; Esmaeil, D.A.; Ahmed, R.A.; Ateia, I.M.; Hany, E.; Elkhier, M.T.A. Emerging therapeutic modality enhancing the efficiency of chemotherapeutic agents against head and neck squamous cell carcinoma cell lines. Cancer Treat. Res. Commun. 2020, 25, 100242. [Google Scholar] [CrossRef]
- Sangboonruang, S.; Kitidee, K.; Chantawannakul, P.; Tragoolpua, K.; Tragoolpua, Y. Melittin from Apis florea Venom as a Promising Therapeutic Agent for Skin Cancer Treatment. Antibiotics 2020, 9, 517. [Google Scholar] [CrossRef]
- Salama, M.A.; Younis, M.A.; Talaat, R.M. Cytokine and inflammatory mediators are associated with cytotoxic, anti-inflammatory and apoptotic activity of honeybee venom. J. Complement. Integr. Med. 2020, 18, 75–86. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.W.; Park, H.W.; Lee, H.W.; Chun, K.H. Bee venom inhibits the proliferation and migration of cervical-cancer cells in an HPV E6/E7-dependent manner. BMB Rep. 2020, 53, 419–424. [Google Scholar] [CrossRef]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin—A Natural Peptide from Bee Venom which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, Y.Y.; Park, K.K.; Choi, Y.H.; Kim, C.H.; Chang, Y.C. Bee Venom Suppresses EGF-Induced Epithelial-Mesenchymal Transition and Tumor Invasion in Lung Cancer Cells. Am. J. Chin. Med. 2019, 47, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, e0224028. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic Effects of Melittin and Plasma Treatment: A Promising Approach for Cancer Therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef]
- Shiassi Arani, F.; Karimzadeh, L.; Ghafoori, S.M.; Nabiuni, M. Antimutagenic and Synergistic Cytotoxic Effect of Cisplatin and Honey Bee Venom on 4T1 Invasive Mammary Carcinoma Cell Line. Adv. Pharmacol. Sci. 2019, 2019, 7581318. [Google Scholar] [CrossRef]
- Jung, G.B.; Huh, J.E.; Lee, H.J.; Kim, D.; Lee, G.J.; Park, H.K.; Lee, J.D. Anti-cancer effect of bee venom on human MDA-MB-231 breast cancer cells using Raman spectroscopy. Biomed. Opt. Express 2018, 9, 5703–5718. [Google Scholar] [CrossRef]
- Zarrinnahad, H.; Mahmoodzadeh, A.; Hamidi, M.P.; Mahdavi, M.; Moradi, A.; Bagheri, K.P.; Shahbazzadeh, D. Apoptotic Effect of Melittin Purified from Iranian Honey Bee Venom on Human Cervical Cancer HeLa Cell Line. Int. J. Pept. Res. Ther. 2018, 24, 563–570. [Google Scholar] [CrossRef]
- Khamis, A.A.A.; Ali, E.M.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.M.; El-Magd, M.A.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar] [CrossRef]
- Mohseni-Kouchesfahani, H.; Nabioni, M.; Khosravi, Z.; Rahimi, M. Honey bee venom combined with 1,25-dihydroxyvitamin D3 as a highly efficient inducer of differentiation in human acute myeloid leukemia cells. J. Cancer Res. Ther. 2017, 13, 544–549. [Google Scholar]
- Zhang, S.F.; Chen, Z. Melittin exerts an antitumor effect on non-small cell lung cancer cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef]
- Alonezi, S.; Tusiimire, J.; Wallace, J.; Dufton, M.J.; Parkinson, J.A.; Young, L.C.; Clements, C.J.; Park, J.K.; Jeon, J.W.; Ferro, V.A.; et al. Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, F.; Tan, J.; Peng, X.; Sun, L.; Wang, P.; Jia, S.; Yu, Q.; Huo, H.; Zhao, H. Melittin inhibits the invasion of MCF-7 cells by downregulating CD147 and MMP-9 expression. Oncol. Lett. 2017, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Alonezi, S.; Tusiimire, J.; Wallace, J.; Dufton, M.J.; Parkinson, J.A.; Young, L.C.; Clements, C.J.; Park, J.K.; Jeon, J.W.; Ferro, V.A.; et al. Metabolomic Profiling of the Effects of Melittin on Cisplatin Resistant and Cisplatin Sensitive Ovarian Cancer Cells Using Mass Spectrometry and Biolog Microarray Technology. Metabolites 2016, 6, 35. [Google Scholar] [CrossRef]
- Drigla, F.; Balacescu, O.; Visan, S.; Bisboaca, S.E.; Berindan-Neagoe, I.; Marghitas, L.A. Synergistic Effects Induced by Combined Treatments of Aqueous Extract of Propolis and Venom. Clujul Med. 2016, 89, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Osmak, M.; Garaj-Vrhovac, V. Antitumour action on human glioblastoma A1235 cells through cooperation of bee venom and cisplatin. Cytotechnology 2016, 68, 1197–1205. [Google Scholar] [CrossRef]
- Zheng, J.; Lee, H.L.; Ham, Y.W.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 2015, 6, 44437–44451. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Zarrinnahad, H.; Bagheri, K.P.; Moradia, A.; Shahbazzadeh, D. First report on the isolation of melittin from Iranian honey bee venom and evaluation of its toxicity on gastric cancer AGS cells. J. Chin. Med. Assoc. 2015, 78, 574–583. [Google Scholar] [CrossRef]
- Kim, Y.W.; Chaturvedi, P.K.; Chun, S.N.; Lee, Y.G.; Ahn, W.S. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncol. Rep. 2015, 33, 1675–1682. [Google Scholar] [CrossRef]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Liu, J.; Ge, Y.; Qin, Q.; Lu, J.; Zhan, L.; Liu, Z.; Zhang, H.; Chen, X.; et al. Melittin radiosensitizes esophageal squamous cell carcinoma with induction of apoptosis in vitro and in vivo. Tumour Biol. 2014, 35, 8699–8705. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, B.; Huang, C.; Meng, X.M.; Bian, E.B.; Li, J. Melittin restores PTEN expression by down-regulating HDAC2 in human hepatocelluar carcinoma HepG2 cells. PLoS ONE 2014, 9, e95520. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Choi, Y.; Shin, J.M.; Cho, H.J.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Bae, Y.S.; Han, S.M.; Kim, C.H.; et al. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, M.M.; Marin-Morales, M.A. Anti-genotoxicity and anti-mutagenicity of Apis mellifera venom. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 762, 43–48. [Google Scholar] [CrossRef]
- Kollipara, P.S.; Kim, J.H.; Won, D.; Lee, S.M.; Sung, H.C.; Chang, H.S.; Lee, K.T.; Lee, K.S.; Park, M.H.; Song, M.J.; et al. Co-culture with NK-92MI cells enhanced the anti- cancer effect of bee venom on NSCLC cells by inactivation of NF-κB. Arch. Pharm. Res. 2014, 37, 379–389. [Google Scholar] [CrossRef]
- Safaeinejad, Z.; Nabiuni, M.; Nazari, Z. Potentiation of a novel palladium (II) complex lethality with bee venom on the human T-cell acute lymphoblastic leukemia cell line (MOLT-4). J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Jeong, Y.J.; Cho, H.J.; Park, K.K.; Chung, I.K.; Lee, I.K.; Kwak, J.Y.; Chang, H.W.; Kim, C.H.; Moon, S.K.; et al. Melittin suppresses HIF-1α/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS ONE 2013, 8, e69380. [Google Scholar] [CrossRef]
- Rocha, M.M.; Dariva, I.; Zornoff, G.C.; De Laurentis, G.S.; Mendes, G.C.; Santana, M.G.; de Miguel, G.C.; Ferreira, R.S.; Sciani, J.M.; Priolli, D.G. A new therapeutic approach for bone metastasis in colorectal cancer: Intratumoral melittin. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210067. [Google Scholar] [CrossRef]
- El-Beltagy, A.E.B.M.; Elsyyad, H.I.H.; Abdelaziz, K.K.; Madany, A.S.; Elghazaly, M.M. Therapeutic Role of Annona muricata Fruit and Bee Venom Against MNU- Induced Breast Cancer in Pregnant Rats and its Complications on the Ovaries. Breast Cancer 2021, 13, 431–445. [Google Scholar] [CrossRef]
- El Bakary, N.M.; Alsharkawy, A.Z.; Shouaib, Z.A.; Barakat, E.M.S. Role of Bee Venom and Melittin on Restraining Angiogenesis and Metastasis in γ-Irradiated Solid Ehrlich Carcinoma-Bearing Mice. Integr. Cancer Ther. 2020, 19, 1534735420944476. [Google Scholar] [CrossRef]
- Lee, C.; Bae, S.S.; Joo, H.; Bae, H. Melittin suppresses tumor progression by regulating tumor-associated macrophages in a Lewis lung carcinoma mouse model. Oncotarget 2017, 8, 54951–54965. [Google Scholar] [CrossRef]
- Lee, H.L.; Park, S.H.; Kim, T.M.; Jung, Y.Y.; Park, M.H.; Oh, S.H.; Yun, H.S.; Jun, H.O.; Yoo, H.S.; Han, S.B.; et al. Bee venom inhibits growth of human cervical tumors in mice. Oncotarget 2015, 6, 7280–7292. [Google Scholar] [CrossRef]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- Melero, I.; Castanon, E.; Alvarez, M.; Champiat, S.; Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021, 18, 558–576. [Google Scholar] [CrossRef]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Kim, M.K.; Han, S.M.; Park, K.K. Anti-Inflammatory Effect of Melittin on Porphyromonas Gingivalis LPS-Stimulated Human Keratinocytes. Molecules 2018, 23, 332. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.H.; Yang, S.C.; Lee, S.M.; Choi, S.M. Melittin restores proteasome function in an animal model of ALS. J. Neuroinflamm. 2011, 8, 69. [Google Scholar] [CrossRef]
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef]
- Lee, W.R.; Pak, S.C.; Park, K.K. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins 2015, 7, 4758–4772. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.H.; Kim, K.H.; Lee, W.R.; Chang, Y.C.; Park, K.K.; Lee, K.G.; Han, S.M.; Yeo, J.H.; Pak, S.C. Bee venom inhibits hepatic fibrosis through suppression of pro-fibrogenic cytokine expression. Am. J. Chin. Med. 2010, 38, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, Y.W.; Kim, H.; Chung, D.K. Bee venom alleviates atopic dermatitis symptoms through the upregulation of decay-accelerating factor (DAF/CD55). Toxins 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Nascimento de Souza, R.; Silva, F.K.; Alves de Medeiros, M. Bee Venom Acupuncture Reduces Interleukin-6, Increases Interleukin-10, and Induces Locomotor Recovery in a Model of Spinal Cord Compression. J. Acupunct. Meridian Stud. 2017, 10, 204–210. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First characterization of the venom from Apis mellifera syriaca, a honeybee from the Middle East region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef]
- Abd El-Hakam, F.E.; Abo Laban, G.; Badr El-Din, S.; Abd El-Hamid, H.; Farouk, M.H. Apitherapy combination improvement of blood pressure, cardiovascular protection, and antioxidant and anti-inflammatory responses in dexamethasone model hypertensive rats. Sci. Rep. 2022, 12, 20765. [Google Scholar] [CrossRef]
- Dartnell, L.R. Ionizing radiation and life. Astrobiology 2011, 11, 551–582. [Google Scholar] [CrossRef] [PubMed]
- El Adham, E.K.; Hassan, A.I.; ADawoud, M.M. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 2022, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.; Lee, G. Neuroprotective Activity of Melittin-The Main Component of Bee Venom-Against Oxidative Stress Induced by Aβ25-35 in In Vitro and In Vivo Models. Antioxidants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Jamasbi, E.; Batinovic, S.; Sharples, R.A.; Sani, M.A.; Robins-Browne, R.M.; Wade, J.D.; Separovic, F.; Hossain, M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 2014, 46, 2759–2766. [Google Scholar] [CrossRef]
- Dosler, S.; Gerceker, A.A. In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 2012, 24, 137–143. [Google Scholar] [CrossRef]
- Socarras, K.M.; Theophilus, P.A.S.; Torres, J.P.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef]
- McGhee, S.; Visovksy, C.; Zambroski, C.; Finnegan, A. Lyme disease: Recognition and management for emergency nurses. Emerg. Nurse 2018, 26, 17–34. [Google Scholar] [CrossRef]
- Da Mata, É.C.; Mourão, C.B.; Rangel, M.; Schwartz, E.F. Antiviral activity of animal venom peptides and related compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.B.; Lee, B.H.; Nikapitiya, C.; Kim, J.H.; Kim, T.H.; Lee, H.C.; Kim, C.J.; Lee, J.-S.; Kim, C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Tarallo, R.; Russo, L.; Galdiero, E.; Cantisani, M.; Morelli, G.; Galdiero, M. Peptide inhibitors against herpes simplex virus infections. J. Pept. Sci. 2013, 19, 148–158. [Google Scholar] [CrossRef]
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Niedbała, G.; Alqarni, M.; Zirintunda, G.; Ssempijja, F.; Musinguzi, S.P.; Usman, I.M.; Matama, K.; Hetta, H.F.; Mbiydzenyuy, N.E.; et al. Bee Venom—A Potential Complementary Medicine Candidate for SARS-CoV-2 Infections. Front. Public. Health 2020, 8, 594458. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cel development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Caramalho, I.; Melo, A.; Pedro, E.; Barbosa, M.M.P.; Victorino RM, M.; Pereira Santos, M.C.; Sousa, A.E. Bee venom enhances thdifferentiation of human regulatory T cells. Allergy 2015, 70, 1340–1345. [Google Scholar] [CrossRef]
| No. | Author | Year | Cell Culture | Species | BV Component or Crude BV | Molecular Mechanism of Acting | Effect |
|---|---|---|---|---|---|---|---|
| 1 | Tetikoğlu [28] | 2023 | Breast cancer | Human | BV | Interaction of γH2AX and β-actin | Genotoxicity |
| 2 | Obeidat [11] | 2023 | Leukemia | Human | BV, melittin | Modulation NF-κB and MAPK pathway, CDK4 inhibition | Apoptosis, necrosis, cell cycle arresting |
| 3 | Sevin [29] | 2023 | TNBC | Human | BV | Unknown | Apoptosis |
| 4 | Hwang [30] | 2023 | Lung cancer | Human | BV | Modulation of expression PARP, caspase-9, p53, Bcl2, Box | Cell death, cell cycle arresting |
| 5 | Yu [31] | 2023 | Lung cancer Glioblastoma TNBC Liver cancer | Human | BV | Inhibition of mTOR pathway | Autophagy induction |
| 6 | Sevin [32] | 2023 | Glioblastoma | Human | BV | Decrease of pro-inflammatory cytokine levels | Lack of cytotoxic effect |
| 7 | Ertilav [33] | 2023 | Glioblastoma | Human | Melittin | Stimulation of TRPM2 channel, prooxidative effect | Apoptosis |
| 8 | Erkoc [34] | 2022 | TNBC | Human | Melittin | Induction of calcium signaling apoptosis, inhibition of cAMP | Apoptosis, anti-proliferative effect |
| 9 | Li [35] | 2022 | Lung cancer | Human | Melittin | Upregulation of ROS production, increasing of intracellular ferrum level, disruption of GPx4, mitochondria damage | Apoptosis (ferroptosis) |
| 10 | Zhao [36] | 2022 | Pancreatic cancer | Human | BV | Modulation of cyclins and cyclin-dependent kinases (CDKs) expression, p53-p21 pathway activation | Apoptosis, cell cycle arresting |
| 11 | Duarte [37] | 2022 | Colon cancer Breast cancer | Human | BV | Unknown | Cytotoxic together with 5-FU and fluphenazine |
| 12 | Małek [38] | 2022 | Glioblastoma | Human | BV | Reduction of MMP2 and MMP9 secretion | Cytotoxic |
| 13 | Yaacoub [39] | 2022 | Cervical cancer | Human | BV, melittin | Unknown | Cytotoxic |
| 14 | Lischer [40] | 2021 | Breast cancer | Human | Melittin | Unknown | Cytotoxic |
| 15 | Gasanoff [41] | 2021 | Leukemia | Human | Melittin | Melittin-induced decline of mitochondrial bioenergetics | Cytotoxic |
| 16 | Mansour [42] | 2021 | Hepatocellular carcinoma | Human | BV, melittin | Upregulation of p53, Bax, Cas3, Cas7, PTEN. Downregulation Bcl-2, Cyclin-D1, Rac1, Nf-κB, HIF-1a, VEGF, MMP9. Oxidative stress induction. | Cell cycle arresting, apoptosis |
| 17 | Huang [43] | 2021 | Gastric cancer | Human | Melittin | MMP2 and MMP9 activity inhibition, decreasing of Wnt/BMP and MMP-2 signaling pathway activity. Inhibition of adhesion molecules. | Cytotoxic, adhesion and invasion inhibition |
| 18 | Lebel [44] | 2021 | Glioblastoma | Human | BV, melittin | Influence on Bak, Bax and Cas3 | Apoptosis, necrosis |
| 19 | Yaacoub [45] | 2021 | Colon cancer | Human | BV, melittin, PLA2 | Unknown | Synergistic activity of melittin and PLA2, cytotoxic |
| 20 | Borojeni [46] | 2020 | Cervical cancer Breast cancer | Human | BV | Unknown | Apoptosis |
| 21 | Kreinest [47] | 2020 | Hodgkin Lymphoma | Human | Melittin | Unknown | Cytotoxic, increase sensitivity of cisplatin |
| 22 | Grawish [48] | 2020 | Head and neck squamous cell carcinoma | Human | BV | Upregulation of Bax, downregulation of Bcl2 and EGFR, influence on cell cycle | Cytotoxic, cell cycle arresting, increasing of cisplatin activity |
| 23 | Sangboonruang [49] | 2020 | Malignant melanoma | Human | Melittin | Upregulation of cytochrome c and its translocation of cytosol, up regulation of Cas3 and Cas9. Reduction of EGFR expression. | Apoptosis |
| 24 | Salama [50] | 2020 | Liver carcinoma Breast cancer Cervical cancer | Human | BV | Influence on IL-10, TNF, IFN-γ. Elevation of Cas3 level. | Apoptosis |
| 25 | Kim [51] | 2020 | Cervical cancer | Human | BV | Increase in p53, p21, p27, Bax. Decrease in cyclin A, cyclin B, Bcl-2, Bcl-XL. Influence on caspases and intercellular signaling pathways. | Cytotoxic to HPVpositive cervical-cancer cell lines |
| 26 | Ceremuga [52] | 2020 | Leukemia | Human | BV | Effect on mitochondrial membrane potential, Annexin V binding and Caspases 3/7 activity | Apoptosis |
| 27 | Jeong [53] | 2019 | Non-small cell lung cancer | Human | BV | Inhibition of EGF-induced F-actin reorganization and cell invasion, inhibited EGF-induced ERK, JNK, FAK and mTOR phosphorylation | Cytotoxic |
| 28 | Soliman [54] | 2019 | Gastric cancer Colon cancer | Human | Melittin | Membrane affecting | Cytotoxic |
| 29 | Shaw [55] | 2019 | Breast cancer Malignant melanoma | Human | Melittin | Synergic effect with cold atmospheric plasma | Cytotoxic |
| 30 | Lim [56] | 2019 | Malignant melanoma | Human | BV, melittin | Inhibition of PI3K/AKT/mTOR and MAPK pathways. Upregulation of Cas3, Cas9. | Apoptosis, inhibition of migration and invasion |
| 31 | Shiassi Arani [57] | 2019 | Breast cancer | Mouse | BV | Unknown | Cytotoxic, synergy with cisplatin |
| 32 | Jung [58] | 2018 | TNBC | Human | BV | Reduction of Cas8, Cas9, Cas3 and PARP expression. Effect of cell morphology, DNA and protein fragmentation. | Apoptosis |
| 33 | Zarrinnahad [59] | 2018 | Cervical cancer | Human | Melittin | Unknown | Apoptosis |
| 34 | Khamis [60] | 2018 | Breast cancer | Human | BV | Upregulation of Bax, downregulation of Bcl2, EGFR, ERα. Influence of cell cycle. | Cytotoxic, synergy with hesperidin and piperine |
| 35 | Mohseni-Kouchesfahani [61] | 2017 | Acute myeloid leukemia | Human | BV | Unknown | Cytotoxic |
| 36 | Zhang [62] | 2017 | Non-small cell lung cancer | Human | Melittin | Decreasing of HIF-1α and VEGF level | Apoptosis, migration inhibiting |
| 37 | Alonezi [63] | 2017 | Ovarian cancer | Human | Melittin | Reduction in the levels of metabolites in TCA cycle, oxidative phosphorylation, purine and pyrimidine metabolism, and the arginine/proline pathway | Cytotoxic, synergy with cisplatin |
| 38 | Wang [64] | 2017 | Breast cancer | Human | Melittin | Downregulating CD147 and MMP-9 expression | Inhibition of migration and invasion |
| 39 | Alonezi [65] | 2016 | Ovarian cancer | Human | Melittin | Reduction in amino acids in the proline/glutamine/arginine pathway. Decreased levels of carnitines, polyamines, adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+). | Cytotoxic |
| 40 | Drigla [66] | 2016 | Breast cancer | Human | BV | Unknown | Antiproliferative |
| 41 | Gajski [67] | 2016 | Glioblastoma | Human | BV | Unknown | Cytotoxic, synergy with cisplatin |
| 42 | Zheng [68] | 2015 | Colon cancer | Human | BV | Increasing in DR4, DR5, p53, p21, Bax, cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9 expression. NF-κB inhibition. | Apoptosis, growth inhibition |
| 43 | Mahmoodzadeh [69] | 2015 | Gastric cancer | Human | Melittin | Unknown | Necrosis |
| 44 | Kim [70] | 2015 | Cervical cancer | Human | BV | Inhibition of HPV E6 and E7 expression | Cytotoxic, antiviral |
| 45 | Choi [71] | 2014 | Non-small cell lung cancer | Human | BV | Increasing in DR3 expression. NF-κB pathway inhibition. | Apoptosis |
| 46 | Zhu [72] | 2014 | Esophageal squamous cell carcinoma | Human | Melittin | Influence of Tax and Bcl-2 proteins. Lack influence of cell cycle. | Apoptosis, radiosensitization of cells |
| 47 | Zhang [73] | 2014 | Liver cancer | Human | Melittin | CyclinD1 and CDK4 downregulation. Upregulation of PTEN. Attenuation of HDAC2 expression. PI3K/Akt signaling pathways inhibition. | Apoptosis |
| 48 | Jeong [74] | 2014 | Breast cancer | Human | Melittin | Inhibition of EGF-induced MMP-9 expression. Inhibition of NF-κB and PI3K/Akt/mTOR pathway. Inhibition mTOR/p70S6K/4E-BP1 pathway. | Apoptosis, inhibition of migration |
| 49 | Hoshina [75] | 2014 | Liver immortal cells | Human | BV | Unknown | Induction of genotoxicity and mutagenicity in human cells |
| 50 | Kollipara [76] | 2014 | Non-small cell lung cancer | Human | BV | Increasing in DR3, DR6, Fas, Bax, cleaved caspase-3, cleaved caspase-8 | Enhancement of cytotoxicity against tumor of natural killer cells, apoptosis |
| 51 | Safaeinejad [77] | 2014 | Leukemia | Human | BV | Morphological changes, caspase-3-independent apoptosis | Potentiation of a novel palladium (II) complex, anti-proliferative, apoptosis |
| 52 | Shin [78] | 2013 | Cervical cancer | Human | Melittin | Decreasing of VEGF secretion, HIF-1ɑ inhibition | Inhibition of angiogenesis |
| No. | Author | Year | Cancer | Species | BV Component or Crude BV | Mechanism of Acting | Effect |
|---|---|---|---|---|---|---|---|
| 1 | Rocha [79] | 2022 | Colorectal cancer | Mouse | Melittin | Unknown | Inhibition of metastasis growth |
| 2 | El-Beltagy [80] | 2021 | Ovarian cancer Breast cancer | Rat | BV | Decreasing of serum MMP1, NF-κB, and TNF. Increasing in caspase 3. Influence on MDA, SOD, CAT. | Restoration of histological changes |
| 3 | El Bakary [81] | 2020 | Ehrlich ascites carcinoma | Mouse | BV, melittin | Decrease of Cas3, MMP2 and MMP9 activities. Decrease of TNF, VEGF, and NO levels. | Suppression of tumor proliferation, inhibition of angiogenesis |
| 4 | Lee [82] | 2017 | Lung carcinoma | Mouse | Melittin | Decrease the macrophage count in tumor environment, reduction of VEGF and CD206 expression in bone marrow-derived M2 macrophages | Reduction of tumor size, antiangiogenic effect |
| 5 | Zhang [62] | 2017 | Non-small cell lung cancer | Mouse | Melittin | Unknown | Inhibition of tumor growth |
| 6 | Lee [83] | 2015 | Cervical cancer | Mouse | BV | Increasing in FAS, DR3 and DR6 expression. Inhibition of NF-κB pathway. | Apoptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, A.; Strzemski, M.; Kurzepa, J.; Kurzepa, J. Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers 2023, 15, 3714. https://doi.org/10.3390/cancers15143714
Małek A, Strzemski M, Kurzepa J, Kurzepa J. Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers. 2023; 15(14):3714. https://doi.org/10.3390/cancers15143714
Chicago/Turabian StyleMałek, Agata, Maciej Strzemski, Joanna Kurzepa, and Jacek Kurzepa. 2023. "Can Bee Venom Be Used as Anticancer Agent in Modern Medicine?" Cancers 15, no. 14: 3714. https://doi.org/10.3390/cancers15143714
APA StyleMałek, A., Strzemski, M., Kurzepa, J., & Kurzepa, J. (2023). Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers, 15(14), 3714. https://doi.org/10.3390/cancers15143714











